Iron-containing nanomaterials: synthesis, properties, and environmental applications
Boris I.
Kharisov
a,
H. V.
Rasika Dias
b,
Oxana V.
Kharissova
*a,
Victor
Manuel Jiménez-Pérez
a,
Betsabee
Olvera Pérez
a and
Blanca
Muñoz Flores
a
aUniversidad Autónoma de Nuevo León, Monterrey, Mexico. E-mail: bkhariss@mail.ru
bDepartment of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, Texas 76019, USA
First published on 25th July 2012
Abstract
Available data on the iron-containing nanomaterials are reviewed. Main attention is paid to the following themes: synthetic methods, structures, composition and properties of the nano zerovalent iron (NZVI), and polymorphic forms of iron oxides and FeOOH. Synthetic methods summarized here include a series of physico-chemical methods such as microwave heating, electrodeposition, laser ablation, radiolytical techniques, arc discharge, metal-membrane incorporation, pyrolysis, combustion, reverse micelle and co-deposition routes. We have also included a few “greener” methods. Coated, doped, supported with polymers or inert inorganic materials, core–shell nanostructures, in particular those of iron and its oxides with gold, are discussed. Studies of remediation involving iron-containing nanomaterials are discussed and special attention is paid to the processes of remediation of organic contaminants (chlorine-containing pollutants, benzoic and formic acids, dyes) and inorganic cations (Zn(II), Cu(II), Cd(II) and Pb(II)) and anions (nitrates, bromates, arsenates). Water disinfection (against viruses and bacteria), toxicity and risks of iron nanomaterials application are also examined.
 Boris I. Kharisov | Dr Boris I. Kharisov (born in Russia in 1964) is currently a Professor and Researcher at the Universidad Autónoma de Nuevo León (UANL). Degrees: An MS in 1986, in radiochemistry and a PhD in inorganic chemistry in 1993, from the Moscow State University, Russia; Dr Hab. in physical chemistry in 2006 from Rostov State University, Russia. He is the co-author of six books, 122 articles, five book chapters, and has two patents. Co-editor: Three invited special issues of international journals. He is the member of the Editorial board of four journals. Specialties: Coordination and inorganic chemistry, phthalocyanines, ultrasound, and nanotechnology. |
 H. V. Rasika Dias | Dr Rasika Dias is a Professor of Chemistry at the University of Texas at Arlington. Born in Colombo, Sri Lanka, he received his BSc Degree from University of Peradeniya (Sri Lanka) and PhD from University of California, Davis (USA). Dr Dias was a Visiting Research Scientist at the DuPont Central Research & Development, Delaware before joining the University of Texas at Arlington faculty in 1992. Professor Dias specializes in inorganic and organometallic chemistry, and has been the author or co-author of several patents and over 160 publications. He has won several awards including the 2009 Southwest Regional American Chemical Society Award. |
 Oxana V. Kharissova | Dr Oxana V. Kharissova (born in 1969 in Ucrania, former USSR) is currently a Professor and Researcher at the UANL. Degrees: An MS in 1994, in crystallography from Moscow State University, Russia, and a Ph.D. in Materials from the Universidad Autónoma de Nuevo León, 2001, Mexico. Memberships: National Researchers System (Level I), Materials Research Society. She is the co-author of three books, four book chapters, 60 articles, and has two patents. Specialties: Nanotechnology (carbon nanotubes, nanometals, fullerenes), microwave irradiation and crystallography. |
 Victor Manuel Jiménez-Pérez | Dr Victor Manuel Jiménez-Pérez was born in Tlaxcala (México, 1974). He received his BSc (1998) in Chemistry at the Universidad Autonóma de Tlaxcala. In 2005, he received his PhD in Chemistry at the CINVESTAV under the supervision of Prof. Rosalinda Contreras in the field of tin chemistry. After postdoctoral work in the Prof. Herbert Roeskýs group at Universität Göttingen (2007), he jointed as a full-professor in Chemistry Faculty at the Universidad Autónoma de Nuevo León. So far, he is the author of more than 20 publications. His research is mainly focused on the design and synthesis of luminescent and sensor organometallic compounds and “green” chemistry. |
 Betsabee Olvera Pérez | Betsabee Olvera Pérez studied Industrial Chemistry at the Universidad Autonóma de Nuevo León (2011). After working for a short time in a private chemical company in Monterrey, Mexico, she is currently an active MSc student at the same University, working in the area of fabrication of iron nanoparticles by “green” chemical and biological methods. She is co-author of two submitted manuscripts. |
 Blanca Muñoz Flores | Dr Blanca Muñoz Flores (born in Tlaxcala, México, 1976) studied Industrial Chemistry at the Universidad Autonóma de Tlaxcala (1999). She received her PhD in Organic Chemistry (2008) at the CINVESTAV-IPN under direction of Prof. Norberto Farfán García. She moved to the Chemistry Faculty of the Universidad Autónoma de Nuevo León (2009) as a full professor. Presently, her research group is working on the design, synthesis, characterization and determination nonlinear optical (NLO) properties of organic and organometallic compounds, as well as on nanoalloys. So far, she is the author of more than 10 research publications in leading scientific journals. |
Introduction
According to the classic definition, nanomaterials are those materials whose key physical characteristics are dictated by the nano-objects they contain. Nanomaterials are classified into compact materials and nanodispersions. The first type includes so-called “nanostructured” materials, i.e., materials isotropic in the macroscopic composition and consisting of closely packed nanometer-sized units as repeating structural elements. Among nanomaterials, the iron-containing nanomaterials have attracted a great deal of interest due to their magnetic properties, allowing such applications as targeted drug delivery1 and other areas of nanomedicine. Core–shell iron (iron oxide)/gold nanoparticles are of special interest due to possibility to be controlled by magnetic fields (iron core) and functionalization (gold shell).2 Some applications of iron-containing nanomaterials have been recently reviewed,3,4 including their usefulness in water treatment (remediation of pollutants and bacteria).5–7 In this review, we pay main attention to the synthetic methods for obtaining Fe0 and FexOy based nanomaterials and their application in water treatment8 (remediation of (in)organic contaminants and disinfection of groundwater).General data on the iron-containing nanostructures
Iron-containing nanomaterials (Table 1) are mainly consisted of zerovalent iron (ZVI is currently a classic term, as well as NZVI (nano zerovalent iron)), iron nanoalloys or core–shell nanoparticles, and oxides, among others, such as, for example, ferrites. Iron typically exists in the environment as iron(II) and iron(III) oxides.9 Iron oxides (iron oxide nanoparticles are also referred in several reports to as superparamagnetic iron-oxide nanoparticles (SPIONs) although SPIONS have inducible magnetic properties),10,11 are one of the most important transition metal oxides of technological importance. Iron oxides is also a collective term for oxides, hydroxides and oxy-hydroxides composed of Fe(II) and/or Fe(III) cations and O2− and/or OH− anions. Sixteen pure phases of iron oxides, i.e., oxides, hydroxides or oxy-hydroxides are known to date. These are Fe(OH)3, Fe(OH)2, Fe5HO8·4H2O, Fe3O4, FeO, five polymorphs of FeOOH and four of Fe2O3. Characteristics of these oxide compounds include mostly the trivalent state of the iron, low solubility and brilliant colors. The main advantages of nano-iron among other nanomaterials are relatively low toxicity and biodegradability. In addition, iron is a relatively cheap and a widespread material.12| Composition | Description |
| Fe | The preparation of nanoparticles consisting of pure iron is a complicated task, because they always contain oxides, carbides and other impurities. |
| BCC-Fe (α-Fe) | α-Fe nanoparticles with a body-centered cubic (BCC) lattice can be prepared by (a) grinding a high-purity (99.999%) Fe powder, (b) evaporation of the metal in an Ar or He atmosphere followed by deposition on a substrate, (c) laser vaporization of pure iron. |
| FCC-Fe (γ-Fe) | In the phase diagram of a bulk Fe sample, this phase exists at the ambient pressure in the temperature range of 1183–1663 K, i.e., above the Curie point (1096 K). In some special alloys, this phase, which exhibits antiferromagnetic properties (the Neel temperature is in the 40–67 K range), was observed at room temperature. These nanoparticles, in several syntheses, can contain substantial amounts of carbon (up to 14 mass%). γ-Fe nanoparticles, synthesized by treatment of Fe(CO)5 with a CO2 laser radiation, may contain, for example, γ-Fe (30 atom%), α-Fe (25 atom%) and iron oxides (45 atom%). |
| “Amorphous” Fe (metallic glass) | Synthesis by: (a) the ultrasonic treatment of Fe(CO)5 in the gas phase or of a solution of Fe(CO)5 in decane under an inert atmosphere; (b) thermal decomposition of Fe(CO)5 in decalin (460 K) in the presence of surfactants. |
| Fe2O3 | Among several crystalline modifications of Fe2O3, there are two magnetic phases, namely, rhombohedral α-Fe2O3 (hematite) and cubic γ-Fe2O3 (maghemite) phases. The α-Fe2O3 and FeOOH (goethite) nanoparticles are obtained by controlled hydrolysis of Fe3+ salts. The γ-Fe2O3 nanoparticles are obtained by (a) mild oxidation (on treatment with Me3NO) of pre-formed metallic nanoparticles, (b) by direct introduction of Fe(CO)5 into a heated solution of Me3NO, (c) thermal decomposition of Fe3+ salts in various media, (d) vaporization of iron(III) oxide in a solar furnace with subsequent condensation, (e) mechanochemical synthesis milling iron powder in a planetary mill with water. |
| Fe3O4 (magnetite) | The cubic spinel Fe3O4 is ferrimagnetic at temperatures below 858 K. The route to these particles most often involves treatment of a solution of a mixture of iron salts (Fe2+ and Fe3+) with a base under an inert atmosphere. |
| In some cases, thermal decomposition of compounds containing Fe3+ ions under oxygen-deficient conditions is accompanied by partial reduction of Fe3+ to Fe2+. Fe3O4 nanoparticles can be also prepared by thermal decomposition of Fe2(C2O4)3·5H2O or by the controlled reduction of ultradispersed α-Fe2O3 in a hydrogen stream. | |
| FeO (wustite) | Cubic Fe2+ oxide is antiferromagnetic (Tc = 185 K) in the bulk state. Synthesis technique: joint milling of Fe and Fe2O3 powders taken in a definite ratio gives nanoparticles consisting of FeO and Fe. On heating of these particles at temperatures of 250-400 °C, the metastable FeO phase disproportionates to Fe3O4 and Fe, while above 550 °C it is again converted into nanocrystalline FeO. |
| α-FeOOH (goethite) | The orthorhombic α-FeOOH (goethite) is antiferromagnetic in the bulk state and has Tc = 393 K, β-FeOOH (akagenite) is paramagnetic at 300 K, γ-FeOOH (lipidocrokite) is paramagnetic at 300 K and δ-FeOOH (ferroxyhite) is ferrimagnetic. As a rule, α-FeOOH is present in iron nanoparticles as an admixture phase. |
| FexCy | Iron carbide is often present in Fe-containing nanoparticles. It is formed either upon decomposition of organic ligands present in the starting MCC or upon the reaction of Fe with the organic medium. Thermal decomposition of Fe(CO)5 on a carbon support forms Fe78C22 nanoparticles. |
| Ferrofluids | Ferrofluids or so-called magnetic liquids are suspensions of colloid magnetic particles stabilized by surfactants in liquid media. The magnetic phase in ferrofluids can be represented by magnetite, ferrites (group of nonmetallic, ceramic like, usually ferromagnetic compounds of ferric oxide with other oxides) and FexCy particles resulting from the thermal decomposition of Fe(CO)5. |
| Fe–Co alloys | The saturation magnetization of Fe–Co alloys reaches a maximum at a Co content of 35 atom%. Fe, Co and Fe–Co (20, 40, 60, 80 atom%) nanoparticles with a structure similar to the corresponding bulk phases can be prepared in a stream of hydrogen plasma. |
| Fe–Ni | Bulk samples of iron–nickel alloys are either nonmagnetic or are magnetically soft ferromagnets (for example, permalloys containing >30% of Ni and various doping additives). |
| Fe–Pt | Fe–Pt nanoparticles can be prepared by joint thermolysis of Fe(CO)5 and Pt(acac)2 in the presence of oleic acid and oleylamine. The reaction of FePt nanoparticles with Fe3O4 followed by heating of the samples at 650 °C in an Ar + 5% H2 stream resulted in a FePt–Fe3Pt nanocomposite with unusual magnetic characteristics. |
Metallic or zerovalent iron (Fe0) is a moderate reducing reagent, which can react with dissolved oxygen and to some extent with water (eqn (1) and (2)):
| 2Fe0(s) + 4H+(aq) + O2(aq) → 2Fe2+(aq) + 2H2O(l) | (1) |
| Fe0(s) + 2H2O(aq) → Fe2+(aq) + H2(g) + 2OH−(aq) | (2) |
The above equations are the classical electrochemical/corrosion reactions by which iron is oxidized from exposure to oxygen and water. The corrosion reactions can be accelerated or inhibited by manipulating the solution chemistry and/or solid (metal) composition.
NZVI particles range from 10 to 100 nm in diameter.13 They exhibit a typical core–shell structure. The core consists primarily of zerovalent or metallic iron whereas the mixed valent [i.e., Fe(II) and Fe(III)] oxide shell is formed as a result of oxidation of the metallic iron (Fig. 1). The presence of excess stabilizers was shown to stabilize the core/shell nanoparticles from further oxidation.14 Various organic molecules can be used for functionalization of NZVI to improve the stability of their aqueous dispersions (strong inhibition of the agglomeration of the iron nanoparticles), for instance PEG, methoxyethoxyethoxyacetic acid (MEEA), polyacrylic acid and 1,4-butanediphosphonic acid (Fig. 2) have been used for this purpose.15
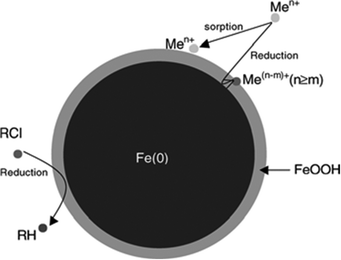 | ||
| Fig. 1 Schematic diagram of zerovalent iron nanoparticle. Reproduced with permission. | ||
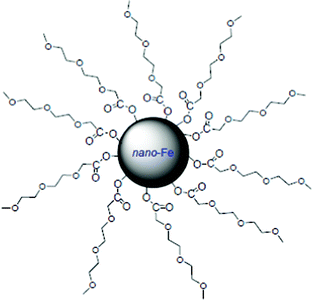 | ||
| Fig. 2 Scheme of a nanoscale iron particle covered by MEEA. | ||
Among iron oxides (SPIONs), magnetite (Fe3O4) and maghemite (γ-Fe2O3) are of a particular interest. Magnetite is an inverse spinel ferrite. The oxygen ions form a close-packed cubic lattice with the iron ions located at two different interstices between them, tetrahedral (A) sites and octahedral (B) sites. Chemically, magnetite/maghemite can be represented by the formula: Fe3+ [Fe2+1−yFe3+1−yFe3+1.67y□0.33y]O4, where y = 0 for pure magnetite and y = 1 for pure maghemite (fully oxidized magnetite). In the temperature range from room to Curie temperature (Tc = 860 K) the A sites are populated by Fe3+ ions and the B sites are populated equally by Fe3+ and Fe2+ ions. That way, twice as many sites are populated with Fe3+ than with Fe2+ ions. Although a simple dehydration of lepidocrocite (γ-FeOOH) topotactically transforms into γ-Fe2O3, commercial manufacturing of maghemite follows the multistep process (3):
| (α and/or γ)-FeOOH (oxidation) → α-Fe2O3 (reduction) → Fe3O4 (controlled oxidation) → γ-Fe2O3 | (3) |
SPIONs typically consist of two components, an iron oxide core of one or more magnetic crystallites embedded in a coating. The SPIONs' core can be composed of magnetite (Fe3O4) and/or maghemite (γ-Fe2O3). The size of SPIONs makes important contribution to their fate in organism. Categories of SPIONs, based on their overall diameter (including iron oxide core and hydrated coating), are noted in the literature as oral or micron-sized SPIONs between 300 nm and 3.5 μm; standard or small SPIONs (SSPIONs) at approximately 60–150 nm; ultrasmall SPIONs (USPIONs) of approximately 10–50 nm; and monocrystalline iron oxide nanoparticles (MION—a subset of USPIONs) of approximately 10–30 nm. MION are so named to underline the single crystal nature of their core. This is in contrast to SPIONs greater than 50 nm that are comprised of multiple iron oxide crystals.16 SPIONs have much larger magnetic susceptibilities (compared with strictly paramagnetic materials) as the entire crystal aligns with the applied field due to its single crystal nature. Hence SPIONs are useful as contrast agents or for hyperthermic treatment of malignant tumors.17
Magnetic nanoparticles offer advantages over non-magnetic nanoparticles because they can easily be separated from water using an external magnetic field. Separation using magnetic gradients, the so-called high magnetic gradient separation (HGMS), is a process widely used in medicine and ore processing.18 This technique allows one to design processes where the particles can not only be used to remove compounds from water but also be separated and then be recycled or regenerated. This approach has been proposed with magnetite (Fe3O4), maghemite (γ-Fe2O3) and jacobsite (MnFe2O4) nanoparticles for removal of chromium(VI) from wastewater. In addition, the following iron-based compositions are usually considered in the literature as media for magnetic information recording: α-Fe, γ-Fe2O3, Fe2O3–Fe3O4, Co–γ-Fe2O3, CrO2, BaFe12O19. In recent years, nanocrystalline (5–10 nm) CoPr, FePt, CoPt, CoSm, SmFeSiC and SmFeAlC films are regarded as the most promising candidates for magnetic materials.
Types of materials containing iron-based magnetic nanoparticles are, in general, as follows: (1) nanoparticles on a substrate surface (for instance, α-Fe or Fe3O4 on ZrO2), (2) iron-containing nanoparticles in matrices, including (a) inorganic matrices (such as zeolites, molecular sieves, glass, xerogels and silica gel, dispersed carbon, highly dispersed SiO2 and Al2O3, magnetic nanoparticles in non-magnetic metals (for example, Fe–Ag or Fe–Hg samples) and organic polymer matrices (ion exchange resins, soluble polymers (such as PVA), polybutadiene, polystyrene and styrene copolymers with butadiene, 4-vinylpyridine, N-vinylpyrrolidone, phenylvinylketoxime, carbochain polymers without heteroatoms and functional groups, polymers containing heteroatoms (such as polyimines or polyvinylpyrrolidone) or block copolymers (for example, [NORCOOH]30[MTD]300 block copolymers (NORCOOH is 2-norbornerne-5,6-dicarboxylic acid, MTD is methyltetracyclododecene)).
In addition to the NZVI and SPIONs, a variety of composite inorganic iron-based nanomaterials have been discovered, in particular core–shell Fe (or FexOy)/Au (see section below) or more complex trimetallic nanoparticles such as (Fe60Co49)core/Au.19 Figuerola et al.20 classified them based on their levels of compositional and/or structural complexity: (1) nanostructures made of an iron-based magnetic material different from iron oxide; (2) nanostructures whose morphology is not a sphere (e.g. hollow structure); (3) multi-material nanostructures, i.e. each of them is made of two or more domains of different inorganic materials joined together. Some examples of these approaches are as follows. Thus, the strategy for functionalization of CoFe2O4 superparamagnetic Nps (nanoparticles) with a mixture of amino and thiol groups that facilitate the electrostatic attraction and further chemisorption of gold Nps, respectively, is shown in Fig. 3.21 Related MnFe2O4 nanoparticles have surpassed SPIONs as contrast agents for MRI in vivo.22 The enhanced sensitivity of MnFe2O4 nanoparticles has been proven in vivo, enabling detection of a tumor mass as small as 50 mg. The composite iron-based nanocrystals can be used for treatment of malignant tumors. Thus, FePt nanoparticles functionalized with luteinizing hormone-releasing hormone (LHRH) peptide have enhanced cytotoxicity against ovarian cancer cells that express LHRH-receptors.23 In the acidic environment of lysosomes, these nanoparticles release toxic iron species, which catalyze the formation of reactive oxygen. The latter is toxic for cells as it can damage lipid membranes, DNAs and proteins. Nanoshells CoS2@FePt also possess better antitumor activity than that of cis-platin.24
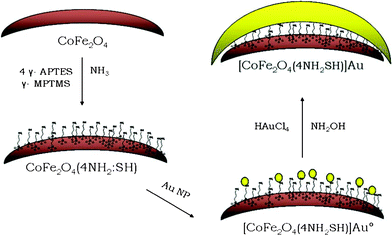 | ||
Fig. 3 Reaction strategy showing the successive steps for gold covering process onto 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 amino 1 amino![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mercapto functionalized cobalt ferrite Nps. Reproduced with permission from Elsevier Science. mercapto functionalized cobalt ferrite Nps. Reproduced with permission from Elsevier Science. | ||
Several groups revealed that ZVI nanoparticles exhibit antimicrobial properties (see section below) against Gram-negative E. coli, Pseudomonas fluorescens and Gram-positive Bacillus subtilis var. niger microorganisms.25,26 The inactivation of E. coli by ZVI nanoparticles could be because of the penetration of the small particles (size ranging from 10–80 nm) into E. coli membranes. ZVI nanoparticles could then react with intracellular oxygen, leading to oxidative stress and causing disruption of the cell membrane. In addition, iron oxide nanoparticles also possess antimicrobial properties (see the section on water disinfection below). In general, iron-based nanomaterials are thoroughly investigated according to their relatively low toxicity integrated with unique properties in order to exploit them in such biomedical applications as remediation of environment, development of novel diagnostic tools and methods for individualized treatment.
Synthesis techniques for NZVI and nano-iron oxides
There is a series of special techniques for preparation of iron and other nanomaterials, which could be artificially divided into “physical”, “physico-chemical”, “chemical” and “biological” methods. Of course, such a separation is conditional; almost any physical process in these transformations is accompanied in these reactions by a chemical transformation. We consider as “physical” methods those requiring special equipment, for example a 60Co source for γ-irradiation of samples or sputtering equipment. Application of different methods has led to formation of iron-containing nanostructures having distinct compositions, iron oxidation states and structures. It is worth mentioning that the use of Mössbauer spectroscopy is almost a strict requirement in working with Fe-nanostructures in order to determine exact iron oxidation number. Tables 2 and 3 contain examples of methods used and the corresponding products.| Method | Description | Examples of products |
| Physical methods | ||
| Condensation methods | The method of nanoparticle synthesis from supersaturated metal vapors is based on the classical nucleation theory in which the nascent phase clusters are described by the spherical liquid drop model. Nanoparticles (clusters) are prepared using various means of metal evaporation: laser vaporization, thermal vaporization, arc discharge, plasma vaporization and solar energy-induced evaporation. Special installations are needed. In the classical thermal vaporization method, a metal or alloy sample is heated in a tungsten boat in an argon or helium stream. The atoms of the vaporized metal lose kinetic energy upon collisions with inert gas atoms, gather in clusters and condense on a cooled substrate as a nanodispersed powder. | Heterometallic nanoparticles (∼30 nm) with the composition Fe–M (M = Ni, Mn, Pt, Cr). |
| Nanodispersion of a compact material | The mechanochemical dispersion of a compact material in mills of various designs is applied, as well as electrolytic erosion and electrochemical generation. | γ-Fe2O3 nanoparticles. |
| Chemical synthesis of magnetic nanoparticles | ||
| Thermolysis of iron-containing compounds | Metal organic chemical vapor deposition (MOCVD) technique. | Nanodispersed Fe oxides from [Fe(OtBu)3]2 precursor. |
| α-Fe nanoparticles from Fe(CO)5 as a precursor. | ||
| Cold-plasma chemical vapor deposition | The growth occurs on an Fe catalyst supported by kanthal (iron–chromium–aluminium (FeCrAl) alloys) wires while heating under a hydrocarbon precursor gas and plasma created by a bias. A possible application for such nanotubes on metallic wires is in luminescent tubes. | Growth of CNTs on Fe wires from hydrocarbon precursor. |
| Aerosol/vapor methods | In spray pyrolysis, a solution of ferric salts and a reducing agent in organic solvent is sprayed into a series of reactors, where the aerosol solute condenses and the solvent evaporates. The resulting dried product consists of particles whose size depends upon the initial size of the original droplets. | Maghemite γ-Fe2O3 nanoparticles. |
| Combustion synthesis | Reaction process is completed within a few minutes. The method uses no additional fuel and nitrate, which is present in the precursor itself, to drive the reaction. | Iron oxide/iron (γ-Fe2O3, α-Fe2O3, Fe3O4 and Fe) coated carbons (activated carbon, anthracite, cellulose fiber) or silica. |
| Chemical precipitation | Addition of alkali to iron salt solutions and keeping the suspensions for ageing. A large amount of nanoparticles can be synthesized. | FeOOH, Fe3O4 or γ-Fe2O3. |
| Coprecipitation | Alkaline coprecipitation of Fe(III) and Fe(II) salts in aqueous media: | Fe3O4 |
| 2Fe3+ + Fe2+ + 8OH− → Fe3O4 + 4H2O | ||
| It is essential to adjust the stoichiometric ratio of Fe(II)/2Fe(III) at the initial stage because it is difficult to control the oxidation kinetics of Fe(II) afterward. Oxygen also plays an important role in the formation of single phase magnetite; usually bubbling nitrogen gas directly into the media prior to reaction is efficient enough for removal of oxygen. | ||
| Precipitation by anhydrous solution | Anhydrous solution approaches for the production of metal oxide nanoparticles and in particular iron oxide. | γ-Fe2O3 nanoparticles (direct oxidation of iron pentacarbonyl in the presence of oleic acid with trimethylamine oxide as an oxidant). |
| Decomposition of iron-containing compounds by ultrasonic treatment | Use of strong sources of ultrasound. At very high temperatures, hot spots generated by the rapid collapse of sonically generated cavities, allows for the conversion of salts into nanoparticles. | Iron nanoparticles from Fe(CO)5 as a precursor, Fe2O3 and Fe3O4 nanoparticles. |
| The reduction of iron-containing compounds | Magnetic metallic nanoparticles can be prepared from metal salts using strong reducing agents, namely, alkali metal dispersions in ethers or hydrocarbons, alkali metal complexes with organic electron acceptors (e.g., naphthalene), NaBH4 and other complex hydrides. | Using NaBH4 as a reductant, both homo- (Fe, Co, Ni) and heterometallic (Fe–Co, Fe–Cu, Co–Cu) nanoparticles are obtained as amorphous powders containing substantial amounts of boron (20 mass% or more). |
| Biomimetic mineralization | Bio-mimetic mineralization within protein cages provides an attractive alternative approach for synthesizing monodisperse nanosized particles. Since maximum particle sizes are limited by the cage inner diameter, reactions carried out in cages can lead to highly monodisperse size distributions, particularly if nucleation rather than growth is the rate-limiting stage of the synthesis. | Fe2O3 nanoparticles. |
| Synthesis in reverse micelles | A microemulsion is defined as the thermodynamically stable isotropic dispersion of two immiscible liquids, stabilized by a monolayer of surfactant at the interface of the two liquids. Synthesis of nanoparticles in nanosized “reactors” as the size of “nanoreactors” can be controlled within certain limits. A micelle is an example of these nanoreactors. Reverse micelles are tiny drops of water stabilized in a hydrophobic liquid phase due to the formation of a surfactant monolayer on their surface. | |
| Microemulsion technique | Microemulsions may consist of oil-in-water or water-in-oil, depending on the concentration of the different components. By varying the concentration of the dispersed phase and the surfactant, it is possible to tailor the size of the droplets in the approximate range of 1–100 nm. | α-FeOOH, magnetite nanoparticles. |
| Sol–gel method and forced hydrolysis technique | Extremely versatile method since it allows the formation of a large variety of metal oxides at relatively low temperatures via the processing of metal salt or metal alkoxide precursors. | α-FeOOH, γ-FeOOH and Fe3O4 (prepared by forced hydrolysis technique). |
Reduction of Ni2+ and Fe2+ ions inserted in silica gel in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with hydrogen at 733–923 K results in Ni3Fe nanoparticles (4–19 nm) within the SiO2 matrix. 1 ratio with hydrogen at 733–923 K results in Ni3Fe nanoparticles (4–19 nm) within the SiO2 matrix. |
||
| Surfactant mediated/template synthesis | This technique uses neutral or charged template molecules. | α-Fe2O3 nanorods and nanotubes (polyisobutylene bissuccinimide or Span80 as surfactants). |
| Synthesis of magnetic nanoparticles at a gas–liquid interface | Nanoparticles can also be synthesized in the absence of solid substrates or matrices by redox reactions at an interface between two phases, one containing a metal compound (precursor) and the other, the reducing agent. | γ-Fe2O3 nanoparticles from Fe(CO)5 as a precursor. |
| UV-irradiation | Iron pentacarbonyl is exposed to an UV-irradiation. | γ-Fe2O3 nanoparticles Fe(CO)5 as a precursor. |
| Hydrothermal technique | Hydrothermal treatment of iron salt could generate iron oxides when the applied conditions are appropriate. | Decomposition of α-FeOOH to α-Fe2O3. |
| Flow injection | The technique consists of continuous or segmented mixing of reagents under laminar flow regime in a capillary reactor. | Magnetite nanoparticles. |
| Electrochemical methods | Electrons act as reactant. It is an environmental friendly process with no pollution. However, the costly platinum is used as an electrode and not for reuse in aqueous solution. | Maghemite or γ-Fe2O3 nanoparticles. |
| Biosynthesis | Biosynthesis of iron oxide nanoparticles with the help of Fe(III)-reducing bacteria (Shewanella sps., Geobacter sps., Thermoanaerobacter ethanolicus etc.), SRB (Archaeoglobus fulgidus, Desulfuromonas acetoxidans) and MTB (Magnetospirillum magnetotacticum, M. gryphiswaldense), are reliable, ecofriendly and economic at ambient temperatures, pressures and neutral pH, that can be used in environmental remediation. | Iron oxides. |
| Method | Description | Examples of products |
| Specific methods for the preparation of particular types of iron-containing nanoparticles | ||
| Heterometallic nanoparticles | As a rule, these particles are prepared by simultaneous thermal decomposition of two metal complexes of different compositions (hydrogen is used most often as the reducing agent). | Heterometallic nanoparticles, Fe48Pt52 and Fe70Pt30, from Pt(acac)2 and Fe(CO)5. |
| Fe–Co nanoparticles from cobalt ferrite CoFe2O4 nanoparticles or (η5-C5H5)CoFe2(CO)9. | ||
| Ferrites | The key method for the preparation of powders of magnetic hexagonal ferrites with a grain size of more than 1 μm includes heating of a mixture of the starting compounds at temperature above 1000 °C (so-called ceramic method). | MnFe2O4 (40 nm), MgFe2O4 (6–18 nm), Co0.2Zn0.8Fe2O4 (2–45 nm), BaFe12−2xSnxZnxO19 (45 nm), SrFe12O19 (30–80 nm). |
| Magnetic nanoparticles with anisotropic shapes | (1) Decomposition of metalloorganic compounds, in particular by ultrasound in magnetic fields. | Fe nanoparticles from Fe(CO)5 as a precursor. |
| (2) The standard way of synthesis of nanothreads and nanowires composed of anisotropic Fe and Co nanoparticles includes the electrolysis of solutions of the metal salts at an aluminium cathode, which is pre-coated by an Al2O3 layer containing channels with a diameter of 18–35 nm and a depth of up to 500 nm. During the electrolysis, these channels are filled by the reduced metal. After completion of the process, the matrix is dissolved in a mixture of acids to separate the nanoparticles. | ||
| 3) Electric arc decomposition of Fe(CO)5 resulted in thread-like (10 to 100 nm in diameter) compounds also consisting of α-Fe and Fe3C nanoparticles. | ||
| Methods for the synthesis of stoichiometrically inhomogeneous magnetic nanoparticles | ||
| Oxidation of nanoparticles | Oxidation of formed nanoparticles by oxygen-containing gas mixtures. | Iron oxide nanoparticles from Fe nanoparticles (contact with air). Iron passivation is possible. α- and γ-Fe2O3 by oxidation of Fe1−xCx nanoparticles. |
| Chemisorption of small molecules on a nanoparticle surface | The interaction of α-Fe nanoparticles with small CO, H2 and O2 molecules. Chemisorption of these molecules on a nanoparticle surface induces only minor changes in the parameters of the Mössbauer spectra. Studies of the reaction of α-Fe nanoparticles with nitrogen showed that only the Fe atoms of the surface layer participate in the chemical binding of nitrogen (the process starts at 300 K). | Chemisorption of N2 on α-Fe surface. |
| Targeted modification of the surface of magnetic nanoparticles | The immobilisation of biological molecules (amino acids, DNA, simple peptides, polysaccharides, lipids) on the surface of magnetic nanoparticles. | γ-Fe2O3 nanoparticles containing enzymes on the surface. |
A host of modern techniques, as described below, are being currently used for obtaining nanoparticles of Fe-containing nanomaterials, although well-known classic wet chemical routes are not forgotten and continue to be applied for production of NZVI27 (in particular, by classic borohydride reduction),28 Fe2O3 (sol–gel method29 or electrochemical deposition from solution30), or Fe3O4 (hydrolysis of Fe3+ and Fe2+ salts in the presence of urea and NaOH with the following ultrasonic treatment of FeO(OH)/Fe(OH)2). Morphology and sizes of the resulting products, produced by various methods, can vary depending on the synthetic method used and conditions. For instance, it was shown that the Fe3O4 nanoparticles31 produced by radiofrequency nitrogen plasma had regular spherical form while the particles produced by wet chemical synthesis had well shaped cubic form. The size distribution of the plasma prepared oxides was wider, producing small particles with size in the range of 25–80 nm, and larger particles with size above 100 nm. The wider particle size distribution is characteristic of plasma prepared powders and it was explained by the authors by different growth conditions of particles due to temperature and velocity gradients of the plasma flow. This does not take place in the wet chemical route: the liquid phase synthesis provides preparation of Fe3O4 nanoparticles with average particles size in the range of 16–26 nm. Similarly, the shape, size and production rate of Fe2O3 nanoparticles, obtained by electrodeposition, were strongly influenced by the electrochemical conditions (e.g. FeCl3 precursor concentration and current density).
NZVI and Fe–M nanoalloys or core–shell nanostructures
Among physical methods, the method of electrical explosion of wires for production of nanopowders was reported.32,33 Intermetallic species can be also prepared by the arc discharge method, for instance Fe–Sn nanoparticles.34 The synthesized nanoparticles have a shell/core structure with a SnO2 shell of 5–10 nm in thickness and a core of polycrystalline intermetallic compounds. It was found that the intermetallic compounds FeSn2 and Fe3Sn2 were generated and coexist with the Sn phase as a single nanoparticle. Microwave treatment (used to produce mainly inorganic compounds/composites/materials, and to a lesser extent, organic/organometallic compounds), is an alternative method to common heating of mixtures/precursors. The transformations of metals in MW field are generalized in a comprehensive recent book.35 Metallic nanoparticles in distinct forms can also be produced using MW. For instance, Fe based nanoparticles have been produced using the microwave–polyol process in ethylene glycol at 100 and 150 °C in the presence of poly(vinyl pyrrolidone) and dodecyl amine.36 In addition, pulsed excimer laser radiation at 248 nm wavelength was used to ablate ~2 μm feedstock of permalloy (Ni 81%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe 19%) under both normal atmospheric conditions and in other gases and pressures.37 α-Fe particles were also prepared using a modified metal–membrane incorporation technique based on diffusing metal ions through a dialysis membrane.38 The diffusion-time varied up to 15 min.
Fe 19%) under both normal atmospheric conditions and in other gases and pressures.37 α-Fe particles were also prepared using a modified metal–membrane incorporation technique based on diffusing metal ions through a dialysis membrane.38 The diffusion-time varied up to 15 min.
For ZVI,39 the easiest, standard and most popular synthetic method is through the reduction of ferric Fe(III) or ferrous Fe(II) salts with NaBH4, NaAlH4 or LiAlH4 as reducing agents. Thus, NZVI was synthesized (Fig. 4, reaction (4))40 in ethanol medium by the method of reduction of FeXn (X = Cl, OH, OR, CN, OCN, SCN) using sodium borohydride under atmospheric conditions.41 The resulting iron nanoparticles were found to be mainly in zerovalent oxidation state and remained without significant oxidation for weeks. Among other reductants used for obtaining iron nanoparticles, poly(vinyl alcohol-co-vinyl acetate-coitaconic acid) is an example.42
| 2FeCl3 + 6NaBH4 + 18H2O → 2Fe0 + 6NaCl + 6B(OH)3 + 21H2 | (4) |
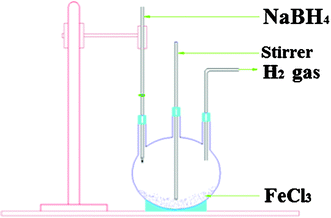 | ||
| Fig. 4 Schematic diagram for synthesis of iron nanoparticles. Reproduced with permission. | ||
A patent43 describes a route to metal nanoparticles by thermal decomposition of iron acetate Fe(OOCCH3)2, placed in a reaction vessel with a passivating solvent such as a glycol ether. The contents of the reaction vessel were mixed for a period of time to form a substantially homogenous mixture. The contents of the reaction vessel were then refluxed at a temperature above the melting point of the iron acetate. The desired particle size was achieved by controlling the concentration of metal salt in the passivating solvent and by varying the amount of reflux time. As an application of the pyrolysis technique, we would like to mention obtaining iron nanoparticles embedded in a carbon matrix from metal phthalocyanine,44 and carbon-encapsulated Fe nanoparticles (size between 5 and 20 nm) via a picric acid-detonation-induced pyrolysis of ferrocene, which is characterized by a self-heating and extremely fast process.45
There are also “green” techniques46–50 for nanoparticle synthesis. Among these methods, use of plant extracts and other natural products as reductants and capping agents for NZVI and various other Fe-containing nanoparticle preparation is interesting51 as well. For example, the use of herbal tea extracts to reduce iron(III) chloride to iron nanoparticles (50 nm) has been reported.52 This process as well as several others involving Ag and Au salts employs the principle that the tea extracts (green tea, chamomile tea, hibiscus tea and the normal table tea) contain phenolic compounds that can act as reducing and capping agents in their application. A similar green single-step preparation of Fe nanoparticles using tea (Camellia sinensis) polyphenols has been described.53 The expedient reaction between polyphenols and ferric nitrate occurs within a few minutes at room temperature and is indicated by color changes from pale yellow to dark greenish/black in the formation of Fe nanoparticles. The nanoparticles were utilized to catalyze H2O2 for treatment of organic contamination. In addition, nanoscale zerovalent iron (NZVI) particles, prepared using tea polyphenols,54 were assessed with methyl tetrazolium (MTS, (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)) and lactate dehydrogenase (LDH) toxicological assays, and some of them were found to be nontoxic when compared with control samples prepared using the conventional borohydride reduction protocols. The authors anticipated the development of various technological and environmental remediation applications for these stable particles including dechlorination of dibenzo-P-dioxins, reduction of chlorinated ethanes, arsenic and hexavalent chromium removal.
A variety of mainly physico-chemical methods have been used for the synthesis of Fe-containing bi- and polymetallic alloys and core–shell nanostructures. For example, high-entropy Nd–Fe–Co–Ni–Mn alloy nanostructure films were synthesized55 by electrodeposition at room temperature. It was shown that the surfaces of the films, prepared at −2.2 V on Ti substrates for 5 min, were composed of great deal of close-grained and homogeneous nanoparticles with size about 200 nm. The film becomes more compact with a longer deposition time. The EDS indicated that the five elements were co-deposited. Monodisperse crystalline zerovalent Fe, Fe–Ni, Fe–Pd nanowires were synthesized similarly.56 Prior to nanowire fabrication, alumina nanotemplates with controlled pore structure (e.g. pore diameter and porosity) were fabricated by anodizing high purity Al foil in H2SO4. After fabrication of alumina nanotemplates, Fe, Fe–Ni and Fe–Pd nanowires were electrodeposited within the pore structure. 200 nm-thick Fe–Pt nanocrystalline magnetic films having planar texture were prepared using magnetron sputtering and crystalline annealing in a magnetic field.57 The prepared films were used to manufacture trial samples of magnetic memory devices having open Kittel domain structure for super-high density recording heads. For Fe3Pt, FePt and FePt3, heat formation was determined.58 X-Ray diffraction, magnetic measurements and Mössbauer spectroscopy were used to study magnetic properties and hyperfine interaction parameters of nanocrystalline (<10 nm) and bulk bcc Fe, Fe90Ge10 and Fe77Al23 alloys.59 It was established that the nanocrystalline state does not influence the formation of specific saturation magnetization, Curie temperature, isomer shift and hyperfine magnetic field. A slight increase (∼20%) of the width of the nanocrystalline iron Mössbauer spectral lines was observed.
Iron–gold alloys and core–shell nanoparticles
A special case, which should be discussed apart, corresponds to core–shell nanoparticles on the basis of iron and its oxides with gold due to the following justification. Finely divided iron has long been known to be pyrophoric, which is major reason that Fe nanoparticles have not been more fully studied.60 This extreme reactivity has traditionally made Fe Nps difficult to study and problematic for practical applications. However, iron has a great deal to offer at the nanoscale, including very potent magnetic, catalytic and medical applications, so methods for reducing its reactivity with simultaneous retention of magnetic and catalytic properties have been developed. Although pure metal iron Nps are unstable in air, by coating the Nps surface with a noble metal, the formed air-stable Nps are protected from oxidation and retain most of the favorable magnetic properties, which possess the potential for applications mentioned above, as well as in high density memory devices by forming self-assembling nanoarrays.61Gold is very promising coating for magnetic and other particles to be functionalized for targeted drug delivery. This metal (Au) as well as iron oxides Fe2O3 and Fe3O4, are biocompatible and suitable for human use. It is also possible to track within human body, and can be functionalized with organic and bioorganic molecules, such as proteins or enzymes. At the same time, Nps of iron and its oxides possessing magnetic properties can be delivered to an organ, tissue, or cancer tumor by using an external magnetic field. So, the unique combination of the nanoscale magnetic core and the functional shell makes Fe-doped Au Nps ideal for biological and biomedical applications due to their conjugation chemistry, optical properties, and surface chemistry.
During the last decade, considerable experimental efforts have been dedicated to biomedical applications of coated magnetic Nps, such as in magnetic resonance imaging contrast enhancement, tissue repair, immunoassay, detoxification of biological fluids, hyperthermia, targeted drug delivery, and cell separation. Results of the investigations in the area of gold-coated iron (iron oxides) core Nps are detailed in a series of monographs,62–68 as well as in reviews69,70 and patents71,72 describing strategies for the synthesis of these Nps, characterization of the core/shell nanostructures, and exploration of potential applications of the core/shell nanomaterials in terms of biological and catalytic interfacial reactivities.
Types of Fe/Au Nps
Water-soluble Fe/Au alloy Nps (Fe/Au alloy Nps are solid solutions where iron atoms substitute gold sites in the face center cubic lattice) have been reported.73 The diameter of these alloy Nps was 4.9 ± 1.0 and 3.8 ± 1.0 nm for two different precursors of iron, ferrous sulfate heptahydrate and iron pentacarbonyl;74 while the estimated particle iron content was 14.8 ± 4.7 mol%. Formation of alloy nanostructure with a narrow distribution of particle sizes and three compositions Au0.25Fe0.75, Au0.5Fe0.5 and Au0.75Fe0.25 by a polyol process while retaining the optical and magnetic properties of the individual components has been discussed.75 Fe/Au Nps of L10 type (mean composition Fe-22 atom% Au) were fabricated by annealing Fe/Au particles.76 Au-rich (>32 atom%) and Au-poor (<16 atom%) regions were formed in most of particles.The major part of publications has been devoted to Fe cores coated with Au shells.77–80 For instance, nanosized iron–gold magnetic Nps with an average particle size 5–25 nm were prepared by a reverse micelle method, which is the principal technique in obtaining core–shell Nps, together with the polylol technique and homogenous solution reduction, efficiently used in many reports. The magnetic properties measurements confirmed behavior typical of a superparamagnetic system.81,82 The XPS studies of the core–shell Fe/Au Nps indicate that besides Fe0 inside the cores, small amounts of Fe(II,III), located onto the gold surface, were also formed during the sample preparation. A certain number of reports have mentioned pure iron core, for instance Fe core/Au shell (11 nm Fe core, 2.5 nm Au shell) without iron oxides.83
The general method for obtaining Fe core/Au shell Nps consists of subsequent reduction of iron and Au salts by NaBH4 and other reductants in aqueous or organic media in the presence of surfactants for preventing agglomeration of the resulting Nps. In a series of reports, the characterized Nps were considered as containing pure metallic iron as a core. However, it should be especially emphasized that the authors of the communications who applied Mössbauer and X-ray absorption (XAS) spectroscopy, together with SEM, TEM and other techniques, noted that only the use of two additional methods mentioned above can give complete information on the state of iron in the core. Thus, gold passivated Fe Nps were prepared in a reverse micelle of cetyltrimethylammonium bromide,84 and their studies by a series of methods above, including Mössbauer spectral study (57Fe), showed the presence of iron in the forms of α-Fe (major part), unexpected Fe1-xBx alloy and several poorly crystallized ordered Fe2O3 components, as well as residual paramagnetic Fe(II) and Fe(III), indicating a more complex structure than had been believed, although the powder X-ray diffraction of Fe/Au Nps-containing samples revealed both the presence of crystalline α-Fe and gold and the absence of any crystalline iron oxides or other crystalline products. In another report,85 the oxidation state of Fe in Fe/Au core–shell Nps was analyzed showing that, in contrast to many previous reports assuming the metallic state of iron in Fe/Au Nps obtained from reverse micelles, the iron component was found to be fully oxidized, according to X-ray absorption spectroscopy data (this method was considered as “an ideal tool for characterizing iron Nps”).
Additionally to the above mentioned reverse micelle technique, other methods have also been well-developed for obtaining Fe/Au Nps. Thus, Fe/Au Nps (8 nm) were prepared by sequential high temperature decomposition of organometallic compounds in a coordinating solvent and functionalized with alkanethiolate ligands preventing aggregation, enabling solubility in a series of both hydrophilic and hydrophobic solvents and allowing further derivatization via ligand exchange reactions.86 Fe/Au Nps, confirmed to be solid solutions, were electrodeposited on an amorphous carbon electrode in a three-compartment glass-made electrochemical cell from aqueous electrolytes at room temperature using 1 mM FeSO4 and HAuCl4 solutions as precursors and CsClO4 as supporting electrolyte in Ar atmosphere.87 It has been reported that these Nps were homogeneously alloyed and the composition of the alloyed Nps was dependent on the electrodepositing potential. In comparison with similarly prepared Au/Ni and Au/Co Nps, the chain length for Fe/Au Nps is lower: Fe/Au < Ni/Au < Co/Au (the largest length was observed for pure Au Nps); this suggests that the transition metals in the alloyed Nps may prohibit the agglomeration of the alloyed particles. Radiolytical reduction of mixed AuIII/FeII ethylene glycol solutions led to two kinds of particles: 2 nm Fe rich Nps as well as large rods (a few tens of nm) and faceted particles rich in gold and containing a small amount of iron.88 The adjunction of iron to gold enhances remarkably its electro-catalytic properties toward oxygen and proton reduction. The authors noted that Au–Fe system is very promising for application in fuel cells. Pulsed laser deposition of the Fe–Au alloy (35% Fe) in a mesoporous alumina membrane template allowed formation of strongly paramagnetic Fe/Au Nps (17% Fe, 46 ± 13 nm), which can be easily functionalized with biological macromolecules.89
Current and proposed uses
Fe/Au Nps, in particular those supported onto HY- and NaY-type zeolites have been investigated for use as catalysts or cleaning surfaces.90 Thus, interactions of Au with Fe species, introduced into the NaY zeolite by two methods (wet-impregnation and ion exchange), in Fe/Au-imp/NaY zeolite system were studied.91 1%Au-0.5%Fe/HY sample, possessing high activity in the CO oxidation, was prepared by coexchange in HY zeolite using Fe(II) ethylenediamine and Au(III) ethylenediamine complex ions.92 Photoinduced sulfur desorption from the surfaces of Au Nps loaded on a series on metal oxides, in particular Fe2O3, was studied.93 Elemental sulfur S8 was selectively adsorbed on the Au Nps surfaces of Au/metal oxides in an atomic state. This phenomenon is applicable to the low-temperature cleaning of sulfur-poisoned metal catalysts.In addition to core–shell FexOy/Au Nps, Fe/Au Nps have also been used in biomedicine. Thus, the method for synthesizing antibody-magnetic Nps with gold shell and iron core for recognition and separation of tumor cells was elaborated.94 Also, their utility as T1 contrast agents in the unoxidized form,95 capturers for pathogens,96 carriers of streptavidin,97etc, have been reported. The 7 nm Fe/Au Nps (1.5% of Fe), obtained98 by simultaneous reduction of Fe3+ and Au3+, can be easily conjugated to thiolated DNA; moreover, and heated in solution to temperatures above 40 °C, indicating suitability for hyperthermia.
Fe/Au Nps can serve as precursors for other Nps or functionalized derivatives. Thus, 200–350 nm C-encapsulated magnetic Nps possessing surfaces with functional groups such as –OH were prepared by hydrothermal heating an aqueous glucose solution of FeAu Nps at 160–180 °C for 2 h.99,100 An organometallic approach to the synthesis of CO-protected Fe/Au Nps led to colloids with hydrodynamic diameters between 4 and 300 nm, from which [Au21{Fe(CO)4}10]5−, [Au22{Fe(CO)4}12]6−, [Au28{Fe(CO)3}4{Fe(CO)4}10]8− and [Au34{Fe(CO)3}6{Fe(CO)4}8]8− were isolated.101 Chemical oxidation in solution of Au Nps, protected by octanethiol and 12-(N-pyrrolyl)dodecanethiol, with FeCl3 leads to cross-linked Au Nps with chainlike structures.102 The authors proposed that this approach may also be applied for Fe/Au.
Coated and supported iron nanoparticles
Fe0 composite nanoparticles, coated with inorganic compounds and minerals, are common and prepared by distinct techniques. Thus, iron nanoparticles coated with boron nitride (BN) nanomaterials were synthesized by using Fe4N and B powders as raw materials.103 The Fe4N was reduced to α-Fe during annealing at 1000 °C for several hours with flowing N2 gas. NZVI/montmorillonite heterostructures were synthesized and their textural evolution under different Fe loadings was investigated.104 The hybridized Fe nanoparticles were well dispersed on the montmorillonite surface, size adjustable, and resistant to oxidation under the protection of native Fe-oxide shells. It was revealed that as the Fe loadings increased, the total pore and mesopore volumes remained almost unchanged but the total, micropore and external surface areas as well as the micropore volume decreased and the average pore diameter increased. With increasing Fe loadings, the mesoporous character was enhanced for these heterostructures. Nanosized composite magnetic particles MgO/Fe were in situ combustion-synthesized at 620 °C for the Mg-70.9 wt% Fe3O4 system.105 It was identified that Mg (29.1 wt%) was the suitable reactant ratio, the sintered composite spherical particles with mean diameter 40 nm were distributed evenly, the particles had good soft magnetic properties, and could serve in the future as drug carrier materials. In addition, well-protected, isolated bcc-iron nanoparticles embedded in silicon dioxide were prepared by e-beam evaporation and post-annealing of multilayers in an ultrahigh vacuum system. The spherical shape and isolation of the particles were confirmed by plan-view and cross-sectional transmission electron microscopy.106 Similarly, an approach using a low-energy electron beam radiation system was investigated to synthesize carbon hybrid structures in amorphous carbon thin films.107Large-size 2D nano-TiO2 and iron-doped nano-TiO2 thin films were prepared108 in a low-temperature aqueous system by using a molecular self-assembly method. The photocatalytic activity was evaluated with the degradation of methyl orange solution under UV and visible light radiation. The doped iron could obviously improve photocatalytic activity of TiO2. The degradation data of methyl orange were respectively 98.62% and 89.24% under illumination by using UV lamp and visible light. Fe-nano ZrO2 composite coating was prepared109 by adopting iron electroplating technology, choosing nano ZrO2 as second phase particle, and using asymmetrical AC–DC electroplating. It was indicated that this method can lead to compact coatings (body-centered cubic α-Fe crystal structure) with small inner stress. Al2O3 coated polyhedral Fe nanocapsules were prepared by arc-discharging a Fe–Al (8 atom% Al) alloy.110 In addition, mobilization and deposition of iron nano and sub-micrometer particles (INSMP) in a porous medium were investigated using a water-saturated glass micromodel.111 It was shown that there were dense aggregations at the pores as the concentration of INSMP increased. The presence of dissolved humic substances (>1 ppm) significantly reduced deposition of suspended particles and enhanced detachment of the deposited particles.
A host of reports are dedicated to carbon-supported ZVI nanomaterials.112 Such encapsulation of iron nanoparticles in protective carbon cages can lead to unique hybrid core–shell nanomaterials.113 Thus, special carbon encapsulated Fe core–shell nanoparticles (15–40 nm) with a size range of 15–40 nm were prepared via a confined arc plasma method.114 It was shown that the carbon encapsulated Fe nanoparticles had clear core–shell structure, the core (16 nm diameter) of the particles is bcc-structure Fe, and the shell (thickness 6–8 nm) of the particles is disordered carbon. The related arc discharge technique115 is also common for production of nanomaterials, in particular for iron. Thus, a simple, inexpensive and one-step synthesis method of metal-containing carbon nanocapsules using an arc discharge in aqueous solution is reported.116 It was found that Fe nanoparticles could be in situ encapsulated in carbon shells when the arc was performed respectively in aqueous solutions of FeSO4. To explain the formation mechanism of metal-containing carbon nanocapsules, a model of discharge in solution was proposed. Iron nanoparticles, coated with graphite nanolayers, were synthesized by annealing mixtures of hematite and carbon under nitrogen atmosphere.117 Hematite was reduced to Fe3O4, FeO, and finally to Fe completely at 1200 °C. High-resolution electron micrographs reveal that the Fe particles are ~200 nm in diameter and coated with graphite nanolayers of ~30 nm. Combustion synthesis of iron oxide/iron coated carbons such as activated carbon, anthracite, cellulose fiber and silica using a Panasonic kitchen microwave with inverter technology was described.118 The size of the iron oxide/iron nanoparticle-coated activated carbon, anthracite, cellulose fiber and silica samples were found to be in the nano range (50–400 nm). Iron oxide/iron nano particles existed in four major phases: γ-Fe2O3, α-Fe2O3, Fe3O4 and Fe, some of which showed significant arsenic adsorption. In addition, carbon-coated iron nanoparticles with well-developed quasi-spherical shape were prepared with Fe(NO3)3·9H2O and starch as carbon source (Fig. 5).119,120 This is an efficient approach for the mass production of nanocage structures under mild conditions, which needs to be further explored for preparing various carbon coated metal nanomaterials. Radiation methods are being applied more widely in the last decade, in particular for iron carbon-supported nanostructures fabrication. Thus, two types of amorphous carbon films, a 15 atom% iron containing film and with column/inter-column structures, were deposited onto Si substrates by a sputtering technique and subsequently exposed to an electron shower of which the energy and dose rate were much smaller compared to an intense electron beam used in transmission electron microscopy. Graphitic structures were formed in an amorphous matrix at a relatively low temperature up to 450 K. It was found that the graphitization progressed more in the electron irradiation than in annealing at 773 K, and it was attributed to thermal and catalytic effects which are strongly related to grain growth of metal clusters.
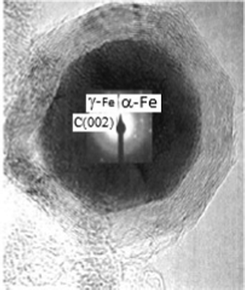 | ||
| Fig. 5 HRTEM of carbon-encapsulated iron nanoparticles from starch (scale bar 10 nm) and inset corresponding electron diffraction pattern taken from iron core regions. Reproduced with permission. | ||
Significant attention is paid to ferrocene as a metal precursor for carbon-supported nanoparticle synthesis121 and the resulting carbon support can exist in distinct forms. For example, carbon nanotubes containing iron nanoparticles were fabricated using microwave heating.122,123 Carbon-encapsulated iron nanoparticles with uniform diameters were synthesized on a large scale by co-carbonization of an aromatic heavy oil and ferrocene at 480 °C under autogenous pressure (Fig. 6).124 It was found that, by increasing the amount of ferrocene added from 2 to 45 wt%, the size of the nanoparticles increased from 15 to 50 nm and the morphologies of the resulting products changed from spherical-type to iron-filled carbon nanorods when the ferrocene loading was higher than 30 wt%. The iron particles pyrolyzed from ferrocene existed mainly in the form of α-Fe and small amounts of Fe3C were also formed when the ferrocene content was higher than 20 wt%.
![HREM images of carbon-encapsulated iron nanomaterials obtained at 480 °C in the presence of ferrocene contents of 13.0 wt% [(a) and (b)] and 40.0 wt% (c). Reproduced with permission.](/image/article/2012/RA/c2ra20812a/c2ra20812a-f6.gif) | ||
| Fig. 6 HREM images of carbon-encapsulated iron nanomaterials obtained at 480 °C in the presence of ferrocene contents of 13.0 wt% [(a) and (b)] and 40.0 wt% (c). Reproduced with permission. | ||
Ferric alginate fibers were prepared by wet spinning of sodium alginate into a coagulating bath containing ferric chloride.125 Carbon-supported nanoscale zerovalent iron fibers (CSNZVIF, with high surface areas of 352 m2 g−1) were obtained through thermal degradation of ferric alginate fibers at 900 °C under an N2 atmosphere. It was found that zerovalent iron particles were well dispersed in the amorphous carbon fibers. The authors stated that the existence of carboxylic and hydroxyl groups in the ferric alginate structure unit play a key role in the formation of carbon-supported nanoscale zerovalent iron fibers. Fe3+ was reduced to Fe0 by hydroxyl group and as-formed amorphous carbon during heating under N2.
Polymer-supported Fe nanoparticles are also widespread. Thus, the procedure developed to produce polymer-supported nano Fe metal particles involved thermal decomposition of Fe(CO)5 in the presence of a monomer (styrene) (reaction (5), which can be better described as a combination of reactions (6) and (7)) that was allowed to concurrently polymerize.126
| nH2C=C(H)Ph + Fe(CO)5 → (–CH2–CH(Ph)–)n + 5CO + Fe0 | (5) |
| Fe(CO)5 → “Fe” + 5CO | (6) |
| nH2C=C(H)Ph + “Fe” → “Fe”–(–CH2–CH(Ph)–)n | (7) |
The effect of pH on the aggregation of polymer-coated NZVI and its deposition onto sand and clay (kaolinite) surfaces was studied.127 NZVI coatings included a high molecular weight (90 kg mol−1) strong polyanion, poly(methylacrylic acid)-b-(methyl methacrylate)-b-(styrenesulfonate) (PMAA-PMMA-PSS) and a low molecular weight (2.5 kg mol−1) weak polyanion, polyaspartate. Enhanced deposition at lower pH was indicated because the elutability of polyaspartate-modified hematite (which did not aggregate) also decreased at lower pH. The greater deposition onto clay minerals compared to similar sized fine silica is attributed to charge heterogeneity on clay mineral surfaces, which is sensitive to pH.
Nano-Fe2O3 phases and their composites
Distinct iron oxide nanostructures and composites based upon them have been prepared by a variety of conventional techniques. Thus, iron oxide nanowires were grown by resistive heating of iron wire (with a diameter of 0.25 mm) under ambient laboratory conditions.128 The synthesis can be easily controlled by observing the color of the wire and by varying the applied heating power. The wire was completely covered by nanowires, having a sword-like shape, i.e., they are belt-like structures, which are thicker at the base and thinner at the end. Maghemite (γ-Fe2O3) nanoparticles were fabricated by microwave heating of (acetylacetonato)iron(III) precursor.129 This precursor is very easy to make, and there is no need for controlling the reaction conditions and heat treatments. Upon irradiation by microwaves it decomposes to maghemite nanoparticles. The β-Fe2O3 cubic phase, one of the least studied Fe–O systems, was obtained by CVD method using a Fe(II) β-diketonate diamine complex, Fe(hfa)2TMEDA, as the molecular source (hfa = 1,1,1,5,5,5-hexafluoro-2,4-pentanedionate, TMEDA = N,N,N′,N′-tetramethylethylenediamine).130 Also, a Fe(II) 1D coordination polymer, [Fe(pyterpy)2](SCN)2·MeOH (pyterpy = 4′-(4-pyridyl)-2,2′:6′,2′-terpyridine), was synthesized by using a branched tube and used as a precursor to fabricate Fe(III) oxide nanoparticles131 by its thermolysis in oleic acid (surfactant) at 286 °C in air. Magnetic iron oxide nanopowders were synthesized electrochemically,132 using a low-carbon steel electrode immersed in a NaCl aqueous solution, at constant temperature of the electrolyte, pH and current density. In the second step, portions of the starting admixture were boiled at ∼360 K during 2 h and autoclaved at various temperatures. In addition, nanostructured iron oxide/hydroxide materials were synthesized by sol–gel technology, starting from the ternary system Fe(NO3)3·9H2O–ethanol–propylene oxide.133 The produced materials were composed by aggregates of nanometric crystallites: ∼1 nm for xerogels and ∼5 nm for aerogels. Their high porosity and surface area (xerogels ∼50% and 150 m2 g−1; aerogels ∼90% and 400 m2 g−1) make them suitable for surface-dependent processes, with the aerogels far superior. It was shown that the 2-line ferrihydrite is their most probable constituent phase.As well as in case of Fe0-containing nanomaterials (see above), radiation methods have been applied for the generation of oxides and composites. Thus, to prepare nanocrystalline iron(III) oxide at room temperature and ambient pressure without using any kind of catalysts, a 2 MeV 10 mA GJ-2-11 electronic accelerator was used as the radiation source.134 The optimal conditions were pH of the solution = 6.54 and a 300 kGy radiation dose. It was shown that the α-Fe2O3 particles had hexagonal structures, spherical shape morphology and good dispersity, and average diameter of ∼30–60 nm. Alternatively, Fe2O3 was fabricated135 from metallic iron under electron beam irradiation and further reaction with oxygen. Iron oxide nanowires were rapidly synthesized in large quantities at room temperature by pulsed-laser ablation (248 nm) of iron powder under methanol.136 At high collection rates, a lamellate nanobelt morphology was observed, whereas at low collection rates nanowires dominated. The as-synthesized products had the stoichiometry of the goethite [FeO(OH)] phase (proposal formation reactions are shown by reactions (8) and (9)) which after annealing at temperatures above 400 °C crystallizes into hematite (α-Fe2O3).
| 2Fe + 12CH3OH→ 6C2H5OH + 2Fe(OH)3 + 3H2 | (8) |
| Fe(OH)3 →FeO(OH) + H2O | (9) |
Composite nanoparticles consisting of gold and iron oxide were synthesized in aqueous solution systems by using a high-energy electron beam.137 The electron irradiation induced radiation-chemical reaction to form metallic gold nanoparticles (reaction mechanism is shown by eqns (10)–(14)), which were firmly immobilized on the surface of the support iron oxide nanoparticles. The size of these gold nanoparticles depended on the concentrations of gold ions, polymers and iron oxide nanoparticles in the solutions before the irradiation. Many other examples of core–shell iron oxide/gold nanoparticles were reviewed,138 where many possible synthetic techniques for core–shell structures on the basis of iron or its oxides with gold have been discussed. Among them, we emphasize that, additionally to the mostly represented “standard” iron oxide core/gold shell Nps, some reports concerned interesting unexpected nanostructures. Thus, iron oxide coated gold nanorods were prepared (Fig. 7) starting from gold nanorods, synthesized by a three-step seed mediated protocol and coated with a layer of poly(sodium 4-styrenesulfonate).139 Here, on the contrary, the gold metal acts as a core and the iron oxide as a shell. The negatively charged polymer on the nanorod surface electrostatically attracted a mixture of aqueous Fe(II) and Fe(III) ions. Coprecipitation of these iron salts was used to form uniform coatings of iron oxide Nps on the surface of the gold nanorods. The oxidation state of iron in the iron oxide coatings was determined and was consistent with Fe2O3 rather than Fe3O4. Multifunctional Fe3O4/polymer/Au shell Nps140 with preserved strong magnetization and good NIR absorption display good dispersibility and stability in aqueous solution. The authors supposed that these features should facilitate biomedical applications, combining the benefits of MRI diagnosis, magnetically targeted delivery and photothermal ablation.
| H2O (γ-rays or e-beam) → e−aq, H·, OH·, etc. | (10) |
| (CH3)2CHOH + OH· → (CH3)2·COH + H2O | (11) |
| (CH3)2·CHOH + H· → (CH3)2·COH + H2 | (12) |
| e−aq + Mn+ → M(n−1)+ | (13) |
| (CH3)2·COH + Mn+ → (CH3)2CO + M(n−1)+ + H+ | (14) |
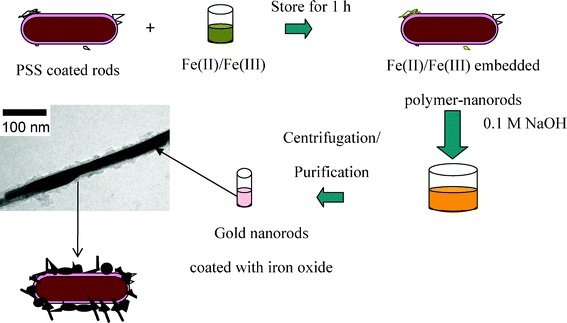 | ||
| Fig. 7 General protocol used to prepare in situ iron oxide coated gold nanorods. Reproduced with permission from ACS (Journal of Electron Microscopy; jmicro.oxfordjournals.org). | ||
Zeolite and related supporting materials serve as an ideal basis for iron oxide composites. Thus, the loading of zeolite with nano-iron oxide (Fig. 8) by a simple chemical route has been described.141 The average crystallite sizes of the doped nanomaterials were 4–6 nm. It was revealed that zeolite acquire magnetic properties after doping with nano-iron oxide. Mesoporous iron oxide silicate nanocomposites Fe2O3-SBA-15 (SBA-15 is hexagonally ordered mesoporous silica) with iron loadings in the range of 1.2–35.8 wt% were synthesized by a direct hydrothermal method.142 It was shown that these mesoporous nanocomposites with well-dispersed nanoclusters of iron oxide in the walls of ordered mesoporous silica structures and high surface area were attained under the condition of adjustment of Fe/Si molar ratios and appropriate Fe loadings.
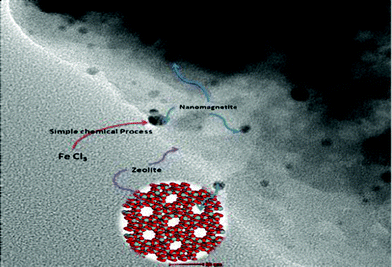 | ||
| Fig. 8 Loading of iron oxide nanoparticles by zeolite. Reproduced with permission. | ||
Fe3O4 and other iron-containing nanomaterials
A certain number of composite nanomaterials based on Fe3O4 is known, for instance core/shell Fe3O4 coated gold nanoparticles (diameter 50–100 nm).143 Their possible formation mechanism was proposed as follows: pH-sensitive polymer owing to a shrunken or stretched structure of polyethyleneimine (PEI), led to the aggregation of Fe3O4–gold seed nanoparticles, then gold reduces onto the surface of Fe3O4–gold seed nanoparticles. It was concluded that these core/shell multifunction nanomaterials will not only have external magnetic separation by the core of Fe3O4, but also detect large biological molecules using the shell of gold. In addition, iron phthalocyanine prepolymer/Fe3O4 nano hybrid magnetic material144 can be applied as high temperature-resistant polymer magnetic composite material.Use of “Greener” methods superparamagnetic Fe3O4 nanoparticles (blocking temperature (TB) of 150 K and saturation magnetization of 37.1 emu g−1) have been synthesized via soya bean sprouts (SBS) templates at room temperature and at normal atmospheric pressure.145 Spherical Fe3O4 nanoparticles with an average diameter of 8 nm simultaneously formed on the epidermal surface and the interior stem wall of SBS, which were responsible for size and morphological control during the whole formation of nanoparticles. In a related report,146 similar Fe3O4 superparamagnetic nanoparticles (12.5 nm, inverse spinel structure) were prepared using α-D-glucose as the reducing agent and gluconic acid (a product of glucose oxidation) as the stabilizer and dispersant. In this case, the TB was found to be higher (190 K) and the magnetic hysteresis loop at 300 K showed a saturation magnetization of 60.5 emu g−1.
Among other iron-containing nanomaterials, we note carbon encapsulated iron carbide nanoparticles (5–40 nm) were synthesized from ferrocene in a quite narrow temperature range (about 600–800 °C) under high (4 GPa) static pressure.147 The material produced under optimum parameters consists of round carbide particles covered with onion-like carbon shells that are embedded into the carbon matrix. In the presence of an excess of carbon a solid-state Fe3C → Fe7C3 transformation is possible in nanoparticles under high (13 GPa) pressure and temperature (1200 °C). Other examples of Fe-containing nanomaterials are shown in Table 2.
Remediation of groundwater using iron-based nanomaterials
Research over the past two decades has demonstrated the efficiency of Fe for the removal of a wide range of chemical and microbial contaminants (e.g. bacteria, chlorinated organics, dyes, emerging contaminants, heavy metals, radionuclides, viruses) from water.152 The prevailing concept considers that the mechanism of Fe0 remediation varies depending on the contaminant of interest. This concept was recently revisited and Fe was proven as a universal material for water treatment.The following methods are currently considered as conventional treatment technologies for water treatment:153 (a) filtration (using ceramic or biosand filters, charcoal and activated carbon filters, granular media and rapid rate filters, fiber and fabric filters), (b) heat and UV-radiation, (c) chemical treatment (coagulation–flocculation, chemical disinfection and flocculant–disinfection), desalination and arsenic removal (reverse osmosis, distillation, adsorptive filter media for arsenic removal). The nanotechnology-based water treatment technologies include: (a) carbon nanotube-based technologies (CNTs membranes, nanomesh), (b) other nanofiltration approaches (nanofiltration membranes and devices, nanofibrous alumina filters and nanofiber gravity-flow devices), (c) nanoporous ceramics, clays and other adsorbents, (d) zeolites, (e) nanocatalyst-based technologies (nanostructured ZVI, TiO2 and iron oxide adsorbents) and (f) magnetic nanoparticles (magnetoferritin and ZVI).
Starting from the 1990s, inorganic nanoparticles,154 in particular NZVI and iron oxides have been used for elimination of certain inorganic and organic substances from groundwater,155 sediments and soils, despite that the NZVI utility in full-scale remediation projects is limited by material costs. ZVI nanoparticles react with a wide range of contaminants, and the effect of particle size on the reactivity of NZVI is as important as other effects such as particle composition, contaminant structure and solution chemistry. It has been established that NZVI is very effective, covering the broadest range of environmental contaminants. Common environmental contaminants (Tables 4–6) that can be transformed by nanoscale iron particles are as follows:156 (a) chlorinated methanes (CHnCl4−n), (b) chlorinated benzenes (C6HnCl6−n), (c) pesticides (DDT, lindane), (d) organic dyes (Orange II, Chrysoidine, Tropaeolin O, Acid Orange, Acid Red), (e) heavy metal ions (Hg2+, Ni2+, Ag+, Cd2+), (f) trihalomethanes (CHBr3, CHBr2Cl, CHBrCl2), (g) chlorinated ethenes (C2Cl4), C2HCl3, cis- or trans-C2H2Cl2, 1,1-dichloroethene C2H2Cl2, vinyl chloride), (h) other polychlorinated hydrocarbons (PCBs, for example hexachlorocyclohexanes157), dioxines, pentachlorophenol (C6HCl5O), (i) other organic contaminants (N-nitrosodimethylamine (NDMA) (C4H10N2O), TNT (C7H5N3O6) and dinitrotoluene,158 (j) inorganic anions (Cr2O72−, AsO43−, ClO4−, NO3−, SeO42−), (k) also removing dissolved metals from solution, e.g. Pb,2+ Ag+ and Ni2+. It may also be able to reduce radionuclides.
Nanoscale zerovalent iron (NZVI) is used for both in situ and ex situ treatment of contaminated groundwater. It functions simultaneously as an adsorbent and a reducing agent, causing organic contaminants to break down into less toxic simple carbon compounds and heavy metals to agglomerate and stick to the soil surface. NZVI can be injected directly into the source of contaminated groundwater as slurry for in situ treatment, or it can be used in membranes for ex situ applications. Bimetallic NZVI, in which the iron nanoparticles are coated with a second metal such as palladium to further increase the reactivity of the iron, is also available. NZVI is more reactive and has a large surface area than granular ZVI.
Among many important investigations in this area, we note recent contributions of Tratnyek et al.159–168 In relation to chlorohydrocarbons, the mechanism of reactivity for ZVI is similar to the mechanism of corrosion (i.e., oxidation of iron) and involves the generation of electrons which in turn reduces the organic species through dechlorination (reaction (15)):
| Fe0 + RCl + H+ → Fe2+ + RH + Cl− | (15) |
Similarly, ZVI acts as a reducing agent for sequestration of metal ions having reduction potential higher than Fe. The chemistry can be understood by an example of interaction of Ni2+ with ZVI (Fig. 9). In the beginning of the reaction, Ni2+ is physically adsorbed (outer sphere interaction) and then is chemisorbed (inner sphere interaction) and finally reduced to metallic nickel. Similar chemistry has been applied for sequestration of many metal ions and metalloids: Pb, Cd, Cr, Co, Cu, Hg, Ni and Se.
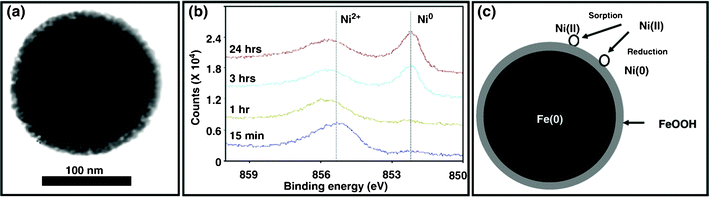 | ||
Fig. 9 (a) TEM images of iron nanoparticles, (b) HR-XPS survey on the Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2p3/2 of iron nanoparticles and (c) a conceptual model for nickel deposition on iron nanoparticles.170 2p3/2 of iron nanoparticles and (c) a conceptual model for nickel deposition on iron nanoparticles.170 | ||
There is an ambiguity regarding the relationship between NZVI and conventional (micron- to millimeter-sized) ZVI.169 NZVI exhibits unique properties like those of some other nanostructured materials, when the behavior of NZVI under environmental remediation conditions is, in fact, not very much different than conventional ZVI. It was shown that: (a) the NZVI used in groundwater remediation that are much larger than particles that exhibit “true” nanosize effects, (b) the higher reactivity of this NZVI is mainly due to its high specific surface area, (c) one factor that limits the intrinsic reactivity of NZVI is the high degree of passivation by enveloping oxides, and (d) the mobility of NZVI is less than a few meters under almost all relevant conditions.
NZVI and iron oxide magnetic nanoparticles have also been chosen171 as promising adsorbents for the removal of (in)organic contaminants from polluted water, because: (a) they can be produced in large quantities using physicochemical methods, (b) it is expected that their adsorption capacity and affinity for pollutants is higher considering the larger surface area and possibly highly active surface sites, (c) the separation of metal-loaded magnetic nano-adsorbent from treated water can be achieved via an external magnetic field, and (d) nanoparticles might be regenerated and reused. Also, iron oxide nanoparticles can be surface-coated with organic compounds to increase their sorption efficiency and specificity for pollutants. In this case, it is worth noting that the covalent attachment of molecules to the surface of nanoparticles and the stability of the coating are important. Some adsorption processes for wastewater treatment have utilized ferrites and a variety of iron containing minerals, such as akaganeite, feroxyhyte, ferrihydrite, goethite, hematite, lepidocrocite, maghemite and magnetite.172 Adsorption of organics to the nanoparticle media was extremely rapid. More than 90% of the organics were adsorbed within 30 min. The isotherm studies indicated that, on a surface area basis, the adsorption capacities of the nanoparticle media were significantly (>2 fold) higher than the ferric oxide media typically used in water treatment.
Remediation of metal ions
NZVI (20–80 nm), obtained using the borohydride reduction method, was examined for aqueous Co2+ removal over a wide concentration range, 1–1000 mg L−1.173 Fe nanoparticles demonstrated very rapid uptake and large removal capacity for Co2+. It was indicated that Co2+ fixation occurred by the interaction of cobalt ions with oxohydroxyl groups on Fe nanoparticle surfaces as well as spontaneous precipitation formation at high loads. The sorption properties of Zn(II), Cu(II), Cd(II) and Pb(II) to nanohematite in single- and binary-adsorbate systems were investigated.174 It was indicated that the presence of a secondary metal can affect the sorption process depending upon the molar ratios, such as increased or reduced adsorption. Also, Pb and Cu adsorption to nanohematite is an endothermic, and a physical adsorption process; however, it is only spontaneous at higher temperatures.Remediation of inorganic anions
The elimination of nitrates175 and arsenic-containing anions is the main application of the NZVI in respect to inorganic matter; a host of reports are dedicated to this area. Thus, the NZVI in different forms (freshly synthesized, dried, and dried-sonicated) was studied as an effective nitrate reduction material.176 As a result, different types of NZVIs were found to be able effectively reduce highly concentrated nitrate without requiring pH control. The authors suggested that the aggregate size and catalyst prominently affect the nitrate reduction rate and that the aggregation effect is more important than the catalyst effect as the aggregate size becomes smaller. The final product of the reaction was ammonium, with nitrite being produced as a byproduct; NZVI changed into different shapes of magnetite (Fe3O4) after the reaction, depending on the reaction conditions. The mechanism for nitrate reduction by nano-zerovalent iron was also studied in ref. 177. Bimetallic iron-containing nanoparticles react similarly; thus, improved N2 selectivity of NZVI via NO3− denitrification using bimetallic Ni/Fe nanoparticles was discussed.178 NO3− was first adsorbed on the nano-Ni/Fe surface and reduced to NO2−. With the rapid reduction of NO2− to NH4+, this NH4+ was desorbed into solution due to its smaller affinity for the particle surface. In addition, microbial reduction of nitrate in the presence of NZVI was evaluated to assess the feasibility of using NZVI in the biological nitrate treatment.179 Nitrate was completely reduced within 3 days in a nanoscale Fe(0)-cell reactor, while only 50% of the nitrate was abiotically reduced over 7 days at 25 °C. Efficient removal of nitrate by Fe(II)-supported anaerobic culture in 14 days indicated that Fe(II), which was produced during anaerobic Fe corrosion in the Fe0-cell system, might act as an electron donor for nitrate. In addition, microbial reduction of nitrate was not significantly affected by low temperature conditions.For arsenic elimination (this is a very important problem in many countries180), nanophase Fe3O4 (magnetite, 17 nm) and Fe2O3 (hematite, 12 nm) were synthesized through a precipitation method181 and were utilized for the removal of either arsenic(III) or (V) from aqueous solution as a possible method for drinking water treatment. The binding was observed to be pH independent from pH 6 through pH 9 and a significant drop in the binding was observed at pH 10. Batch isotherm studies were performed using the Fe2O3 and Fe3O4 to detect the binding capacity of As(III) and As(V) to the iron oxide nanomaterials. Among other studies, XPS and X-ray absorption spectroscopy were used for NZVI treated with Cr(III) and Cr(VI).182 It was shown that (a) the crystalline Fe(III) phase is composed of lepidocrocite (γ-FeOOH), (b) Cr(VI) was entirely reduced to Cr(III) by nano Fe0 with no residual Cr(VI) after reaction. Cr(III) precipitated as Cr(OH)3 in the presence of corroding nano Fe0 was nearly identical to the Cr(VI)-nano Fe0 reaction product and both Cr(III)- and Cr(VI)-treated nano Fe0 yielded a predominantly hydroxylated Cr(OH)3 and/or a mixed phase CrxFe1−x(OH)3 product, both of which are highly insoluble under environmental conditions. In addition, NNZVI was also evaluated for the reduction of bromate that is a highly persistent and carcinogenic oxyhalide formed as an ozonization byproduct during oxidative disinfection in drinking water treatment.183 It was revealed that in a 20 min bromate reduction NZVI mostly converted to Fe2O3 and Fe3O4 corrosion products mixed with Fe hydroxides. Humic acid was the most influencing factor to decrease NZVI reactivity in bromate reduction. In addition, the effect of sonication pretreatment showed that the bromate reduction efficiency could be enhanced by increasing the actual reactive surface area.
Remediation of organic pollutants
A large variety of organic pollutants, especially chlorinated hydrocarbons, have been used in remediation experiments using NZVI, Fe/M nanoparticles and iron oxides. Thus, the oxidative capability of NZVI was analytically compared via experiments using the model compounds benzoic acid and formic acid.184 Rapid production of H2O2 with concomitant loss of the model compound was observed upon addition of NZVI and bimetallic nanoparticles to oxygenated solutions with eventual loss of formic acid. On the other hand, functionalization of NZVI with polymeric stabilizers starch, carboxylmethylcellulose and alginate slowed down the rate of H2O2 production and the consumption of formic acid. Fe nanoparticles were used to remediate PCB-contaminated soil and an attempt was made to maximize PCB destruction in each treatment step.185 The destruction efficiency during the preliminary treatment (mixing of soil and Fe nanoparticles in water) can be increased by increasing the water temperature. A minimum total PCB destruction efficiency of 95% can be achieved. At 300 °C in air, Fe2O3 is also a good catalyst for remediating PCB-contaminated soils. Nanoscale palladized iron (Pd/Fe) bimetallic particles (spherical granules with diameter of 47 ± 11.5 nm connected with one another to form chains and the chains composed nanoscale Pd/Fe bimetallic particles), prepared by reductive deposition method186 and containing α-Fe0, revealed the best dechlorination effect in elimination of monochloroacetic acid by different reductants followed the trend: nanoscale Pd/Fe bimetallic particles of 0.182% Pd/Fe > nanoscale Fe > reductive Fe. The effectiveness of NZVI to dechlorinate atrazine (1-chloro-3-ethylamino-5-isopropylamino-2,4,6-triazine) in contaminated water and soil was studied, analyzing the influence of iron sources, solution pH, Pd catalyst and presence of Fe or Al sulfate salts.187 The results indicated nano ZVI can be successfully used to remediate atrazine in water and soil. Atrazine destruction kinetic rates were greatly enhanced in both contaminated water and soil treatments by NZVI when sulfate salts of Fe(II), Fe(III) or Al(III) was added with the following order of removal rates: Al(III) 2.23 > Fe(III) 2.04 > Fe(II). Another example of higher activity of composite Fe nanoparticles in comparison to free Fe0 samples is a one-pot method,188 developed to prepare Fe/FeS nanoparticles using dithionite at r.t. The FeS precipitations on the Fe surface were formed by the interaction between dissolved Fe species and H2S, one of the decomposition products of dithionite in solution. The resulting Fe/FeS nanoparticles showed a much higher reactivity toward contaminants than the pure Fe nanoparticles and were applied for the rapid removal of TCE from water.Supporting NZVI or iron oxides on natural materials or compounds having high surface area leads to their longer activity. Thus, NZVI was reported as an effective material for azo dye removal, however, similar to other nanomaterials, ultra-fine powder has a strong tendency to agglomerate into larger particles, resulting in an adverse effect on both effective surface area and catalyst performance. Nanosized Fe0 particles dispersed onto the surface of natural bentonites were fabricated189 and their ability to decolorize Orange II (OII) was evaluated. Spherical individual Fe0 particles were observed after dispersion onto bentonites, and these samples were used for Orange II decolorization over a wide pH range. Higher reactivity was attributed to the good dispersion of Fe0 particles on clay mineral surface. The same pollutant (Orange II) was also removed by use of non-supported self-assembled Fe3O4 hierarchical nanostructures (obtained by hydrothermal approach),190 which could be easily transformed to γ-Fe2O3 and α-Fe2O3 without changing its original morphology by calcination in air. Another example is the study of the kinetics of nitrobenzene reduction by Fe(II) sorbed on α-Al2O3, measured while simultaneously characterizing the Fe oxidation product with Mössbauer spectroscopy and electron microscopy.191 The onset of nitrobenzene reduction coincided with a change in particle suspension color from white to yellow–ochre due to formation of nanogoethite rods (α-FeOOH) from oxidation of sorbed Fe(II). Formation of nanogoethite on the α-Al2O3 particles appears to promote the rapid reduction of nitrobenzene. In addition, an encapsulation approach that relies upon Gum Arabic to stabilize high quantities of NZVI (∼12 g L−1) in the dispersed phase of a soybean oil-in-water emulsion was offered.192 The formed emulsion is kinetically stable due to substantial repulsive barriers to droplet–droplet induced deformation and subsequent coalescence. Sedimentation time scales were found to be on the order of hours (τ = 4.77 ± 0.02 h). NZVI within the emulsion was shown to be reactive with both trichloroethane degradation and H2 production observed. A special case is the use of iron oxide/TiO2 nanoparticles.193 Thus, photocatalytic oxidation with TiO2 nanoparticles (6–20 nm) was investigated as a promising water-treatment process.194195 When irradiated with UV light, TiO2 nanoparticles can adsorb and degrade a wide variety of environmental organic pollutants. For instance, the strong affinity between the surface of TiO2 nanoparticles for organic arsenic species (monomethylarsonic [MMA] and dimethylarsinic [DMA] acids) leads to covalent bonding between MMA or DMA and the surface of nanoparticles through bidentate (AsMMA-Ti 3.32 Å) and monodentate (AsDMA-Ti 3.37 Å) inner-sphere complexes, respectively. Doping TiO2 nanoparticles with Fe3+ ions at 0.1–0.5% may significantly increase the photocatalytic activity. The doped ions act as charge separators of the photoinduced electron–hole pair and enhanced interfacial charge transfers. In a related work, iron-doped-TiO2 was prepared by the hydrothermal method.196 Titanium(IV) tetra-tert-butoxide and FeCl3 or FeCl2 dissolved in n-octanol was heated at 230 °C for 2 h in the presence of water. The resulting powders were rinsed, dried and calcined at 560 °C. Photocatalyst doped with FeCl3 had better photoactivity for degradation of dye in aqueous solution under UV and visible light. It was found that the amount of doped iron ions plays a significant role in affecting its photocatalytic activity.
Special studies of remediation applying iron-containing nanomaterials
Generally, in soils the NZVI particles remain active towards the contaminants for a period of 6–8 weeks. During this time, the NZVI particles can aggregate and attach to soil grains in the subsurface leading to reduced mobility, thereby limiting their remediation potential. NZVI has been shown to be effective across a broad range of soil pHs, temperatures, and nutrient levels. Competing anions, however, may reduce its effectiveness. The amount of groundwater that NZVI can treat may depend on the quality of the iron, including the number of times it has been reused, the type of substrate used (for ex situ use), and the quality of the water used to make the injectable slurry, including the amount of oxygen and the amounts and types of particulates in contains (for in situ use). The effects of reaction conditions such as reaction time and NZVI concentration on NZVI characteristics and reactivity were studied.206 The particle size was dramatically decreased from 87.4 to 9.5 nm under short reaction time and high reductant concentration. The reactivity of NZVI (evaluated by a nitrate reduction test) was increased in the direction of high reductant concentration and fast synthesis, although deactivation increased in the same direction. Also, column and batch sedimentation studies were conducted207 to study the transport of nanoscale NZVI particles stabilized by three polyelectrolytes: polyvinyl alcohol-co-vinyl acetate-co-itaconic acid (PV3A), poly(acrylic acid) (PAA) and soy proteins. It was revealed that the both PV3A and PAA can increase NZVI mobility by reducing particle size and generating negative charged surfaces of NZVI. PV3A stabilized NZVI has the best transport performance among the three materials. Due to the large surface area of NZVI, large amounts of polyelectrolytes are often needed. In addition, to understand more the factors that limit NZVI mobility, several field-scale tests were performed208 using carboxylmethyl cellulose (CMC) stabilized NZVI.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 Fe
3 Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr mole ratio, C–Fe reduced a 10 ppm Cr6+ solution to ∼1 ppm within 3 days. This method can be made in a scalable process from inexpensive starting materials by carbo-thermal reduction. Another approach without any chemical process is as follows. Unlike conventional methods such as chemical synthesis and vapor phase condensation, which typically involve toxic chemicals, sophisticated equipment and extensive labor, the precision milling method relies solely on the mechanical impact forces generated by stainless steel beads in a high-speed rotary chamber to break down the iron microparticles.215 The system used nontoxic solvents, was completely scalable to large-scale manufacturing. After 8 h of milling, the feed micro iron was effectively reduced to particles with sizes below 50 nm. A result of application of such techniques is the use of NZVI at industrial scale. Thus, application of NZVI technology for remediation of groundwater at an operating industrial facility, containing chlorinated solvent contaminant, was offered.216 As a result, the nonaqueous phase source area in the groundwater was successfully treated and the chlorinated solvent degraded to benign byproducts within a year. Use of the nanoscale iron facilitated distribution of the NZVI into the silty clay soil pores using high pressure injection. The work was performed adjacent to active plant buildings and roadways with no loss of plant production.
Cr mole ratio, C–Fe reduced a 10 ppm Cr6+ solution to ∼1 ppm within 3 days. This method can be made in a scalable process from inexpensive starting materials by carbo-thermal reduction. Another approach without any chemical process is as follows. Unlike conventional methods such as chemical synthesis and vapor phase condensation, which typically involve toxic chemicals, sophisticated equipment and extensive labor, the precision milling method relies solely on the mechanical impact forces generated by stainless steel beads in a high-speed rotary chamber to break down the iron microparticles.215 The system used nontoxic solvents, was completely scalable to large-scale manufacturing. After 8 h of milling, the feed micro iron was effectively reduced to particles with sizes below 50 nm. A result of application of such techniques is the use of NZVI at industrial scale. Thus, application of NZVI technology for remediation of groundwater at an operating industrial facility, containing chlorinated solvent contaminant, was offered.216 As a result, the nonaqueous phase source area in the groundwater was successfully treated and the chlorinated solvent degraded to benign byproducts within a year. Use of the nanoscale iron facilitated distribution of the NZVI into the silty clay soil pores using high pressure injection. The work was performed adjacent to active plant buildings and roadways with no loss of plant production.
Among other studies on NZVI particles, we note their in situ modification techniques217 and potentiometric detection.218
Tables 4–6 list examples of examples of pollutant remediation using NZVI, supported and alloyed NZVI and iron oxides and FeOOH.
| System | Ion/substance to be eliminated | Description | Ref. |
| NZVI | Cr(VI) | 0.10 g L−1 NZVI completely reduces Cr(VI) within 120 min following pseudo-first order kinetics. NZVI showed significant Cr(VI) reduction at field also, indicating it an effective tool for managing sites contaminated with Cr(VI). | 219 |
| NZVI (size 30–400 nm) | Heavy metals (Cd, Cr, Hg) | Reduction of heavy metals in the heavy metal-polluted soil into lower valent fixed heavy metals. | 220 |
| NZVI | Cd(II) | The adsorption of Cd2+ on NZVI increased significantly with increasing pH. Zn2+, Co2+ and Mg2+ are potential inhibitors to Cd2+ adsorption by NZVI. | 221 |
| NZVI | Chlorinated organic contaminants (e.g., solvents, pesticides) and inorganic anions or metals. | Use of NZVI for remediation of a series of substances. | 222 |
| NZVI (10 μm particles) | Orange 6 | 98% of the dye can be degraded within 30 min of contact. | 223 |
| NZVI | Pyrene | NZVI particles were found to be more efficient in removing pyrene than commercially available microscale ZVI (MZVI, <10 μm) particles. | 224 |
| NZVI | Lindane (classified by the United States Environment Protection Agency as a potent carcinogen and teratogen). | Lindane (10 μ/g) completely disappeared from spiked soil within 24 h at nZVI concentration of 1.6 g L−1, indicating its possible use in environmental cleanup. | 225 |
| NZVI | Malodorous sulfides | NZVI has high capacity for the removal and sequestration of malodorous sulfides such as hydrogen sulfide and dimethyl disulfide. The core–shell structure of iron nanoparticles plays a key role during the reactions. Both iron in the core and FeOOH in the shell provide rich surface sites for the sorption of sulfides, while the surface associated sulfide can further react with hydrogen sulfide and evolve to iron polysulfide. | 226 |
| NZVI | Xanthan | The rheological properties of NZVI-xanthan suspensions were extensively tested under two different flow conditions (simple shear flow and flow through a porous medium), showing a shear thinning behavior that is dependent on iron concentration. | 227 |
| NZVI (40 nm) | Arsenite [As(III)] | NZVI demonstrated very rapid adsorption and large capacity for the removal of As(III). The maximum As(III) adsorption capacity in batch experiments calculated by Langmuir adsorption isotherm was 76.3 mg L−1 of As(III)/g of NZVI. | 228 |
| NZVI | Chlorinated ethenes | The concentration of chlorinated ethenes decreased by 30–50%. The oxidizing iron reduces chlorinated hydrocarbons and forms non-chlorinated hydrocarbons. Organic matter in water is thus reduced and treated water is safe for living organisms without health danger. | 229 |
| NZVI | Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) | RDX is a common contaminant of soil and water at military facilities. Its degradation with NZVI nanoparticles in water in the presence or absence of a stabilizer additive such as CM-cellulose (CMC) or poly(acrylic acid) (PAA) was studied, showing that the degraded RDX to the corresponding nitroso derivatives prior to completed decomposition. | 230 |
| NZVI | Anions (NO3−, Cl−, SO42−, HCO3−) and natural organic matter (NOM). | More than 95% of 1.8 mM TCE was removed within 20 h with a NZVI dosage of 25 g L−1 (k = 0.15 h−1). TCE degradation reactions were not substantially affected by the presence of each anion with concentrations as high as 100 times the average field concentrations. However, when four anions (NO3−, Cl−, SO42−, HCO3−) were present simultaneously, the degradation reactivity was decreased by 60% (k = 0.069 h−1). | 231 |
| NZVI, Fe/Ni or Fe3O4 | Uranium ions. | The Fe nanoparticles removed 98% of the total uranium from solution, resulting in a final U-concentration of <4 μg L−1. In all the systems, uranium was reduced to U(IV) and retained on the surfaces of the nanoparticulate solids for up to 48 h; the U-stability was not affected by annealing the Fe or the FeNi nanoparticles before use. | 232, 233 |
| NZVI | Vinyl chloride (VC) | The degradation efficiency of vinyl chloride (VC) detected at most of monitoring wells was 50–99%. | 234 |
| System | Ion/substance to be eliminated | Description | Ref. |
| Fe 0 /M nanoparticles and their composites with supports | |||
| Pd/Fe and Fe (NZVI and MZVI (microscale ZVI)) | Water disinfection byproducts; trihalomethanes (THMs) including chloroform, bromoform, dichlorobromomethane, dibromomethane and dibromochloromethane | Complete dehalogenation of THMs by nanoscale Pd/Fe particles was achieved in less than one hour. Methane, accounting for 60–90% of the THMs lost, was the major product. Nanoscale Pd/Fe particles lasted over 700 h without exhausting their ability to dehalogenate THMs in a closed batch system. | 235 |
| Pd/Fe | Pentachlorophenol (PCP) | Degradation of PCP by Pd/Fe nanoparticles was carried out in aqueous solutions containing different cations in sulfate form, Na2SO4, CuSO4, NiSO4 and Fe2(SO4)3, respectively. The observed inhibitory effect of Na2SO4 on degradation of PCP was contributed to the existence of SO42−. Overcoming the inhibitory effect of SO42−, Cu2+, Ni2+ and Fe3+ could facilitate the degradation kinetics and efficiencies of PCP by Pd/Fe nanoparticles. The enhancement effect of Cu2+ and Ni2+ result from the presence of reduced forms of Cu and Ni on Pd/Fe surfaces. | 236 |
| FePd nanoparticle suspensions in water the presence of various stabilizers (i.e., CM-cellulose (CMC), polyvinylpyrrolidone (PVP) and guar gum). | Trichloroethylene | A dramatic improvement in FePd suspension stability was observed when the stabilizer is present in the matrix during the nanoparticle synthesis step. In case of guar gum, gelation during synthesis significantly increased suspension viscosity, enhancing suspension stability. | 237 |
| NZVI, bimetallic nanoparticles (NZVI/Pd), and NZVI/Pd impregnated activated carbon (NZVI/Pd-AC) composite particles | Polybrominated di-Ph ethers (PBDEs) and/or polychlorinated biphenyls (PCBs). | Compared to NZVI, the Fe-normalized rate constants for NZVI/Pd were about 2-, 3- and 4-orders of magnitude greater for tri-, di- and mono-BDEs, respectively, with di-Ph ether as a main reaction product. Pd mainly deposits on the outer part of particles, while Fe was present throughout the AC particles. It was suggested that ∼7% of the total Fe within the AC was zerovalent, which shows the difficulty with in situ synthesis of a significant fraction of zerovalent Fe in the microporous material. | 238 |
| Fe/Cu (Cu amended zerovalent iron bimetallic nanoparticles), supported in activated carbon (AC). | γ-HCH (γ-hexachlorocyclohexane) | Cu amended zerovalent iron bimetallic nanoparticles were synthesized by doping Cu on the surface of iron and incorporated with granular activated carbon to prepare supported particles (AC–Fe0–Cu), which were used to remove γ-HCH. Cu on the surface of iron enhanced the dechlorination activity of Fe0. The dechlorination rate constant increased with the Cu loading on the surface of iron and the maximum was achieved with 6.073% Cu. AC could adsorb the degradation products. | 239 |
| FexNi1−x nanostructures (0 < x < 1.0) | 1,1,1,2-Tetrachloroethane | Fe1.0, Fe0.71Ni0.29 and Fe0.55Ni0.45 exhibited reactivity toward 1,1,1,2-tetrachloroethane, with Fe1.0 yielding the greatest rate of reductive dechlorination. | 240 |
| Na CM-cellulose (CMC)-stabilized Pd/Fe nanoparticles | 2,4-Dichlorophenoxyacetic acid (2,4-D), 2-chlorophenoxyacetic acid (2-CPA) | Considerable enhancements in particle stability and chemical reactivity with the addition of CMC to Pd/Fe nanoparticles were established. CMC-stabilized Pd/Fe nanoparticles (0.6 g Fe L−1) were able to remove much higher levels of 2,4-D with only one intermediate 2-chlorophenoxyacetic acid (2-CPA) and the final organic product phenoxyacetic acid (PA), than non-stabilized Pd/Fe nanoparticles or microsized Pd/Fe particles. | 241 |
| Fe–carbon composites | |||
| Fe–C nanocomposites, in particular Fe NPs on the external surface of carbon microspheres (synthesis see Fig. 10) | Chlorinated hydrocarbons | Spherical Fe–C nanocomposites were synthesized through a facile aerosol-based process and a subsequent carbothermal reduction. The distribution and immobilization of Fe particles throughout the C microspheres prevents nano-Fe aggregation, allowing the maintenance of particle reactivity. The carbon microspheres allow adsorption of trichloroethylene (TCE), thus removing dissolved TCE rapidly and facilitating reaction by increasing the local concentration of TCE in the vicinity of Fe particles (Fig. 11). | 242–244 |
| Carbon-coated Fe nanoparticles | Cr(VI) | Ferromagnetic carbon-coated Fe nanoparticles (core size of 15 nm) demonstrate strong ability to effectively remove more than 95 wt% of Cr(VI) in wastewater via carbon shell physical adsorption, which is much higher than the commercial available Fe NPs. | 245 |
| Poly(acrylic acid)/poly(vinyl alcohol)/multiwalled carbon nanotube (MWCNT) composite | Dyes of acid fuchsine, acridine orange, and methyl blue. | The produced composite nanofibrous mats exhibited a superior capability to decolorize the dyes of acid fuchsine, acridine orange and methyl blue, which are model dyes in wastewater of the printing and dyeing industry. | 246 |
| Encapsulated and supported NZVI | |||
| NZVI particles (10–90 nm) were encapsulated in biodegradable calcium-alginate capsules | Trichloroethylene | Trichloroethylene (TCE) degradation was 89–91% in 2 h. TCE degradation reaction rates for encapsulated and bare NZVI were similar indicating no adverse affects of encapsulation on degradation kinetics. The shelf-life of encapsulated NZVI was found to be four months with little decrease in TCE removal efficiency. | 247 |
| NZVI/chitosan (CS) composite | Cr(VI) | There is no significant difference between the reaction rates of bare NZVI and entrapped NZVI. The reduction capacity for Cr(VI) increases with increasing temperature and NZVI dosage but decreases with the increase in initial concentration of Cr(VI) and pH. | 248 |
| NZVI/CTMA-Bent composite (CTMA-Bent = organobentonite) | Atrazine | The removal efficiency of atrazine by the composite was compared with that by commercial Fe powder and NZVI itself. For both treatments by NZVI and NZVI/CTMA-Bent, the removal efficiency increased as the pH of the solution decreased, and the removal percentage of atrazine by NZVI/CTMA-Bent reached 63.5% at initial pH 5.0 after 120 min. | 249 |
| NZVI supported on montmorillonite (Mont) and hexadecyl trimethylammonium modified montmorillonite (HDTMA-Mont) | Cr(VI) | The presence of organo-montmorillonite apparently decreased the extent of aggregation and the size of the iron particles. Iron nanoparticles had a core–shell structure with the core being iron, and the shell consisting of iron (hydr)oxides. In contact with Cr(VI), the reduction of Cr(VI) was highest with HDTMA-Mont/iron particles, followed by Mont/iron particles and free iron nanoparticles. | 250 |
| NZVI-kaolinite composite (K-NZVI) | Pb2+ | More than 96% of Pb2+ was removed from aqueous solution using K-NZVI at an initial condition of 500 mg L−1 Pb2+ within 30 min under the conditions of 10 g L−1 of K-NZVI, pH 5.10 and a temperature of 30 °C. | 251 |
| ZVI/TiO2 nanocomposite (+UV light) | Phenol and CCl4 | Nanosized ZVI was synthesized and immobilized on the surface of a TiO2 nanotube, with carbon tetrachloride (CT) and phenol as the target compounds. The coupled degradation of CT and phenol by the NZVI combined with the titanate nanotube under illumination with UV light could be used for the simultaneous degradation of mixed priority pollutants. | 252 |
| Fe 0 –silica composites | |||
| Fe0 nanoparticles (20–110 nm diameter) supported on silica fume (SF–Fe0) | Cr(VI) | Attachment of Fe0 nanoparticles on the commercially available sub-micrometer silica fume prevented them from aggregation while maintaining the particle reactivity. When the Fe0 concentration was 0.4 g L−1, 88.00% of 40 mg L−1 Cr(VI) was removed in 120 min, 22.55% higher than unsupported Fe0. Almost all unsupported Fe0 was retained, whereas 51.50% and 38.29% of SF–Fe0 were eluted from the vertical and horizontal sand column, respectively. | 253 |
| Nanoscale zerovalent iron entrapped in porous silica particles. | Trichloroethylene (TCE). | Nanoscale zerovalent iron entrapped in porous silica particles and prepared through an aerosol-assisted process were used for TCE remediation. The entrapment of iron nanoparticles into the silica matrix prevents their aggregation while maintaining the particles' reactivity. Furthermore, the silica particles are functionalized with alkyl groups and are extremely efficient in adsorbing dissolved TCE. | 254 |
| System | Ion/substance to be eliminated | Description | Ref. |
| Fe 2 O 3 and Fe 3 O 4 | |||
| Alumina–zirconia–titania ceramic membranes coated with iron oxide nanoparticles | Disinfection byproduct precursors (in particular, ozonation byproducts: aldehydes, ketones and ketoacids) | Commercial available ceramic membranes (sintered in air at 900 °C for 30 min) with a nominal molecular weight cut-off of 5 kDa were coated 20, 30, 40 or 45 times with sol suspension-processed Fe2O3 nanoparticles (average diameter of 4–6 nm). Optimum water quality was achieved at 40 layers, which corresponds to a surface coating morphology consisting of a uniform, coarse-grained structure with open, nanosized interconnected pores. | 255, 256 |
| Iron oxide nanoparticles (19.3 nm magnetite and 37.0 nm hematite) | Arsenic (arsenate and arsenite) and metals (V, Cr, Co, Mn, Se, Mo, Cd, Pb, Sb, Tl, Th, U) | Strong adsorption of arsenic to the magnetite nanoparticles in the column. Arsenic and 12 other metals (V, Cr, Co, Mn, Se, Mo, Cd, Pb, Sb, Tl, Th, U) could be simultaneously removed by the iron oxide nanoparticles in soil. Irreversible sorption of arsenic to the iron oxide nanoparticle surface. | 257 |
| Nanomagnetites produced by the bacterial reduction of schwertmannite powder. | Tc(VII) and Cr(VI) | Schwertmannite-derived biomagnetite proved capable of retaining more (∼20%) 99mTc(VII) than ferrihydrite-derived biomagnetite. | 258 |
| Fe3O4 | Nitrates | Residual NO3− concentration of 1.35 mg L−1 is lower than international standards. | 259 |
| FeOOH | |||
| Iron (oxyhydr)oxide nanocrystallines | As(III) and heavy metals | Various phases of iron (oxyhydr)oxide nanocrystallites were synthesized from the phase-controlled transformation of amorphous hydrous ferric- or ferrous-oxide in thermal solution with a certain ethanol–water ratio and with the presence of oleic acid (goethite nanorods, hematite nanocubes and magnetite nanoparticles). The as-synthesized goethite nanorods and magnetite nanoparticles demonstrated extremely strong As(III) affinity, with 5.8 and 54 times of As(III) adsorption, respectively, higher than the micron-sized relatives. | 260 |
| α-FeOOH | Fluorides | Fluoride from contaminated H2O sample could be successfully brought down from 10.25 to 0.5 mg L−1. | 261 |
 | ||
| Fig. 10 (a) Schematic of aerosol-based process and (b) schematic of synthesis route to synthesize iron–carbon composites. Reproduced with permission. | ||
 | ||
| Fig. 11 Conceptual schematic showing potential applicability of Fe–C composites for the adsorption and simultaneous dechlorination of chlorinated hydrocarbon contaminants. Reproduced with permission. | ||
Water disinfection using NZVI and iron oxides
In general, both zerovalent iron (ZVI) and nanoscale ZVI (NZVI) were found to effectively remove many contaminants relevant to drinking water, including viruses, bacteria, chlorine, disinfection byproducts (DBPs), and other chemicals.262 It was proposed that NZVI can be applied to decentralized drinking water systems to improve the performance of point-of-use devices. However, in comparison with use of iron-containing nanomaterials in remediation of chemical pollutants from water, their use for water disinfection is represented by a considerably lesser number of examples.The bactericidal effect of nanoFe is a unique property of nanoFe, which was considerably less observed in other types of Fe-based compounds. An observed exception corresponds to iron coated sands and micro-scale iron powder, which were reported to adsorb viruses via electrostatic attraction and cause viruses to disintegrate or become noninfective. In any case, nanosized iron particles may provide higher efficiency compared to their bulk or micro-scale counterparts.263 It was established that NZVI in aqueous solution is able to rapidly inactivate E. coli.264 A strong bactericidal effect of nanoFe was found under deaerated conditions, with a linear correlation between log inactivation and nanoFe dose (0.82 log inactivation/mg L−1 nanoFe-h). The inactivation of E. coli under air saturation required much higher nanoFe doses due to the corrosion and surface oxidation of nanoFe by dissolved oxygen. The effect that adsorbed synthetic polymers and natural organic matter and aging (partial oxidation) have on the bactericidal properties of NZVI to the Gram-negative bacterium, E. coli, was elucidated.265 Exposure to 100 mg L−1 of bare NZVI with 28% Fe0 content resulted in a 2.2-log inactivation after 10 min and a 5.2-log inactivation after 60 min. In addition to E. coli, iron oxides showed a high inactivation capacity of MS2 virus.266 Thus, the inactivation of MS2 coliphage (MS2) by nanoparticulate NZVI and ferrous ion (Fe(II)) in aqueous solutions was demonstrated.267 It was established that for NZVI, the inactivation efficiency of MS2 under air-saturated conditions was greater than that observed under deaerated conditions, indicating that reactions (16)–(21) associated with the oxidation of NZVI were mainly responsible for the MS2 inactivation. Under air-saturated conditions, the inactivation efficiency increased with decreasing pH for both NZVI and Fe(II), associated with the pH-dependent stability of Fe(II). The ·OH and Fe(IV) (e.g., FeO2+) are mainly responsible for the oxidation of organic compounds and inactivation of pathogenic microorganisms. The authors suggested that the NZVI surfaces interacted directly with the MS2 phages, leading to their inactivation. Also, it was indicated that NZVI caused more capsid damage than Fe(II).
| Fe0s + O2 + 2H+ → Fe(II) + H2O2 | (16) |
| Fe0s + O2 + 2H+ → Fe(II) + H2O | (17) |
| Fe(II) + H2O2 → Fe(III) + ·OH + OH− | (18) |
| Fe(II) + H2O2 → Fe(VI) + H2O | (19) |
| Fe(II) + O2 → Fe(III) + ·O2− | (20) |
| Fe(II) + ·O2− + 2H+ → Fe(III) + H2O2 | (21) |
Other types of bacteria are also available to eliminate using NZVI. Thus, cyanobacteria pose a serious threat to water resources around the world, compounded by the fact that they are extremely resilient, having evolved numerous protective mechanisms to ensure their dominant position in their ecosystem. It was just shown (Fig. 12)268 that treatment with NZVI is an effective and environmentally benign method for destroying and preventing the formation of cyanobacterial water blooms. These nanoparticles have multiple modes of action, including the removal of bioavailable phosphorus, the destruction of cyanobacterial cells, and the immobilization of microcystins, preventing their release into the water column. The primary product of NZVI treatment is nontoxic and highly aggregated Fe(OH)3, which promotes flocculation and gradual settling of the decomposed cyanobacterial biomass. As another example, the bio-toxicity of Fe0 nanoparticles on the denitrifying bacteria Alcaligenes eutrophus was detected by the two methods of detecting inhibition of the growth of microorganisms and nitrification inhibition rate.269 It was shown that Fe0 nanoparticles had (a) obvious toxicity on the growth and nitrification inhibition rate and (b) distinct relationship between dose and toxicity of Fe0 nanoparticles. Fe0 nanoparticles had visible toxicity on the denitrifying bacterial, which could be decreased by surface coating and decoration of nanometal materials.
 | ||
| Fig. 12 (a) SEM images of cyanobacteria before treatment, (b) unused NZVI particles, (c) highly deformed cells after brief exposure to NZVI, and (d) completely destroyed cells surrounded by ferric oxide aggregates. Reproduced with permission. | ||
For the case of supported NZVI, we note first of all Fe-doped TiO2. It is known that TiO2 itself possess antimicrobial properties under UV-irradiation. Nonwoven nanofibers of pure and iron-doped titanium dioxide (TiO2) were tested for evaluation of their antimicrobial attributes for using them as disinfectant gauze for wound healing upon brief activation by UV/IR illumination.270 It was found that the fibers exhibited superior bactericidal affinity when exposed briefly (3–12 s) to either multiphoton laser or IR radiations. On the other hand, exposure to a UV beam for up to 20 min was not effective in mitigating the bacterial colonization of the E. coli. In a related report,271 the Fe/Ce codoped nanometer titanic hydrosol, which could be applied to indoor antisepsis, was prepared under atmosphere pressure by a microwave-assisted peptization process and resulted good antibacterial performance. It was shown that the Fe/Ce codoped nano-TiO2 hydrosol had better antibacterial performance than the pure nano-TiO2 hydrosol. The relative antibacterial rate exceeded 95% when the illumination of natural light irradiation lasted for 6 h. Finally, yellowish (Fe, N)-doped nanocrystalline TiO2 powders (anatase in phase, 10 nm size) were prepared using TiOSO4, CO(NH2)2, Fe(NO3)3·9H2O and CN3H5·HCl as precursors by a hydrothermal method.272 TiO2 powders were mixed with organic silicon and acrylic syrup to test their antibacterial performance by the colony counting method. It was shown that the sterilization ratio of E. coli by the heat-treated (Fe, N)-doped nanocrystalline TiO2 powders was reached up to 94.5% while that of the powders without any heat treatment was 91.1%.
All other known examples of use of iron-containing nanomaterials in water disinfection correspond to iron oxides, supported on distinct materials. Some differences in comparison with NZVI use have been observed. Thus, a material system composed of iron oxide (Fe2O3) nanoparticles loaded onto a fiberglass support displayed excellent antiviral properties against the model virus, MS2 phage, but is ineffective against bacteria, specifically E. coli.273,274 To increase the antibacterial properties and still maintain antiviral activity, silver nanoparticles were added to this system through an aqueous hydrothermal reduction process with 0.25 M silver nitrate (AgNO3). It was revealed that a 0.05 mg mL−1 loading of the Ag modified oligodynamic nanoparticle impregnated fiberglass system consisting of Fe2O3 (9.1 wt%) and Ag (0.1 wt%)/g-fiber, displayed robust antibacterial activity by achieving a 2 log removal of 106 CFU/mL E. coli in 1 min. Site competition between the Ag and other species on the surface of the fiberglass was observed. The fiberglass impregnated with CuO/Ag and FexOy/Ag, both exhibited synergistic disinfection ability greater than that of fiberglass impregnated with only Ag, the latter displaying the highest bactericidal effect. Silver-doped iron oxides were also used in a series of other related reports due to well-known antibacterial properties of this metal both in bulk, nanoforms and Ag+ ion. For instance, silver (10 nm) nanoparticles inlaid Fe3O4-SiO2 magnetic composite (Fe3O4–SiO2–Ag) was synthesized and its potential application as an antibacterial material in water disinfection was investigated.275 The minimum inhibitory concentrations of Fe3O4–SiO2–Ag magnetic composite to E-coli and Staphylococcus aureus were 15.625 and 31.25 mg L−1, respectively, and the minimum bactericidal concentrations were 250 and 500 mg L−1, respectively. This disinfectant in normal saline solution could kill 99.9% of the tested bacteria within 60 min. In addition, the effects of silver(I) and iron(III) doping contents on photocatalytic performance of the titania thin film were studied.276 Ag and Fe doping and co-doping contents on nanotitania photocatalytic bactericidal films were prepared by sol–gel method, thus combining three active antibacterial species (Ag, Fe and TiO2). The photocatalytic activity of titania films was evaluated by the sterilizing rate of the E. coli. For fluorescent light irradiation, optimal doping amounts for silver(I)/titania and iron(III)/titania are 0.05, 0.1%, respectively. More complex Fe–Ag containing systems (a nano-Nd/Fe/B pipe containing ZnO and Ag2O) are also known.277
Metal oxides, zeolites and such classic objects of nanotechnology as carbon nanotubes have been applied as supports for iron oxides. Among metal oxide nanoparticles, zinc oxide demonstrates significant bacterial growth inhibition on a broad spectrum of bacteria, mainly by catalysis of reactive oxygen species formation from water and oxygen. Zinc oxide was combined278 with iron oxide to produce magnetic composite nanoparticles with improved colloidal aqueous stability, together with adequate antibacterial activity. The Zn/Fe oxide composite nanoparticles (containing iron oxide, zinc oxide and zinc ferrite phases) were synthesized by basic hydrolysis of Fe2+ and Zn2+ ions in aqueous continuous phase containing gelatin. Their antibacterial activity, tested against Staphylococcus aureus and E. coli, was found to be dependent on the weight ratio [Zn]/[Fe], i.e., the higher the ratio, the higher the antibacterial activity. The activity against Staphylococcus aureus was significantly higher than that observed against E. coli. The environmental safety effect of nanosized Fe materials including Fe3O4, Fe3O4-zeolite composite and Fe-doped zeolite was studied using a Tetrahymena thermophile model.279 The magnetic nanosized Fe3O4 is most sensitive to the suppression of the growth of Tetrahymena thermophile, as compared with the Fe3O4–zeolite composite and Fe-doped zeolite. In addition, a photocatalytic technique using visible light and carbon nanotubes and nanosized Fe2O3 powder was used to inhibit pathogenic bacterial growth in water.280 It was suggested that after careful design, this system can be used to disinfect drinking water, making it free of pathogenic bacteria.
A special case is the use of polymer supports (membranes) containing the NZVI and iron oxides, whose use precisely for water disinfection has not yet been well developed, in a difference with remediation of chemical pollutants. In contrast to conventional, passive membrane technologies, an approach, reported in ref. 281 utilized two independently controlled, nanostructured membranes in stacked configuration for the generation of the necessary oxidants to purify water (Fig. 13). These include biocatalytic and organic/inorganic (polymer/iron) nanocomposite membranes. The bioactive (top) membrane contains an electrostatically immobilized enzyme for the catalytic production of one of the main reactants, hydrogen peroxide (H2O2), from glucose. The bottom membrane contains either immobilized iron ions or ferrihydrite/iron oxide nanoparticles for the decomposition of hydrogen peroxide to form powerful free radical oxidants. By permeating a solution containing a model organic contaminant, such as trichlorophenol, with glucose in oxygen-saturated water through the membrane stack, significant contaminant degradation was realized. According to the authors, other applications including disinfection and/or virus inactivation are possible.
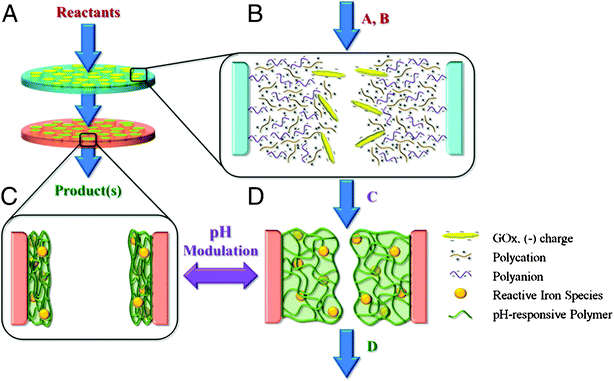 | ||
| Fig. 13 Schematic of reactive nanostructured stacked membrane system. (A) Setup of stacked membrane system consisting of two membranes of different functionality operated via convective flow. (B) Pore of top membrane with layer-by-layer polycation/polyanion assembly containing electrostatically immobilized GOx for the conversion of reactants A + B → C. (C) Pore of bottom membrane consisting of pH-responsive PAA gel with immobilized iron species in collapsed state. (D) Pore of bottom membrane after exposure to increased pH causing gel to swell; reactive iron species catalyzes conversion of C → D. Reproduced with permission. | ||
Toxicity and risks of iron nanomaterials application
The conventional methods for in situ remediation of chlorinated organic solvents, such as trichloroethylene, tend to produce undesirable byproducts, whereas the use of nanoscale bimetallic particles according to the techniques described above has succeeded in eliminating some of these byproducts.282 However, at present the potential environmental risks of NZVI in in situ field scale applications are largely unknown at the present and traditional environmental risk assessment approaches are not yet able to be completed.283 Therefore, it may not yet be fully clear how to consider the environmental benefits and risks of NZVI for in situ applications. At present, there are no significant grounds on which to form the basis that NZVI currently possess a significant, apparent risk to the environment, although the majority of the most serious criteria (i.e. potential for persistency, bioaccumulation, toxicity) are generally unknown. Similar discussion could be applied to SPIONs, including magnetite (Fe3O4), which are widely used in uses such as hyperthermic malignant cell treatment, magnetic resonance imaging, targeted drug delivery, tissue engineering, gene therapy, and cell membrane manipulation.This is rather new area of research. Several reviews284–289 and reports290–294 (some of them described below) on iron-containing nanoparticles could provide a background to this problem. However, as noted in a review295 superparamagnetic iron oxide nanoparticles (SPIONs) are promising materials for various biomedical applications including targeted drug delivery and imaging, hyperthermia, magneto-transfections, gene therapy, stem cell tracking, molecular/cellular tracking, magnetic separation technologies (e.g. rapid DNA sequencing), and detection of liver and lymph node metastases. The most recent applications for SPIONs for early detection of inflammatory, cancer, diabetes and atherosclerosis have also increased their popularity in academia. Another fundamental discussion of SPIONs concerns potential toxicity. Neenu Singh et al.,296 have investigated such aspects as cytotoxicity, protein–SPION interaction, changes in gene expression, impact on cell proliferation, among others.
NZVI (bare and supported)
Several research groups have studied the factors (size, surface, supporting material, synthesis conditions, etc.) influence the possible damage to living cells caused by zerovalent iron particles. The overall conclusion is that the NZVI do possess certain toxicity for animal cells (for example iron nanoparticles showed perturbations in the expression of a set of functional genes297). However, this toxicity is much lower in comparison with nanoparticles of some other metals. Among other studies, the size-dependent properties of inorganic metal (in particular, Fe0) and metal oxide (boehmite) nanoparticles in relation to environmental, health and safety perspectives were defined.298 Major risks concern the ignition, combustion and explosion of the metal particles in reactive atmospheres, like hydrogen and predominantly oxygen and air. So, basic mechanisms of oxidation and reactions at small scale, classification of combustibility, ignition by when initiated by low-energy ignition sources, rates of flame propagation, intensity of hydrogen generation at interaction with water, inflammability initiated by electrostatic discharge were investigated299 for a series of metals, in particular iron. In addition, oxidative stress markers with SiO2-Fe induced cytotoxicity in human endothelial cells were evaluated using iron-doped nanosilica (16 nm).300 Significant modifications for all parameters in cells treated with these nanoparticles were found. Related SiO2/Nd–Fe–B nanoparticles had mild toxicity, and could meet the requirement of the national standard for medical implantation materials.301 Iron-doped TiO2 nanoparticles were tested302 as photosensitizers to kill tumor cells by studying the HL60 cells activity with different nanoparticles dosages, different magnetic field strengths and different conditions of receiving the visible radiation and no radiation. The nanoparticles exhibited toxic/inhibiting effect on leukemia cells, the greater the concentration of nanoparticles was, the more the toxic/inhibiting effect was; while the toxic/inhibiting effect of magnetic field on cells was dependent on the concentration of iron-doped and magnetic induction intensity. In addition, it is necessary to mention that toxicological impacts of iron nanoparticles on the aquatic ecosystem remain poorly understood. In a study the larvae of medaka fish (Oryzias latipes) were treated303 with thoroughly characterized solutions containing CM-cellulose (CMC)-stabilized NZVI, aged nanoscale iron oxides (nFe-oxides) or ferrous ion Fe(II) for 12–14 days aqueous exposure to assess the causal toxic effect(s) of iron NPs on the fish. The authors established that with the CMC-NZVI solution the dissolved oxygen level decreased, and a burst of reactive oxygen species (ROS) was generated as Fe(II) oxidized to ferric ion (Fe(III)); with the other two iron solutions, these parameters did not significantly change.In case of iron oxides (SPIONs), much more research has been carried out, especially on doped and supported iron oxides in distinct nanostructural forms. Thus, to test the hypothesis about nanoparticle-triggered endothelial dysfunction, iron oxide nanoparticles (Fe2O3 and Fe3O4), as two widely used nanomaterials and the main metallic components in particulate matter, were selected to assess their potential risks on human endothelial system.304 The direct effects of iron oxide nanoparticles on human aortic endothelial cells (HAECs) and the possible effects mediated by monocyte (U937 cells) phagocytosis and activation were investigated. It was revealed (Fig. 14) that intravascular iron oxide nanoparticles may induce endothelial system inflammation and dysfunction in three ways: (a) nanoparticles may escape from phagocytosis that interact directly with the endothelial monolayer; (b) nanoparticles are phagocytized by monocytes and then dissolved, thus impact the endothelial cells as free iron ions; or (c) nanoparticles are phagocytized by monocytes to provoke oxidative stress responses. Similarly, the SPIONs (6–12 nm diameter and aggregated clusters of these 6–12 nm nanoparticles), produced using a flame synthesis method, were tested in respect of their biocompatibility with endothelial cells for 24 h.305 It was suggested that flame synthesized iron oxide nanoparticles are comparable to commercially available nanoparticles for biological applications.
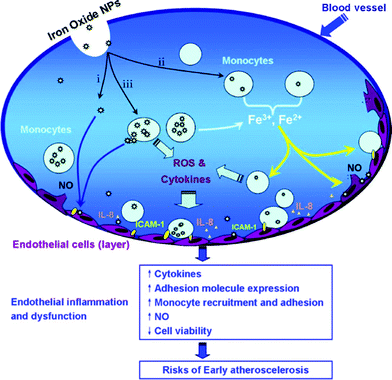 | ||
| Fig. 14 Influence of iron oxides on endothelial cells. Reproduced with permission. | ||
Multiply-replicated cytotoxicity (in vitro) assays utilizing a human epithelial (lung model) cell line (A549) consistently demonstrated varying degrees of cell death for essentially all particulate matter (PM) which was characterized as aggregates of nanoparticulates or primary nanoparticles.306 Cytokine release was detected, in particular, for Fe2O3, as well as reactive oxygen species (ROS) production. Nanoparticulate materials in the indoor and outdoor environments appear to be variously cytotoxic. Cytotoxicity of nanoparticles with iron oxide as core and a biocompatible polymer as “first layer” was analyzed,307 presenting a broad overview of currently available in vitro and in vivo toxicity data. It was indicated that the toxicity data obtained vary significantly depending on size, size distribution, surface (including coating), and subsequent surface derivatization. The acute toxicity and the anti-phagocytosis of folic acid-O-carboxymethyl chitosans superparamagnetic Fe oxide nanoparticles (FA-OCMCS-SPIO-NPs) and O-carboxymethyl chitosans superparamagnetic Fe oxide nanoparticles (OCMCS-SPIO-NPs) were evaluated.308 Apparent toxic reaction and tissue injury did not occur among the animals. Prussian blue stain showed that FA-OCMCS-SPIO-NPs could escape from phagocytosis of liver and spleen completely and most of OCMCS-SPIO-NPs could while dextran-SPIO-NPs could not.
The toxicities (at both cellular and molecular levels) of three forms of SPIONs of various surface chemistries (COOH, plain, and NH2) through the comparison with gene expression patterns of three cell types (i.e., human heart, brain, and kidney) were evaluated.309 It was revealed that SPIONs-COOH altered genes associated with cell proliferative responses due to their reactive oxygen species (ROS) properties. Uptake, toxicity and degradation of magnetic nanowires (200 nm diameter and lengths comprised between 1 and 40 μm, fabricated by controlled assembly of γ-Fe2O3 nanoparticles) by NIH/3T3 mouse fibroblasts were studied.310 It was revealed that (a) the wires do not display acute short-term (<100 h) toxicity towards the cells and (b) the cells are able to degrade the wires and to transform them into smaller aggregates, even in short time periods (days). The in vitro cytotoxicity of 100 nm iron oxide particles (Fe2O3) was evaluated in the human embryonic kidney cell line HEK293.311 Cell viability assays demonstrated that 100 μg mL−1 Fe2O3 exhibited 20% reduction in HEK293 cell viability in 24 h. Comparing the results with those for other targets, at both the cellular and molecular levels, the toxicity was observed in the following order: ZnO > Fe2O3-NPs > MCM-41.
Combination of microinjection techniques and Raman spectroscopy was used to investigate the effects of Ag and Fe3O4 nanoparticles on Hela cells.312 The nanoparticles were microinjected inside the cells and these latter ones were probed by means of Raman spectroscopy after a short incubation time, in order to highlight the first and impulsive mechanisms developed by the cells to counteract the presence of the nanoparticles. A different behavior of the cells treated with nanoparticles in comparison with the control cells was observed. These differences were supposed to be generated by an emerging oxidative stress due to the nanoparticles. γ-Fe2O3 can cause cell death within 24 h of exposure, most likely through oxidative stress.313In vivo exploration suggested that although γ-Fe2O3 nanoparticles are rapidly cleared through the urine, they can lead to toxicity in the liver, kidneys and lungs, while the brain and heart remain unaffected. γ-Fe2O3 could exhibit harmful properties and therefore surface coating, cellular targeting, and local exposure should be considered before developing clinical applications. Acute toxicity tests were selected according to their extensive use in toxicological studies of iron oxide nanoparticles and included phytotoxicity using several seeds, Daphnia magna and a bioluminescent test (Microtox), revealing low toxicity.314 The toxic effects of inhalation exposure to Fe2O3 nanoparticles (together with ZnO) in rats were investigated.315 Iron content in liver and lung tissues was significantly increased at 36 h. The levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein (TP), creatine kinase (CK), and lactate dehydrogenase (LDH) were significantly decreased compared to the unexposed controls. It was shown that both types of nanoparticles caused severe damage in liver and lung tissues.
Zeta potential measurements, common in nanotoxicology, were applied to Fe2O3, among other oxides in respect of to acquaint the effects of pH and time on nanoparticle zeta potential, agglomerate size and cellular viability.316 Fe2O3 increased in zeta potential and agglomerate size over time. Cytotoxicity studies revealed that Fe2O3 caused decreasing cellular viability over 48 h. It was indicated that alterations in the pH have a large effect on zeta potential and agglomerate size which may be used as a predictive measure of nanotoxicity. Cytotoxicity of SPIONs (bare and poly(ethylene glycol)-co-fumarate (PEGF)-coated SPION with narrow size distributions) and their ability to change cell medium components was investigated317 in respect with Dulbecco's modified Eagle's medium (DMEM) and primary mouse fibroblast (L929) cell lines. The potential toxic effects of iron(II,III) oxide nanoparticles were studied.318 While in vitro MTT assay showed a moderate cytotoxic effect, the Fe(II,III) nanoparticles proved to be devoid of mutagenic effect in the bacterial systems tested. In addition, peptide–SPION complexes were proved to be biocompatible and are localized around the cells due to their peptide coating.319
In case of other iron-containing nanostructures, there is little available information on their toxicity. Thus, the toxicity of Mn0.5Zn0.5Fe2O4 nanoparticles in vitro on L-02 cell was studied,320 showing that (a) the activity of L-02 cell decreased with the increasing of Mn0.5Zn0.5Fe2O4 nanoparticle concentration and prolonging of poisoning time and (b) the activity of L-02 cell became stable after poisoning for 48 h. The toxicity of the related compound, stoichiometric Mn0.2Zn0.8Fe2O4 monodisperse nanoparticles, was evaluated by viability assays on human umbilical vein endothelial cells.321
Conclusions
Currently, the use of the NZVI, iron oxides and other Fe-contained nanomaterials for remediation of both organic and inorganic pollutants from groundwater and contaminated soils could be considered as a relatively hot topic in the nanotechnology. During the last decade, a series of distinct methods have been offered to synthesize these nanostructures as by traditional wet chemistry routes and more sophisticated modern techniques as well as “greener” methods using plant extracts. Due to relatively low toxicity of iron-containing nanoparticles, it has been allowed to apply them in free and supported forms to reach a considerable decontamination of the environment.Evaluation of toxicity of the Fe-containing nanomaterials remains an object of permanent research and polemics. Since real long-time effects of the presence of iron-containing nanoparticles in rivers, groundwater, lakes and oceans are still unknown, the idea to introduce iron-containing nanoparticles into oceans,322 in order to stimulate growth of phytoplankton and better adsorption of CO2, was further rejected.
The research field, dedicated to core–shell iron (iron oxide)/gold nanoparticles, is of a special interest due to possibility to be controlled by magnetic fields (iron core) and functionalization (gold shell). These air-stable Nps are protected from the oxidation and retain most of the favorable magnetic properties, which possess the potential for applications in drug delivery, high density memory devices by forming self-assembling nanoarrays, and a series of other applications.
Acknowledgements
BIK is grateful to the Paicyt-UANL-2012 project for financial support.References
- B. Chertok, B. A. Moffat, A. E. David, F. Yu, C. Bergemann, B. D. Ross and V. C. Yang, Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors, Biomaterials, 2008, 29(4), 487–496 CrossRef CAS.
- P. M. Tiwari, K. Vig, V. A. Dennis and S. R. Singh, Functionalized Gold Nanoparticles and Their Biomedical Applications, Nanomaterials, 2011, 1, 31–63 Search PubMed.
- C. S. S. R. Kumar, Magnetic nanomaterials, Wiley-VCH, Weinheim, 2009, 648 pp Search PubMed.
- An-Hui Lu, E. L. Salabas and F. Schuth, Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS.
- K. Watlington, Emerging Nanotechnologies for Site Remediation and Wastewater Treatment. Report for U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response Office of Superfund Remediation and Technology Innovation, Technology Innovation and Field Services Division, Washington, DC, 2005, 47 pp Search PubMed.
- T. E. Cloete, M. B. M. de Kwaadsteniet and J. M. López-Romero, Nanotechnology in water treatment. Applications, Caister Academic Press, 2010, 196 pp Search PubMed.
- B. Tansel, New Technologies for Water and Wastewater Treatment: A Survey of Recent Patents. Recent Patents on Chemical Engineering, 2008, ch. 1, pp. 17–26 Search PubMed.
- I. Ali, New Generation Adsorbents for Water Treatment, Chem. Rev., 2012 DOI:10.102/cr300133d.
- X. Q. Li, D. W. Elliott and W. Zhang, Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: materials and engineering aspects, Crit. Rev. Solid State Mater. Sci., 2006, 31, 111–122 CrossRef CAS.
- M. Hofmann-Amtenbrink, B. von Rechenberg and H. Hofmann, Superparamagnetic nanoparticles for biomedical applications, in Nanostructured Materials for Biomedical Applications, 2009, Transworld Research Network, Kerala, India Search PubMed.
- M. Mahmoudi, M. A. Sahraian, M. A. Shokrgozar and S. Laurent, Superparamagnetic Iron Oxide Nanoparticles: Promises for Diagnosis and Treatment of Multiple Sclerosis, ACS Chem. Neurosci., 2011, 2, 118–140 Search PubMed.
- D. L. Huber, Iron nanoparticles, in Dekker encyclopedia of nanoscience and nanotechnology, ed. J. A. Schwarz, C. I. Contescu and K. Putyera, CRC Press, Taylor and Francis Group, Boca Raton, FL, 2008, vol. 3, pp. 1681–1687 Search PubMed.
- C. S. Rajan, Nanotechnology in Groundwater Remediation, Int. J. Environ. Sci. Dev., 2011, 2(3), 182–187 Search PubMed.
- S. Cheong, P. Ferguson, I. F. Hermans, G. N. L. Jameson, S. Prabakar, D. A. J. Herman and R. D. Tilley, Synthesis and Stability of Highly Crystalline and Stable Iron/Iron Oxide Core/Shell Nanoparticles for Biomedical Applications, ChemPlusChem, 2012, 77, 135–140 Search PubMed.
- U. Harm, J. Schuster and K.-M. Mangold, Modification of iron nanoparticles for ground water remediation, DECHEMA, Karl-Winnacker Institut, 2010, http://kwi.dechema.de/kwi_media/Downloads/ec/F564_Nanoeisen_Harm.pdf. Search PubMed.
- C. F. Geraldes and S. Laurent, Classification and basic properties of contrast agents for magnetic resonance imaging, Contrast Media Mol. Imaging, 2009, 4(1), 1–23 Search PubMed.
- Y. W. Jun, J. W. Seo and J. Cheon, Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences, Acc. Chem. Res., 2008, 41(2), 179–189 CrossRef CAS.
- B. Nowack, Chapter 1. Pollution Prevention and Treatment Using Nanotechnology, Nanotechnology, Volume 2: Environmental Aspects, ed. H. Krug, Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim, 2008.
- J. Bai and J-P. Wang, High-magnetic-moment core–shell-type FeCo-Au/Ag nanoparticles, Appl. Phys. Lett., 2005, 87(15), 1-152502–1-152502 Search PubMed.
- A. Figuerola, R. Di Corato, L. Manna and T. Pellegrino, From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications, Pharmacol. Res., 2010, 62(2), 126–143 CrossRef CAS.
- M. Pita, J. M. Abad, C. Vaz-Dominguez and C. Briones et al., Synthesis of cobalt ferrite core/metallic shell nanoparticles for the development of a specific PNA/DNA biosensor, J. Colloid Interface Sci., 2008, 321, 484–492 CrossRef CAS.
- K. An and T. Hyeon, Synthesis and biomedical applications of hollow nanostructures, Nano Today, 2009, 4(4), 359–373 CrossRef.
- C. Xu, Z. Yuan, N. Kohler, J. Kim, M. A. Chung and S. Sun, FePt nanoparticles as an Fe reservoir for controlled Fe release and tumor inhibition, J. Am. Chem. Soc., 2009, 131(42), 15346–15351 CrossRef CAS.
- J. Gao, G. Liang, B. Zhang, Y. Kuang, X. Zhang and B. Xu, FePt@CoS2 yolk-shell nanocrystals as a potent agent to kill HeLa cells, J. Am. Chem. Soc., 2007, 129(5), 1428–1433 CrossRef CAS.
- M. Diao and M. Yao, Use of zerovalent iron nanoparticles in inactivating microbes, Water Res., 2009, 43(20), 5243–5251 Search PubMed.
- J. Y. Kim, H. J. Park, C. Lee, K. L. Nelson, D. L. Sedlak and J. Yoon, Inactivation of Escherichia coli by nanoparticulate zerovalent iron and ferrous ion, Appl. Environ. Microbiol., 2010, 76(22), 7668–7670 Search PubMed.
- Q. W. Ding, T. W. Qian, H. F. Liu and W. Xue, Preparation of Zero-Valent Iron Nanoparticles and Study of Dispersion, Appl. Mech. Mater., 2011, 55–57, 1748–1752 Search PubMed.
- J.-m. Lee, J.-h. Kim, J.-w. Lee, J.-h. Kim, H.-s. Lee and Y.-s. Chang, Synthesis of Fe-nano Particles Obtained by Borohydride Reduction with Solvent. Paper A-068, in: B. M. Sass (Conference Chair), Remediation of Chlorinated and Recalcitrant Compounds—2008. Proceedings of the Sixth International Conference on Remediation of Chlorinated and Recalcitrant Compounds (Monterey, CA, May 2008). ISBN 1-57477-163-9, published by Battelle, Columbus, OH, www.battelle.org/chlorcon.
- A. Khodabakhshi, M. M. Amin and M. Mozaffari, Synthesis of magnetite nanoparticles and evaluation of its efficiency for arsenic removal from simulated industrial wastewater, Iran. J. Environ. Health. Sci. Eng., 2011, 8(3), 189–200 Search PubMed.
- H. Park, P. Ayala, M. A. Deshusses, A. Mulchandani, H. Choi and N. V. Myung, Electrodeposition of maghemite (γ-Fe2O3) nanoparticles, Chem. Eng. J., 2008, 139, 208–212 Search PubMed.
- J. Grabis, G. Heidemane and D. Rašmane, Preparation of Fe3O4 and γ-Fe2O3 Nanoparticles by Liquid and Gas Phase Processes, Mater. Sci. (MEDŽIAGOTYRA), 2008, 14(4), 292–295 Search PubMed.
- S. G. Ballard, Apparatus and methods for the production of powders, US Pat., US20056972115, 2005 Search PubMed.
- W. -B. Kim, J.-S. Park, C.-Y. Suh, D.-S. Kil and J.-C. Lee, US Pat., 20070209477, 2007 Search PubMed.
- J. P. Lei, X. L. Dong and X. G. Zhu et al., Formation and characterization of intermetallic Fe–Sn nanoparticles synthesized by an arc discharge method, Intermetallics, 2007, 15(12), 1589–1594 Search PubMed.
- M. Gupta, E. Wong and W. Leong, Microwaves and Metals, Wiley-Interscience, New York, 2007, 256 pp Search PubMed.
- S. Komarneni, H. Katsuki, D. Li and A. S. Bhalla, Microwave–polyol process for metal nanophases, J. Phys.: Condens. Matter, 2004, 16, S1305–S1312 Search PubMed.
- M. F. Becker, J. R. Brock and H. Cai et al., Metal nanoparticles generated by laser ablation, Nanostruct. Mater., 1998, 10(5), 853–863 CrossRef CAS.
- A. R. Rodrigues, J. M. Soares, F. L. A. Machado, W. M. de Azevedo and D. D. de Carvalho, Synthesis of α-Fe particles using a modified metalmembrane incorporation technique, J. Magn. Magn. Mater., 2007, 310(2), 2497–2499 Search PubMed.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. J. Lyon, S. Mahendra, M. J. Mclaughlin and J. R. Lead, Nanomaterials in the environment: behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem., 2008, 27(9), 1825–1851 CrossRef CAS.
- R. Yuvakkumar, V. Elango, V. Rajendran and N. Kannan, Preparation and characterization of zerovalent iron nanoparticles, Dig. J. Nanomater. Biostruct., 2011, 6(4), 1771–1776 Search PubMed.
- H. Bönnemann, W. Brijoux, R. Brinkmann, E. Dinjus, T. Joussen and B. Korall, Erzeugung von kolloiden Übergangsmetallen in organischer Phase und ihre Anwendung in der Katalyse, Angew. Chem., Int. Ed. Engl., 1991, 30, 1312–1344 CrossRef.
- S. Yuan-Pang, L. Xiao-Qin, Z. Wei-Xian and H. P. Wang, A method for the preparation of stable dispersion of zerovalent iron nanoparticles, Colloids Surf., A, 2007, 308(1–3), 60–66 Search PubMed.
- A. Harutyunyan, L. Grigorian and T. Tokune, Method for synthesis of metal nanoparticles, US Pat., 20056974493, 2005 Search PubMed.
- C. Klinke and K. Kern, Iron nanoparticle formation in a metal–organic matrix: from ripening to gluttony, Nanotechnology, 2007, 18, 215601 CrossRef.
- Y. Lu, Z. Zhu and Z. Liu, Carbon-encapsulated Fe nanoparticles from detonation-induced pyrolysis of ferrocene, Carbon, 2005, 43(2), 369–374 CrossRef CAS.
- P. T. Anastas and I. T. Horvath, Green Chemistry for a Sustainable Future, Wiley, 1st edn, 2012, 350 pp Search PubMed.
- C.-J. Li and P. T. Anastas, Handbook of Green Chemistry - Green Processes, Wiley-VCH, 3 Volume Set edition, 2012, 1326 pp Search PubMed.
- V. K. Ahluwalia, Green Chemistry: Environmentally Benign Reactions, CRC Press, 2nd edn, 2012, 326 pp Search PubMed.
- J. T. Patel, O. B. Patel and B. P. Raval, Green Chemistry: New Avenues in Chemical Research: Focus in Healthcare, LAP LAMBERT Academic Publishing, 2012, 60 pp Search PubMed.
- R. Luque, Green Chemistry, Nova Science Publishers, 2011 Search PubMed.
- G. E. Hoag, J. B. Collins, R. S. Varma and M. Nadagouda, Green synthesis of metal nanoparticles using plant extracts, PCT Int. Appl., WO 2009140694 A2 20091119, 2009 Search PubMed.
- K. E. Tanui, Green Synthesis and Characterization of Iron Nanoparticles, http://chemistry.uonbi.ac.ke..
- G. E. Hoag, J. B. Collins, J. L. Holcomb, J. R. Hoag, M. N. Nadagouda and R. S. Varma, J. Mater. Chem., 2009, 19(45), 8671–8677 RSC.
- M. N. Nadagouda, A. B. Castle, R. C. Murdock, S. M. Hussain and R. S. Varma, Green Chem., 2010, 12, 114–122 RSC.
- C. Yao, H. Ma and Y. Tong, Electrochemical preparation and magnetic study of amorphous nanostructured Nd–Fe–Co–Ni–Mn high entropy alloy film, Yingyong Huaxue, 2011, 28(10), 1189–1194 Search PubMed.
- B.-Y. Yoo, S. C. Hernandez, B. Koo, Y. Rheem and N. V. Myung, Electrochemically fabricated zerovalent iron, iron-nickel, and iron-palladium nanowires for environmental remediation applications, Water Sci. Technol., 2007, 55, 149–156 Search PubMed (1-2, Wastewater Reclamation and Reuse for Sustainability).
- A. V. Glebov, V. A. Glebov and O. I. Popova, Development of nanofilm Fe-Pt magnets for superdense-recording heads, Tsvetnye Metally, 2009, 12, 67–70 Search PubMed.
- B. Wang, D. C. Berry, Y. Chiari and K. Barmak, Experimental measurements of the heats of formation of Fe3Pt, FePt, and FePt3 using differential scanning calorimetry, J. Appl. Phys., 2011, 110(1), 013903/1–1-013903/8 Search PubMed.
- E. P. Yelsukov, G. N. Konygin, V. E. Porsev Voronina and V. Ye, Mossbauer spectroscopy of Fe-based nanomaterials, Czech. J. Phys., 2006, 56(S3), E31–E44 Search PubMed.
- L. H. Dale, Synthesis, applications, and applications of iron nanoparticles, Small, 2005, 1(5), 482–501 CrossRef CAS.
- W. L. Zhou, E. E. Carpenter, J. Lin, A. Kumbhar, J. Sims and C. J. O'Connor, Nanostructures of gold coated iron core–shell nanoparticles and the nanobands assembled under magnetic field, Eur. Phys. J. D: At., Mol. Opt. Phys., 2001, 16(1–3), 289–292 Search PubMed.
- Nanoparticle Assemblies and Superstructures, ed. N. S. Kotov, CRC Press, 2005, 407 pp Search PubMed.
- Electrochemical Sensors, Biosensors and their Biomedical Applications, ed. X. Zhang, H. Ju and J. Wang, Academic Press, 2007, 446 pp Search PubMed.
- Nanomaterials for Application in Medicine and Biology, ed. M. Giersig and G. B. Khomutov, Springer, 2008, 99 pp Search PubMed.
- Handbook of Microscopy for Nanotechnology, ed. N. Yao and Z. L. Wang, Springer, 2005, 170 pp Search PubMed.
- Principles of Inorganic Materials Design, ed. J. N. Lalena and D. A. Cleary, Wiley-Interscience, 2005, 404 pp Search PubMed.
- Nanocomposite structures and dispersions, ed. I. Capek, Volume 23 (Studies in Interface Science), Elsevier Science, 2006, 155 pp Search PubMed.
- Modern Supramolecular Gold Chemistry: Gold-Metal Interactions and Applications, ed. A. Laguna, Wiley-VCH, 2008, 525 pp Search PubMed.
- L. Guczi, A. Beck, A. Horvath and D. Horvath, From molecular clusters to metal nanoparticles, Top. Catal., 2002, 19(2), 157–163 CrossRef CAS.
- L. Wang, H.-Y. Park and S. I.-I. Lim et al., Core@shell nanomaterials: gold-coated magnetic oxide nanoparticles, J. Mater. Chem., 2008, 18(23), 2629–2635 RSC.
- S. Grancharov, S. O'Brien, G. Held and C. Bruce Murray, Method of preparation of biomagnetic nanoparticles coated with a noble metal layer, US Pat., 20060140868, 2006; World Pat., WO/2004/060580, 2004.
- T. Hyeon, J. Y. Kim, M-.H. Cho, K. Kim Seong and J. Lee, Use of core–shell gold nanoparticle which contains magnetic nanoparticles for MRI T2 contrast agent, cancer diagnostic and therapy, World Pat., WO 2008048074, 2008.
- A. Naitabdi and B. R. Cuenya, Formation, thermal stability, and surface composition of size-selected Au-Fe nanoparticles, Appl. Phys. Lett., 2007, 91(11), 113110–1-113110–3 Search PubMed.
- N. Dahal, V. Chikan, J. Jasinski and V. J. Leppert, Synthesis of Water-Soluble Iron-Gold Alloy Nanoparticles, Chem. Mater., 2008, 20(20), 6389–6395 Search PubMed.
- H. L. Liu, J. H. Wu, J. H. Min and Y. K. Kim, Synthesis of monosized magnetic-optical AuFe alloy nanoparticles, J. Appl. Phys., 2008, 103(7, Pt. 2), 07D529–1–1-07D529/3 Search PubMed.
- K. Sato, B. Bian and Y. Hirotsu, L10 type ordered phase formation in Fe-Au nanoparticles, Jpn. J. Appl. Phys., 2002, 41(Part 2, No. 1A/B), L1–L3 Search PubMed.
- S.-J. Cho, S. M. Kauzlarich and J. Olamit et al., Characterization and magnetic properties of core–shell structured Fe/Au nanoparticles, J. Appl. Phys., 2004, 95(11), 6804–6806 CrossRef CAS.
- J. Lin, W. Zhou and A. Kumbhar et al., Gold-coated iron (Fe/Au) nanoparticles: synthesis, characterization, and magnetic field-induced self-assembly, J. Solid State Chem., 2001, 159(1), 26–31 CrossRef CAS.
- R. P. Andres, Ng, A. T. Fe/Au nanoparticles and methods, World Pat., WO 2003073444, 2003.
- M. Chen, S. Yamamuro, D. Farrell and S. A. Majetich, Gold-coated iron nanoparticles for biomedical applications, J. Appl. Phys., 2003, 93(10), 7551–7553 CrossRef CAS.
- A. Zelenakova, J. Kovac, V. Kavecansky and V. Zelenak, Magnetic study of the Fe coated by Au nanoparticles, Acta Phys. Polonica, A, 2008, 113(1), 533–536 Search PubMed.
- O. Pana, C. M. Teodorescu and O. Chauvet et al., Structure, morphology and magnetic properties of Fe-Au core–shell nanoparticles, Surf. Sci., 2007, 601(18), 4352–4357 Search PubMed.
- Z. Ban, Y. A. Barnakov, F. Li, V. O. Golub and C. J. O'Connor, The synthesis of core–shell iron@gold nanoparticles and their characterization, J. Mater. Chem., 2005, 15(43), 4660–4662 RSC.
- S.-J. Cho, A. M Shahnin and G. J. Long et al., Magnetic and moessbauer study of core/shell Fe/Au nanoparticles, Chem. Mater., 2006, 18(4), 960–967 CrossRef CAS.
- B. Ravel, E. E. Carpenter and V. G. Harris, Oxidation of iron in iron/gold core/shell nanoparticles, J. Appl. Phys., 2002, 91(10), 8195–8197 CrossRef CAS.
- D. A. Fleming, M. Napolitano and M. E. Williams, Chemically functional alkanethiol derivatized magnetic nanoparticles, MRS Symp. Proc., 2003, 746, 207–212 Search PubMed.
- D.-L. Lu, K. Domen and K.-I. Tanaka, Electrodeposited Au-Fe, Au-Ni, and Au-Co alloy nanoparticles from aqueous electrolytes, Langmuir, 2002, 18(8), 3226–3232 CrossRef CAS.
- M. Mirdamadi-Esfahani, M. Mostafavi and B. Keita et al., Au-Fe system: application in electro-catalysis, Gold Bull., 2008, 41(2), 98–104 CAS.
- W.-S. Chang, J.-W. Park, V. Rawat, T. Sands and G.U. Lee, Templated synthesis of gold–iron alloy nanoparticles using pulsed laser deposition, Nanotechnology, 2006, 17(20), 5131–5135 Search PubMed.
- D. B. Akolekar, S. K. Bhargava, G. Foran and M. Takahashi, Studies on gold nanoparticles supported on iron, cobalt, manganese, and cerium oxide catalytic materials, J. Mol. Catal. A: Chem., 2005, 238(1–2), 78–87 CrossRef CAS.
- E. Smolentseva, A. Pestryakov and N. Bogdanchikova et al., Influence of Fe introduction method on gold state in NaY zeolite, Int. J. Mod. Phys. B: Condens. Matter Phys, Stat. Phys, Appl. Phys., 2005, 19(15, 16 & 17, Pt. 1), 2496–2501 Search PubMed.
- D. Horvath, M. Polisset-Thfoin, J. Fraissard and L. Guczi, Novel preparation method and characterization of Au-Fe/HY zeolite containing highly stable gold nanoparticles inside zeolite supercages, Solid State Ionics, 2001, 141–142, 153–156 CrossRef CAS.
- H. Tada, T. Soejima, S. Ito and H. Kobayashi, Photoinduced desorption of sulfur from gold nanoparticles loaded on metal surfaces, J. Am. Chem. Soc., 2004, 126(49), 15952–15953 CrossRef CAS.
- Z. Wang, J. Wang and L. Sun, Method for synthesizing antibody-magnetic nanoparticles with gold shell and iron core for recognition and separation of tumor cells, CN 101303342, 2008.
- S.-J. Cho, B. R. Jarrett, A. Y. Louie and S. M. Kauzlarich, Gold-coated iron nanoparticles: a novel magnetic resonance agent for T1 and T2 weighted imaging, Nanotechnology, 2006, 17(3), 640–644 CrossRef CAS.
- S. V. Jadhav, Synthesis and characterization of iron core – gold shell nanoparticles and their application to pathogen detection. Abstracts, 39th Central Regional Meeting of the ACS, Corvington KY, USA, May 20–23 2007.
- H. Liu, S. Li, N. He and Y. Deng, Preparation of streptavidin coated gold magnetic nanoparticles using cysteamine. Abstracts of Papers, 235th ACS National Meeting, New Orleans, LA, USA, Apr. 6–10 2008, ANYL-137.
- K. A. Brown, A. Wijaya, J. D. Alper and K. Hamad-Schifferli, Synthesis of water-soluble, magnetic Fe/Au nanoparticles, MRS Symp. Proc., 2006 Search PubMed , Volume Date 2005, 900E (Nanoparticles and Nanostructures in Sensors and Catalysis), Paper #: 0900-O07-01.
- N. Y. He, Y. F. Guo, Y. Deng, Z. F. Wang, S. Li and H. N. Liu, Carbon encapsulated magnetic nanoparticles produced by hydrothermal reaction, Chin. Chem. Lett., 2007, 18(4), 487–490 CrossRef CAS.
- Z. Wang, P. Xiao and N. He, Synthesis and characteristics of carbon encapsulated magnetic nanoparticles produced by a hidrotermal reaction, Carbon, 2006, 44(15), 3277–3284 CrossRef CAS.
- C. Femoni, M. C. Iapalucci, G. Longoni, C. Tiozzo and S. Zacchini, An organometallic approach to gold nanoparticles: synthesis and X-ray structure of CO-protected Au21Fe10, Au22Fe12, Au28Fe14 and Au34Fe14 clusters, Angew. Chem., Int. Ed., 2008, 47(35), 6666–6669 CrossRef CAS.
- T. Wang, D. Zhang, W. Xu, S. Li and D. Zhu, New approach to the assembly of gold nanoparticles: formation of stable gold nanoparticle ensemble with chainlike structures by chemical oxidation in solution, Langmuir, 2002, 18(22), 8655–8659 Search PubMed.
- I. Narita, T. Oku1, H. Tokoro and K. Suganuma, Synthesis and structures of iron nanoparticles coated with boron nitride nanomaterials, J. Electron Microsc. Technol., 2006, 55(3), 123–127 Search PubMed.
- M. Fan, P. Yuan, F. Bergaya, H. He, T. Chen, J. Zhu and D. Liu, A critical textural evolution study of zerovalent iron/montmorillonite nanosized heterostructures under various iron loadings, Clays Clay Miner., 2011, 59(5), 490–500 Search PubMed.
- Y. Kong and H. Zhou, Formation and magnetic characterization of magnesium oxide / iron nano composite particles, Adv. Mater. Res., 2011, 236–238 Search PubMed (Pt. 2, Application of Chemical Engineering), 1927–1930.
- F. Wang, M. Malac, R. F. Egerton and A. Meldrum et al., Multilayer route to iron nanoparticle formation in an insulating matrix, J. Appl. Phys., 2007, 101(034314), 7 pp Search PubMed.
- E. Iwamura and M. Yamaguchi, Nano-structural modification of amorphous carbon thin films by low-energy electron beam irradiation, Cailiao Rechuli Xuebao, 2004, 25(5, Pt. 2), 1247–1252 Search PubMed.
- Q.-X. Li, Z.-y. Sun and T. Wang, A study on preparation, characterization and photocatalytic activity of iron-doped nano-TiO2 thin films based on self-assembled monolayers, Kuangwu Xuebao, 2011, 31(1), 102–107 Search PubMed.
- Q.-Y. Xu, F. Liu, J.-T. Lu, G. Kong and C.-S. Che, Microstructure and performance of electroplated Fe-nano ZrO2 composite coating, Jixie Gongcheng Cailiao, 2007, 31(9), 51–54 Search PubMed.
- G. Shi, J. Zhang, D. Yu and L. Chen, Synthesis and microwave-absorbing properties of Al2O3 coated polyhedral Fe nanocapsules prepared by arc-discharge method, Adv. Mater. Res., 2011, 299–300 Search PubMed (Pt. 2, Materials and Manufacturing), 739–742.
- Q. Wang, J.-H. Lee, S.-W. Jeong, A. Jang, S. Lee and H. Choi, Mobilization and deposition of iron nano and sub-micrometer particles in porous media: A glass micromodel study, J. Hazard. Mater., 2011, 192, 1466–1475 Search PubMed.
- J. Wang and R. Cheng, Preparation and application of activated carbon supported iron nanomaterial, with application to pentachlorophenol degradation, CN 101708457, 2010.
- M. Bystrzejewski, Synthesis of carbon-encapsulated iron nanoparticles via solid state reduction of iron oxide nanoparticles, J. Solid State Chem., 2011, 184(6), 1492–1498 Search PubMed.
- Z. Wei, X. Wang and H. Yang, Preparation of carbon-encapsulated Fe core–shell nanostructures by confined arc plasma, Mater. Sci. Forum, 2011, 688, 245 Search PubMed (Nano-Scale and Amorphous Materials), 245–249.
- C. N. R. Rao, P. J. Thomas and G. U. Kulkarni, Nanocrystals: Synthesis, Properties and Applications(Springer Series in Materials Science), Springer, 2007, 182 Search PubMed.
- X. Bingshe, G. Junjie, W. Xiaomin, L. Xuguang and I. Hideki, Synthesis of carbon nanocapsules containing Fe, Ni or Co by arc discharge in aqueous solution, Carbon, 2006, 14(13), 2631–2634 Search PubMed.
- H. Tokoro, S. Fujii and T. Oku, Iron nanoparticles coated with graphite nanolayers and carbon nanotubes, Diamond Relat. Mater., 2004, 13(4–8), 1270–1273 CrossRef.
- M. N. Nadagouda and D. A. Lytle, Microwave-assisted combustion synthesis of nano iron oxide/iron coated activated carbon, anthracite, cellulose fiber, and silica, with arsenic adsorption studies, J. Nanotechnol., 2011, 972486 Search PubMed (8 pp.).
- Y. An and J. Qiu, Synthesis of carbon encapsulated iron nanoparticles by carbonization of starch with iron as catalyst, 2007, The American Carbon Society. http://acs.omnibooksonline.com/data/papers/2004_C085.pdf Search PubMed.
- Y. An, X. Wu, Z. Sui, Y. Xia and Y. Liu, A novel method for synthesis of homogeneous carbon encapsulated Fe nanoparticles based on natural biopolymer, J. Rare Earths, 2007, 25, 452 Search PubMed.
- G. Huang and J. Weng, Syntheses of carbon nanomaterials by ferrocene, Curr. Org. Chem., 2011, 15(21), 3653–3666 Search PubMed.
- O. V. Kharissova, Vertically aligned carbon nanotubes fabricated by microwaves, Rev. Adv. Mater. Sci., 2004, 7(1), 50–54 Search PubMed.
- O. V. Kharissova and J. R. Cardenas, Advance in methods of forming vertically aligned carbon nanotubes by microwave, Phys. Status Solidi C, 2005, 2(8), 3063–3066 Search PubMed.
- J. Huo, H. Song and X. Chen, Preparation of carbon-encapsulated iron nanoparticles by co-carbonization of aromatic heavy oil and ferrocene, Carbon, 2004, 42, 3177–3182 CrossRef CAS.
- Q. Kong, C. Guo, B. Wang, Q. Ji and Y. Xia, A facile preparation of carbon-supported nanoscale zerovalent iron fibers, Mater. Sci. Forum, 2011, 688, 349–352 Search PubMed.
- A. Desai, D. Mahajan and M. Rafailovich, Synthesis and characterization of nanosized iron on a polystyrene support as potential Fischer–Tropsch catalysts, Prepr. Pap.-Am. Chem. Soc., Div. Fuel Chem., 2003, 48(2), 783–784 Search PubMed.
- H.-J. Kim, T. Phenrat, R. D. Tilton and G. V. Lowry, Effect of kaolinite, silica fines and pH on transport of polymer-modified zero valent iron nano-particles in heterogeneous porous media, J. Colloid Interface Sci., 2012, 370(1), 1–10 Search PubMed.
- A. G. Nasibulin, S. Rackauskas, H. Jiang, Y. Tian, P. R. Mudimela, S. D. Shandakov, L. Nasibulina, J. Sainio, E. I. Kauppinen and Y. Jiang, Simple and Rapid Synthesis of α-Fe2O3 Nanowires Under Ambient Conditions, Nano Res., 2010, 2, 373–379.9 Search PubMed.
- M. Kooti and L. Matturi, Microwave-Assisted Fabrication of γ-Fe2O3 Nanoparticles from Tris(acetylacetonato) Iron (III), Int. Nano Lett., 2011, 1(1), 38–42 Search PubMed.
- D. Barreca, G. Carraro, A. Devi, E. Fois, A. Gasparotto, R. Seraglia, C. Maccato, C. Sada, G. Tabacchi, E. Tondello, A. Venzo and M. Winter, β-Fe2O3 nanomaterials from an iron(II) diketonate-diamine complex: a study from molecular precursor to growth process, Dalton Trans., 2012, 41, 149–155 RSC.
- A. Mehrani and A. Morsali, One-dimensional Coordination Polymer as New Precursor for Preparation of Fe2O3 Nanoparticles, J. Inorg. Organomet. Polym. Mater., 2011, 21(3), 476–479 Search PubMed.
- L. Vulićević, N. Ivanović, A. Maričić, M. Srećković, S. Vardić, M. Plazinić and Z. Tomić, Hydrothermal Treatment of Electrochemically Synthesised Nanocrystalline Magnetic Iron Oxide Powder, Sci. Sintering, 2007, 39, 85–91 Search PubMed.
- L. Duraes, A. Moutinho, I. J. Seabra, B. F. O. Costa, H. C. de Sousa and A. Portugal, Characterization of iron(III) oxide/hydroxide nanostructured materials produced by sol–gel technology based on the Fe(NO3)3.9H2O/ethanol/propylene oxide system, Mater. Chem. Phys., 2011, 130(1–2), 548–560 Search PubMed.
- Z. Li, M.-H. Wu, J.-Z. Gu, Z. Jiao, S.-L. Lu and De-Q. Wang, Preparation of nanocrystalline Fe2O3 by radiation technique, Gaoxiao Huaxue Gongcheng Xuebao, 2006, 20(3), 481–484 Search PubMed.
- S. -Q. Liu, K. Furuya,A. Hasegawa,K. Mitsuishi and H. Hashimoto, Manufacture of iron oxide using electron beam, Jpn. Pat., JP 2005247669, 2005 Search PubMed.
- S. Mollah, Laser Synthesis of Iron Oxide Nanowires, Asian J. Chem., 2009, 21(10), S001–003 Search PubMed.
- S. Seino, T. Kinoshita, T. Nakagawa, T. Kojima, R. Taniguci, S. Okuda and T. A. Yamamoto, Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high-energy electron beam, J. Nanopart. Res., 2007, 10(6), 1071–1076 Search PubMed.
- B. I. Kharisov, O. V. Kharissova, M. José Yacamán and U. Ortiz Mendez, State of the art of the bi- and trimetallic nanoparticles on the basis of gold and iron, Recent Pat. Nanotechnol., 2009, 3(2), 81–98 Search PubMed.
- A. Gole, J. W. Stone, W. R. Gemmill, H.-C. zur Loye and C. J. Murphy, Iron oxide coated gold nanorods: synthesis, characterization, and magnetic manipulation, Langmuir, 2008, 24(12), 6232–6237 CrossRef CAS.
- L. Wang, J. Bai, Y. Li and Y. Huang, Multifunctional nanoparticles displaying magnetization and near-IR absorption, Angew. Chem., Int. Ed., 2008, 47(13), 2439–2442 CrossRef CAS.
- T. A. Salah El-Din, A. A. Elzatahry, D. M. Aldhayan, A. M. Al-Enizi and S. S. Al-Deyab, Synthesis and Characterization of Magnetite Zeolite Nano Composite, Int. J. Electrochem. Sci., 2011, 6, 6177–6183 Search PubMed.
- H. Liu, G. Lan, Y. Yan, H. Tang, H. Liu and Y. Li, Direct hydrothermal synthesis of novel ordered magnetic mesoporous nanocomposites with high content of iron, Gongye Cuihua, 2011, 19(8), 11–15 Search PubMed.
- Y.-F. Shi, X. Zhou, L.-B. Zhong, W.-L. Xu, Y. Wang and Q.-Q. Zhang, Synthesis and formation mechanism of core–shell Fe3O4 coated gold nanomaterials, Dongnan Daxue Xuebao, Yixueban, 2011, 30(1), 6–10 Search PubMed.
- X. Liu, M. Xu, R. Zhao and J. Zhong, Iron phthalocyanine prepolymer/Fe3O4 nano hybrid magnetic material and its preparation method, Faming Zhuanli Shenqing, 2011 Search PubMed , CN 102086304.
- Y. Cai, Y. Shen, A. Xie, S. Li and X. Wang, Green synthesis of soya bean sprouts-mediated superparamagnetic Fe3O4 nanoparticles, J. Magn. Magn. Mater., 2010, 322(19), 2938–2943 Search PubMed.
- W. Lu, Y. Shen, A. Xie and W. Zhang, Green synthesis and characterization of superparamagnetic Fe3O4 nanoparticles, J. Magn. Magn. Mater., 2010, 322(13), 1828–1833 CrossRef CAS.
- R. H. Bagramov, V. D. Blank, N. R. Serebryanaya, G. A. Dubitsky, E. V. Tatyanin and V. V. Aksenenkov, High Pressures Synthesis of Iron Carbide Nanoparticles Covered with Onion-Like Carbon Shells, Fullerenes, Nanotubes, Carbon Nanostruct., 2012, 20(1), 41–48 Search PubMed.
- M. Mohapatra and S. Anand, Synthesis and applications of nano-structured iron oxides/hydroxides – a review, Int. J. Eng. Sci. Technol., 2010, 2(8), 127–146 Search PubMed.
- S. P. Gubin, Yu. A. Koksharov, G. B. Khomutov and Y. G. Yurkov, Magnetic nanoparticles: preparation, structure and properties, Russ. Chem. Rev., 2005, 74(6), 489–520 Search PubMed.
- K. A. Revati and B. D. Padney, Microbial synthesis of iron-based nanomaterials—A review, Bull. Mater. Sci., 2011, 34(2), 191–198 Search PubMed.
- O. V. Kharissova and B. I. Kharisov, Synthetic techniques and applications of activated nanostructurized metals: highlights up to 2008, Recent Pat. Nanotechnol., 2008, 2(2), 103–119 Search PubMed.
- C. Noubactep and A. Schoener, Metallic iron: dawn of a new era of drinking water treatment research?, Fresenius Environ. Bull., 2010, 19(8a), 1661–1668 Search PubMed.
- Overview and Comparison of Conventional and Nano-Based water treatment technologies. Meridian Institute Global Dialogue on Nanotechnology and the Poor: Opportunities and Risks. http://www.merid.org/nano/watertechpaper..
- T. B. Scott, Inorganic nanoparticles for environmental remediation, Inorg. Nanopart., 2011, 393–439 Search PubMed.
- J. U. Lee, S. G. Cho, Y. S. Jang, J. H. Kim and S. H. Cho, Method for purifying soil and groundwater using nZVI and DI-PRB, KR 1027140 B1 20110405, 2011.
- Z. Wei-xian, Nanoscale iron particles for environmental remediation: An overview, J. Nanopart. Res., 2003, 5, 323–332 CrossRef CAS.
- D. W. Elliott, H.-L. Lien and W.-x. Zhang, J. Environ. Qual., 2008, 37(6), 2192–2201 CrossRef CAS.
- K. R. Reddy, K. Darko-Kagya and C. Cameselle, Electrokinetic-enhanced transport of lactate-modified nanoscale iron particles for degradation of dinitrotoluene in clayey soils, Sep. Purif. Technol., 2011, 79(2), 230–237 Search PubMed.
- A. J. Salter-Blanc and P. G. Tratnyek, Effects of solution chemistry on the dechlorination of 1,2,3-trichloropropane by zerovalent zinc, Environ. Sci. Technol., 2011, 45, 4073–4079 Search PubMed.
- Z. Shi, J. T. Nurmi and P. G. Tratnyek, Effects of nano zerovalent Iron (nZVI) on oxidation-reduction potential (ORP), Environ. Sci. Technol., 2011, 45, 1586–1592 CrossRef CAS.
- P. G. Tratnyek and A. J. Salter, Response to Comment on “Degradation of 1,2,3-Trichloropropane (TCP): Hydrolysis, Elimination, and Reduction by Iron and Zinc”, Environ. Sci. Technol., 2010, 44, 3198–3199 Search PubMed.
- V. Sarathy, P. G. Tratnyek, J. T. Nurmi and R. L. Johnson G. O. B. Johnson, Degradation of 1,2,3-trichloropropane (TCP): Hydrolysis, elimination, and reduction by iron and zinc, Environ. Sci. Technol., 2010, 44, 787–793 Search PubMed.
- C. A. Gorski, J. T. Nurmi, P. G. Tratnyek, T. B. Hofstetter and M. M. Scherer, Redox behavior of magnetite: Implications for contaminant reduction, Environ. Sci. Technol., 2010, 44, 55–60 Search PubMed.
- R. L. Johnson, R. O'Brien Johnson, J. T. Nurmi and P. G. Tratnyek, Natural organic matter enhanced mobility of nano zerovalent iron, Environ. Sci. Technol., 2009, 43, 5455–5460 Search PubMed.
- V. Sarathy, P. G. Tratnyek, J. T. Nurmi, D. R. Baer, J. E. Amonette, C. Chun, R. L. Penn and E. J. Reardon, Aging of iron nanoparticles in aqueous solution: effects on structure and reactivity, J. Phys. Chem. C, 2008, 112, 2286–2293 CrossRef CAS.
- J.-H. Kim, P. G. Tratnyek and Y.-S. Chang, Rapid dechlorination of polychlorinated dibenzo-p-dioxins (PCDDs) by bimetallic and nano-sized zerovalent iron, Environ. Sci. Technol., 2008, 42, 4106–4112 CrossRef CAS.
- R. L. Johnson, R. B. Thoms, R. O'Brien Johnson, J. T. Nurmi and P. G. Tratnyek, Mineral precipitation and flow reduction up-gradient from a zerovalent iron permeable reactive barrier, Ground Water Monit. Rem., 2008, 28, 56–64 Search PubMed.
- R. L. Johnson, P. G. Tratnyek, R. Miehr, B. B. Thoms and J. Z. Bandstra, Reduction of hydraulic conductivity and reactivity in zerovalent iron columns by oxygen and TNT, Ground Water Monit. Rem., 2005, 25, 129–136 Search PubMed.
- P. G. Tratnyek, V. Sarathy, J. T. Nurmi and R. L. Johnson, Nano ZVI vs. conventional ZVI: The effect of particle size, Abstracts of Papers, 233rd ACS National Meeting, Chicago, IL, United States, Mar. 25–29, 2007, p. IEC-167.
- X.-qin Li and W.-xian Zhang, Iron Nanoparticles: the Core–Shell Structure and Unique Properties for Ni(II) Sequestration, Langmuir, 2006, 22(10), 4638–462 CrossRef CAS.
- M. Auffan, H. J. Shipley, S. Yean, A. T. Kan, M. Tomson, J. Rose and J.-Y. Bottero, Chapter 10. Nanomaterials as Adsorbents, inEnvironmental Nanotechnology, McGraw-Hill, 2007, pp. 371–392 Search PubMed.
- D. K. Tiwari, J. Behari and P. Sen, Application of Nanoparticles in Waste Water Treatment, World Appl. Sci. J., 2008, 3(3), 417–433 Search PubMed.
- C. Uzum, T. Shahwan, A. E. Eroglu, I. Lieberwirth, T. B. Scott and K. R. Hallam, Application of zerovalent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions, Chem. Eng. J., 2008, 144(2), 213–220 CrossRef CAS.
- V. Grover, J. Hu, K. Engates and H. J. Shipley, Sorption of metals to hematite nanoparticles: Competitive and temperature effects, Abstracts of Papers, 241st ACS National Meeting & Exposition, Anaheim, CA, United States, March 27–31, 2011, COLL-458.
- M. Ahmadi, F. Mashhoon, R. Kaveh and F. Tarkian, Use of mechanically prepared iron nano particles for nitrate removal from water, Asian J. Chem., 2011, 23(3), 1205–1208 Search PubMed.
- A. Ryu, S.-W. Jeong, A. Jang and H. Choi, Reduction of highly concentrated nitrate using nanoscale zerovalent iron: Effects of aggregation and catalyst on reactivity, Appl. Catal., B, 2011, 105(1–2), 128–135 Search PubMed.
- Y.-H. Hwang, D.-G. Kim and H.-S. Shin, Mechanism study of nitrate reduction by nano zero valent iron, J. Hazard. Mater., 2011, 185(2–3), 1513–1521 Search PubMed.
- H. Kang, T. Li, Z. Xiu, L. Sun, Z. Liu and Z. Jin, Denitrification of nitrate using bimetallic Ni/Fe nanoparticles. Preprints of Extended Abstracts presented at the ACS National Meeting, American Chemical Society, Division of Environmental Chemistry, 2006, vol. 46(2), pp. 72–75.
- K.-H. Shin and D. K. Cha, Microbial reduction of nitrate in the presence of nanoscale zerovalent iron, Chemosphere, 2008, 72(2), 257–262 CrossRef CAS.
- http://www.msnbc.msn.com/id/16947780/ns/technology_and_science-innovation/t/million-prize-awarded-water-purifier/#.T-t7AvVSSyo .
- S. Luther, N. Borgfeld, J. Kim and J. G. Parsons, Removal of arsenic from aqueous solution: A study of the effects of pH and interfering ions using iron oxide nanomaterials, Microchem. J., 2012, 101, 30–36 Search PubMed.
- B. A. Manning, J. R. Kiser, H. Kwon and S. R. Kanel, Spectroscopic Investigation of Cr(III)- and Cr(VI)-Treated Nanoscale Zerovalent Iron, Environ. Sci. Technol., 2007, 41(2), 586–592 CrossRef CAS.
- Q. Wang, S. Snyder, J. Kim and H. Choi, Aqueous Ethanol modified Nanoscale Zerovalent Iron in Bromate Reduction: Synthesis, Characterization, and Reactivity, Environ. Sci. Technol., 2009, 43(9), 3292–3299 Search PubMed.
- Q. Sun, S. E. Mylon and T. D. Waite, Production of reactive oxygen species from zerovalent iron nanomaterials. Preprints of Extended Abstracts presented at the ACS National Meeting, American Chemical Society, Division of Environmental Chemistry, 2008, vol. 48(1), pp. 379–381.
- P. Varanasi, A. Fullana and S. Sidhu, Remediation of PCB contaminated soils using iron nano-particles, Chemosphere, 2007, 66(6), 1031–1038 CrossRef CAS.
- C. Chen, X. Wang, Y. Chang and H. Liu, Dechlorination of disinfection by-product monochloroacetic acid in drinking water by nanoscale palladized iron bimetallic particle, J. Environ. Sci., 2008, 20(8), 945–951 Search PubMed.
- T. Satapanajaru, P. Anurakpongsatorn, P. Pengthamkeerati and H. Boparai, Remediation of atrazine-contaminated soil and water by nano zerovalent iron, Water, Air, Soil Pollut., 2008, 192(1–4), 349–359 Search PubMed.
- E.-J. Kim, J.-H. Kim, A.-M. Azad and Y.-S. Chang, Facile Synthesis and Characterization of Fe/FeS Nanoparticles for Environmental Applications, ACS Appl. Mater. Interfaces, 2011, 3(5), 1457–1462 Search PubMed.
- Y. Xi, M. Megharaj and R. Naidu, Dispersion of zerovalent iron nanoparticles onto bentonites and use of these catalysts for orange II decolourisation, Appl. Clay Sci., 2011, 53(4), 716–722 Search PubMed.
- J. Ge, J. Tian, L. Zhuo, H. Chen and B. Tang, Fabrication of self-assembled iron oxide hierarchical nanostructures and their application in water treatment, Solid State Sci., 2011, 13(8), 1554–1559 Search PubMed.
- P. Larese-Casanova, D. M. Cwiertny and M. M. Scherer, Nanogoethite Formation from Oxidation of Fe(II) Sorbed on Aluminum Oxide: Implications for Contaminant Reduction, Environ. Sci. Technol., 2010, 44(10), 3765–3771 Search PubMed.
- T. Long and C. A. Ramsburg, Encapsulation of nZVI particles using a Gum Arabic stabilized oil-in-water emulsion, J. Hazard. Mater., 2011, 189(3), 801–808 Search PubMed.
- A. Zaleska, Doped-TiO2: A Review, Recent Pat. Eng., 2008, 2, 157–164 Search PubMed.
- M. Aufan, Nanoparticules d'oxydes mrtalliques: relations entre la reactivite de surface et des reponses biologiques. http://www.tel.archives-ouvertes.fr/docs/00/30/85/03/PDF/auffan_PhD.pdfSimilares. Search PubMed.
- C. Jing, X. Meng, S. Liu, S. Baidas, R. Patraju, C. Christodoulatos and G. P. Korfiatis, Surface complexation of organic arsenic on nanocrystalline titanium oxide, J. Colloid Interface Sci., 2005, 290(1), 14–21 CrossRef CAS.
- J. Zhu, W. Zheng, B. He, J. Zhang and M. Anpo, Characterization of Fe-TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for degradation of XRG dye diluted in water, J. Mol. Catal. A: Chem., 2004, 216, 35–43 CAS.
- O. Celebi, C. Uezuem, T. Shahwan and H. N. Erten, A radiotracer study of the adsorption behavior of aqueous Ba2+ ions on nanoparticles of zerovalent iron, J. Hazard. Mater., 2007, 148(3), 761–767 Search PubMed.
- M. Elsner, M. Chartrand, N. VanStone, G. Lacrampe Couloume and B. Sherwood Lollar, Identifying Abiotic Chlorinated Ethene Degradation: Characteristic Isotope Patterns in Reaction Products with Nanoscale Zero-Valent Iron, Environ. Sci. Technol., 2008, 42(16), 5963–5970 CrossRef CAS.
- M. Elsner, G. L. Couloume, S. Mancini, L. Burns and B. S. Lollar, Carbon isotope analysis to evaluate nanoscale Fe(O) treatment at a chlorohydrocarbon contaminated site, Ground Water Monit. Rem., 2010, 30(3), 79–95 CrossRef CAS.
- S. E. Mylon, Q. Sun and T. D. Waite, Process optimization in use of zero valent iron nanoparticles for oxidative transformations, Chemosphere, 2010, 81(1), 127–131 Search PubMed.
- C. R. Keenan and D. L. Sedlak, Factors affecting the yield of oxidants from the reaction of nanoparticulate zerovalent iron and oxygen. Preprints of Extended Abstracts - ACS National Meeting, American Chemical Society, Division of Environmental Chemistry, 2008, vol. 48(2), pp. 748–751.
- H.-S. Kim, J.-Y. Ahn, K.-Y. Hwang, W.-S. Shin and I. Hwang, Characteristics of nanoscale zerovalent iron (NZVI) particles formed under controlled air contact. Preprints of Extended Abstracts - ACS National Meeting, American Chemical Society, Division of Environmental Chemistry, 2008, 48(2), pp. 198–202.
- M. B. Allabaksh, B. K. Mandal, M. K. Kesarla, K. S. Kumar and P. S. Reddy, Preparation of stable zero valent iron nanoparticles using different chelating agents, J. Chem. Pharm. Res., 2010, 2(5), 67–74 Search PubMed.
- A. Tiehm, S. Krassnitzer, Y. Koltypin and A. Gedanken, Chloroethene dehalogenation with ultrasonically produced air-stable nano iron, Ultrason. Sonochem., 2009, 16(5), 617–621 Search PubMed.
- K. Moore, B. Forsberg, D. R. Baer, W. A. Arnold and R. L. Penn, Zero-Valent Iron: Impact of Anions Present during Synthesis on Subsequent Nanoparticle Reactivity, J. Environ. Eng., 2011, 137(10), 889–896 Search PubMed.
- Y.-H. Hwang, D.-G. Kim and H.-S. Shin, Effects of synthesis conditions on the characteristics and reactivity of nano scale zero valent iron, Appl. Catal., B, 2011, 105(1–2), 144–150 Search PubMed.
- P. Jiemvarangkul, W.-X. Zhang and H.-L. Lien, Enhanced transport of polyelectrolyte stabilized nanoscale zerovalent iron (nZVI) in porous media, Chem. Eng. J., 2011, 170(2–3), 482–491 CrossRef CAS.
- M. M. Krol, A. J. Oleniuk, D. M. O'Carroll and B. E. Sleep, Modelling field scale nano-zero valent iron transport. 85th ACS Colloid and Surface Science Symposium, Montreal, QC, Canada, Jun. 19–22, 2011, COLLSYMP-441 Search PubMed.
- Y.-D. Park, C.-S. Park and J.-W. Park, Interaction between iron reducing bacteria and nano-scale valent iron, Sus. Environ. Res., 2010, 20(4), 233–238 Search PubMed.
- D. M. Cwiertny and Y. Xie, Dithionite as a regenerant for the reducing capacity of nanoscale zerovalent iron in situ treatment zones, ed. W. Sigmund, Abstracts of Papers, 238th ACS National Meeting, Washington, DC, United States, Aug. 16–20, 2009, ENVR-063.
- A. I. Keller, Y. Sheikhaldeen and I. Rampersaud, Environmental remediation of contaminated groundwater using zerovalent iron nanoparticles, ed. M. Laudon and B. Romanowicz, Abstracts, 41st Central Regional Meeting of the American Chemical Society, Dayton, OH, United States, Jun. 16–19, 2010, CERMACS-387.
- X. Li, X. Shen and L. Zhong, Method for delivering nanoparticles series remediation substance into polluted aeration zone using foams, CN 102225427, 2011.
- X. Shen, L. Zhao, Y. Ding, B. Liu, H. Zeng, L. Zhong and X. Li, Foam, a promising vehicle to deliver nanoparticles for vadose zone remediation, J. Hazard. Mater., 2011, 186(2–3), 1773–1780 Search PubMed.
- L. B. Hoch, E. J. Mack, B. W. Hydutsky, J. M. Hershman, J. M. Skluzacek and T. E. Mallouk, Carbothermal Synthesis of Carbon-supported Nanoscale Zerovalent Iron Particles for the Remediation of Hexavalent Chromium, Environ. Sci. Technol., 2008, 42(7), 2600–2605 CrossRef CAS.
- S. Li, W. Yan and W.-X. Zhang, Solvent-free production of nanoscale zerovalent iron (nZVI) with precision milling, Green Chem., 2009, 11(10), 1618–1626 RSC.
- M. Corbin and J. Wolters, Application of nanoparticle technology for environmental cleanup at an industrial facility. Abstracts of Papers, 235th ACS National Meeting, New Orleans, LA, United States, Apr. 6–10, 2008, IEC-023.
- L. Pang, Q. Zhou and X. Su, Progress of in situ modification techniques of nanoscale zerovalent iron, Huagong Jinzhan, 2011, 30(6), 1361–1368 Search PubMed.
- Z. Shi, J. T. Nurmi, R. L. Johnson and P. G. Tratnyek, Potentiometric detection of iron nanoparticles. Abstracts of Papers, 239th ACS National Meeting, San Francisco, CA, United States, Mar. 21–25, 2010, ENVR-402 Search PubMed.
- R. Singh, V. Misra and R. P. Singh, Removal of Cr(VI) by Nanoscale Zerovalent Iron (nZVI) From Soil Contaminated with Tannery Wastes, Bull. Environ. Contam. Toxicol., 2011, 88(2), 210–214 Search PubMed.
- Y. Liu, In situ remediation method for heavy metal-polluted soil, 2011, CN 102101123, 2011.
- H. K. Boparai, M. Joseph and D. M. O'Carroll, Cadmium removal by nano zerovalent iron: Influence of physicochemical parameters. Abstracts of Papers, 239th ACS National Meeting, San Francisco, CA, United States, Mar. 21–25, 2010, ENVR-385.
- N. C. Mueller, J. Braun, J. Bruns, M. Cernik, P. Rissing, D. Rickerby and B. Nowack, Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe, Environ. Sci. Pollut. Res., 2011, 19(2), 550–558 Search PubMed.
- M. G. O. Souza, F. T. Silva and J. F. Oliveira, Organic pollutants in groundwater: remediation by nanoscale iron particles, WIT Trans. Ecol. Environ., 2008, I, 105–113 Search PubMed (Water Pollution).
- M. C. Chang and H. Y. Kang, Remediation of pyrene-contaminated soil by synthesized nanoscale zerovalent iron particles, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2009, 44, 576–582 Search PubMed.
- R. Singh, A.s Singh, V. Misra and R. P. Singh, Degradation of lindane contaminated soil using zerovalent iron nanoparticles, J. Biomed. Nanotechnol., 2011, 7(1), 175–176 Search PubMed.
- X. Li, Sequestration of malodorous sulfides with zerovalent iron nanoparticles, ed. M. Laudon and B. Romanowicz, Abstracts of Papers, 240th ACS National Meeting, Boston, MA, United States, Aug. 22–26, 2010, ENVR-330.
- S. Comba, D. Dalmazzo, E. Santagata and R. Sethi, Rheological characterization of xanthan suspensions of nanoscale iron for injection in porous media, J. Hazard. Mater., 2011, 185(2–3), 598–605 Search PubMed.
- Y. Huang, D. Liu, Z. Qin, X. Liu and L. Zhang, As(III) adsorption from drinking water on nanoscale zerovalent iron (NZVI): Kinetics and isotherm studies, Integr. Ferroelectr., 2011, 127(1), 39–47 Search PubMed.
- I. Halamova, P. Kvapil and L. Benesova, Use of iron nanoparticles for remediation of groundwater by in situ chemical reduction of chlorinated hydrocarbons in the Pisecna landfill locality, Vodni Hospodarstvi, 2011, 61(7), 265–268 Search PubMed.
- G. Naja, A. Halasz, S. Thiboutot, G. Ampleman and J. Hawari, Degradation of Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) Using Zerovalent Iron Nanoparticles, Environ. Sci. Technol., 2008, 42(12), 4364–4370 CrossRef CAS.
- H.-S. Kim, J.-Y. Ahn, D.T. Le, K.-Y. Hwang, J.-Y. Lee, J.-Y. Chun and I. Hwang, Effects of anions and organic matter on reactivity and surface characteristics of nanoscale zerovalent iron particles, Abstracts of Papers, 242nd ACS National Meeting & Exposition, Denver, CO, United States, Aug. 28–Sep. 1, 2011, COLL-23.
- M. Dickinson and T. B. Scott, The effect of vacuum annealing on the remediation abilities of iron and iron-nickel nanoparticles, J. Nanopart. Res., 2011, 13(9), 3699–3711 Search PubMed.
- R. A. Crane, M. Dickinson, I. C. Popescu and T. B. Scott, Magnetite and zerovalent iron nanoparticles for the remediation of uranium contaminated environmental water, Water Res., 2011, 45(9), 2931–2942 Search PubMed.
- Y.-T. Wei, S.-C. Wu, C.-M. Chou, C.-H. Che, S.-M. Tsai and H.-L. Lien, Influence of nanoscale zerovalent iron on geochemical properties of groundwater and vinyl chloride degradation: A field case study, Water Res., 2010, 44(1), 131–140 CrossRef CAS.
- H.-L. Lien, Application and implication of zerovalent iron for transformation of disinfection by-products: a comparison study between nanoscale palladium iron and microscale iron, Zhongguo Huanjing Gongcheng Xuekan, 2005, 15(4), 213–221 Search PubMed.
- Y.-H. Shih, M.-Y. Chen and Y.-F. Su, Pentachlorophenol reduction by Pd/Fe bimetallic nanoparticles: Effects of copper, nickel, and ferric cations, Appl. Catal., B, 2011, 105(1–2), 24–29 Search PubMed.
- N. Sakulchaicharoen, D. M. O'Carroll and J. E. Herrera, Enhanced stability and dechlorination activity of pre-synthesis stabilized nanoscale FePd particles, J. Contam. Hydrol., 2010, 118(3–4), 117–127 CrossRef CAS.
- Y. Zhuang, S. Ahn, A. L. Seyfferth, Y. Masue-Slowey, S. Fendorf and R. G. Luthy, Dehalogenation of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyl by Bimetallic, Impregnated, and Nanoscale Zerovalent Iron, Environ. Sci. Technol., 2011, 45(11), 4896–4903 Search PubMed.
- C. Chang, F. Lian and L. Zhu, Simultaneous adsorption and degradation of γ-HCH by nZVI/Cu bimetallic nanoparticles with activated carbon support, Environ. Pollut., 2011, 159(10), 2507–2514 Search PubMed.
- Y. Hong, Y. Rheem, M. Lai, D. M. Cwiertny, S. L. Walker and N. V. Myung, Electrochemical synthesis of FexNi1-x nanostructures for environmental remediation, Chem. Eng. J., 2009, 151(1–3), 66–72 Search PubMed.
- H. Zhou, J. Han, S. A. Baig and X. Xu, Dechlorination of 2,4-dichlorophenoxyacetic acid by sodium carboxymethyl cellulose-stabilized Pd/Fe nanoparticles, J. Hazard. Mater., 2011, 198, 7–12 Search PubMed.
- J. Zhan, B. Sunkara, J. Tang, Y. Wang, J. He, G. L. McPherson and V. T. John, Carbothermal Synthesis of Aerosol-Based Adsorptive-Reactive Iron-Carbon Particles for the Remediation of Chlorinated Hydrocarbons, Ind. Eng. Chem. Res., 2011, 50(23), 13021–13029 Search PubMed.
- B. Sunkara, J. Zhan, I. Kolesnichenko, Y. Wang, J. He, J. E. Holland, G. L. McPherson and V. T. John, Modifying Metal Nanoparticle Placement on Carbon Supports Using an Aerosol-Based Process, with Application to the Environmental Remediation of Chlorinated Hydrocarbons, Langmuir, 2011, 27(12), 7854–7859 CrossRef CAS.
- B. Sunkara, Jingjing Zhan, Jibao He, G. L. McPherson, G. Piringer and V. T. John, Nanoscale Zerovalent Iron Supported on Uniform Carbon Microspheres for the In situ Remediation of Chlorinated Hydrocarbons, ACS Appl. Mater. Interf., 2010, 2(10), 2854–2862 Search PubMed.
- D. Zhang, S. Wei, C. Kaila, X. Su, J. Wu, A. B. Karki, D. P. Young and Z. Guo, Carbon-stabilized iron nanoparticles for environmental remediation, Nanoscale, 2010, 2(6), 917–919 RSC.
- S. Xiao, M. Shen, R. Guo, S. Wang, X. Shi, Immobilization of zerovalent iron nanoparticles into polymer/carbon nanotube composite nanofibers for environmental applications. Abstracts of Papers, 239th ACS National Meeting, San Francisco, CA, United States, March 21–25, 2010, ENVR-111 Search PubMed.
- A. N. Bezbaruah, S. S. Shanbhogue, S. Simsek and E. Khan, Encapsulation of iron nanoparticles in alginate biopolymer for trichloroethylene remediation, J. Nanopart. Res., 2011, 13(12), 6673–6681 Search PubMed.
- T. Liu, L. Zhao, D. Sun and X. Tan, Entrapment of nanoscale zerovalent iron in chitosan beads for hexavalent chromium removal from wastewater, J. Hazard. Mater., 2010, 184(1–3), 724–730 Search PubMed.
- Y. Zhang, Y. Li and X. Zheng, Removal of atrazine by nanoscale zero valent iron supported on organobentonite, Sci. Total Environ., 2011, 409(3), 625–630 Search PubMed.
- S. Li, P. Wu, H. Li, N. Zhu, P. Li, J. Wu, X. Wang and Z. Dang, Synthesis and characterization of organo-montmorillonite supported iron nanoparticles, Appl. Clay Sci., 2010, 50(3), 330–336 Search PubMed.
- X. Zhang, S. Lin, Z. Chen, M. Megharaj and R. Naidu, Kaolinite-supported nanoscale zerovalent iron for removal of Pb2+ from aqueous solution: Reactivity, characterization and mechanism, Water Res., 2011, 45(11), 3481–3488 Search PubMed.
- C. -C. Lee, I.-L. Kao and R.-A. Doong, Combination of titanate nanotubes and zerovalent iron for the degradation of mixed contaminants, Preprints of Extended Abstracts, ACS National Meeting, American Chemical Society, Division of Environmental Chemistry, 2007, vol. 47(2), pp. 69–72.
- Y. Li, T. Li and Z. Jin, Stabilization of Fe0 nanoparticles with silica fume for enhanced transport and remediation of hexavalent chromium in water and soil, J. Environ. Sci., 2011, 23(7), 1211–1218 Search PubMed.
- J. Zhan, T. Zheng, G. Piringer, C. Day, G. L. McPherson and Y. Lu, Papadopoulos, Kyriakos; John, Vijay T. Transport Characteristics of Nanoscale Functional Zerovalent Iron/Silica Composites for in Situ Remediation of Trichloroethylene, Environ. Sci. Technol., 2008, 42(23), 8871–8876 Search PubMed.
- B. S. Karnik, M. J. Baumann, S. J. Masten and S. H. Davies, AFM and SEM characterization of iron oxide coated ceramic membranes, J. Mater. Sci., 2006, 41(20), 6861–6870 Search PubMed.
- B. S. Karnik, S. H. Davies, M. J. Baumann and S. J. Masten, Fabrication of Catalytic Membranes for the Treatment of Drinking Water Using Combined Ozonation and Ultrafiltration, Environ. Sci. Technol., 2005, 39(19), 7656–7661 CrossRef CAS.
- H. J. Shipley, K. E. Engates and A. M. Guettner, Study of iron oxide nanoparticles in soil for remediation of arsenic, J. Nanopart. Res., 2010, 13(6), 2387–2397 Search PubMed.
- R. S. Cutting, V. S. Coker, N. D. Telling, R. L. Kimber, C. I. Pearce, B. L. Ellis, R. S. Lawson, G. van der Laan, R. A. D. Pattrick and D. J. Vaughan et al., Optimizing Cr(VI) and Tc(VII) Remediation through Nanoscale Biomineral Engineering, Environ. Sci. Technol., 2010, 44(7), 2577–2584 Search PubMed.
- G. C. C. Yang and M.-Y. Wu, Injection of nanoscale Fe3O4 slurry coupled with the electrokinetic process for remediation of NO3− in saturated soil: Remediation performance and reaction behavior, Sep. Purif. Technol., 2011, 79(2), 272–277 Search PubMed.
- X. Zhao, X. Guo, Z. Yang, H. Liu and Q. Qian, Phase-controlled preparation of iron (oxyhydr)oxide nanocrystallines for heavy metal removal, J. Nanopart. Res., 2010, 13(7), 2853–2864 Search PubMed.
- M. Mohapatra, K. Rout, S. K. Gupta, P. Singh, S. Anand and B. K. Mishra, Facile synthesis of additive-assisted nano goethite powder and its application for fluoride remediation, J. Nanopart. Res., 2009, 12(2), 681–686 Search PubMed.
- Chunjian Shi, D. E. Pomeroy, P. C. Chiu, Application of nanoscale zerovalent iron to decentralized drinking water systems. Abstracts of Papers, 242th ACS National Meeting & Exposition, Denver, CO, United States, Aug. 28–Sep. 1, 2011, ENVR-403.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. J. Alvarez, Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications, Water Res., 2008, 42, 4591–4602 CrossRef CAS.
- C. Lee, J. Y. Kim, W. I. L. Lee, K. L. Nelson, J. Yoon and D. L. Sedlak, Bactericidal Effect of Zero-Valent Iron Nanoparticles on Escherichia coli, Environ. Sci. Technol., 2008, 42(13), 4927–4933 CrossRef CAS.
- Z. Li, K. Greden, P. J. J. Alvarez, K. B. Gregory and G. V. Lowry, Adsorbed Polymer and NOM Limits Adhesion and Toxicity of Nano Scale ZeroValent Iron to E. coli, Environ. Sci. Technol., 2010, 44(9), 3462–3467 CrossRef CAS.
- X. Li, J. Wang, L. A. Gutierrez, T. H. Nguyen and J. Economy, Iron oxide nanoparticle coating on glass substrates for both arsenic and MS2 virus removal. Preprints of Extended Abstracts, ACS National Meeting, American Chemical Society, Division of Environmental Chemistry, 2008, vol. 48(1), pp. 444–446.
- J.-Y. Kim, C.-H. Lee, D. C. Love, D. L. Sedlak, J.-Y. Yoon and K. L. Nelson, Inactivation of MS2 Coliphage by Ferrous Ion and Zero-Valent Iron Nanoparticles, Environ. Sci. Technol., 2011, 45(16), 6978–6984 Search PubMed.
- B. Marsalek, D. Jancula, E. Marsalkova, M. Mashlan, K. Safarova, J. Tucek and R. Zboril, Multimodal Action and Selective Toxicity of Zerovalent Iron Nanoparticles against Cyanobacteria, Environ. Sci. Technol., 2012, 46(4), 2316–2323 Search PubMed.
- M. Dong, X. Wang, F. Huang, Z. Jin and T. Li, Toxicity of Fe0 nanoparticles on the denitrifying bacteria-Alcaligenes eutrophus, Adv. Mater. Res., 2012, 343–344(Pt. 2, Materials for Environmental Protection and Energy Application), 889–894 Search PubMed.
- A.-M. Azad, R. Hershey, S. Ali and V. Goel, Bactericidal efficacy of electrospun pure and Fe-doped titania nanofibers, J. Mater. Res., 2011, 25(9), 1761–1770 Search PubMed.
- H. Wang and P. Wang, Preparation and antibacterial property of Fe/Ce codoped nano-titanic hydrosol, Wuji Huaxue Xuebao, 2009, 25(11), 1928–1934 Search PubMed.
- R.-L. He, Y. Wei and W.-B. Cao, Preparation of (Fe, N)-doped TiO2 powders and their antibacterial activities under visible light irradiation, J. Nanosci. Nanotechnol., 2009, 9(2), 1094–1097 Search PubMed.
- G. Nangmenyi, X. Li, S. Mehrabi, E. Mintz and J. Economy, Silver-modified iron oxide nanoparticle impregnated fiberglass for disinfection of bacteria and viruses in water, Mater. Lett., 2011, 65(8), 1191–1193 Search PubMed.
- G. N. Nangmenyi, E. Mintz, X. Li, H. Nguyen and J. Economy, Antibacterial activity of a novel material system consisting of iron oxide and Ag nanoparticles on a fiberglass substrate. Preprints of Extended Abstracts, ACS National Meeting, American Chemical Society, Division of Environmental Chemistry, 2008, vol. 48(1), pp. 708–713.
- X. Zhang, H. Niu, J. Yan and Y. Cai, Immobilizing silver nanoparticles onto the surface of magnetic silica composite to prepare magnetic disinfectant with enhanced stability and antibacterial activity, Colloids Surf., A, 2011, 375(1–3), 186–192 CrossRef CAS.
- J. Han, S.-J. Zhang, Z.-G. Lu and Y.-J. Wang, Doping Fe3+ to nano-Ag TiO2 for photocatalytic performance improvement, Yingyong Guangxue, 2010, 31(5), 718–723 Search PubMed.
- Z. Chen, Water treatment device employing nano-neodymium-iron-boron pipe containing zinc oxide and silver oxide, and steam shower room equipped with the same with antiscaling and descaling effects, CN 101671071, 2010.
- T. Gordon, B. Perlstein, O. Houbara, I. Felner, E. Banin and S. Margel, Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties, Colloids Surf., A, 2011, 374(1–3), 1–8 CrossRef CAS.
- W.-X. Zhang, J.-L. Cao, Q. Sun, Y.-Y. Xiang, J.-Y. Qian, M. Yang, W. Wei and F. Xu, Study on the effect of nano-sized iron materials on environmental safety using a Tetrahymena thermophila system, Huaxue Shijie, 2007, 48(6), 341–344 Search PubMed.
- M. Sharon, B. Pal and D. V. Kamat, Photocatalytic killing of pathogenic bacterial cells using nanosize Fe2O3 and carbon nanotubes, J. Biomed. Nanotechnol., 2005, 1(3), 365–368 Search PubMed.
- S. R. Lewis, S. Datta, M. Gui, E. L. Coker, F. E. Huggins, S. Daunert, L. Bachas and D. Bhattacharyya, Reactive nanostructured membranes for water purification, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8577–8582, S8577/1-1-S8577/5 CrossRef.
- M. A. Omole, I. K'Owino and O. A. Sadik, Nanostructured materials for improving water quality: potentials and risks, Nanotechnol. Appl. Clean Water, 2009, 233–247 Search PubMed.
- K. D. Grieger, A. Fjordboge, N. B. Hartmann, E. Eriksson, P. L. Bjerg and A. Baun, Environmental benefits and risks of zerovalent iron nanoparticles (nZVI) for in situ remediation: Risk mitigation or trade-off?, J. Contam. Hydrol., 2010, 118(3–4), 165–183 Search PubMed.
- J. Ai, E. Biazar, M. Jafarpour, M. Montazeri, A. Majdi, S. Aminifard, M. Zafari, H. R. Akbari and H. G. Rad, Nanotoxicology and nanoparticle safety in biomedical designs, Int. J. Nanomed., 2011, 6, 1117–1127 Search PubMed.
- A. Adamcakova-Dodd, P. S. Thorne and V. H. Grassian, In vivo toxicity studies of metal and metal oxide nanoparticles, Handb. Syst. Toxicol., 2011, 2, 803–833 Search PubMed.
- S. J. H. Soenen and M. De Cuyper, Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects, Nanomedicine, 2010, 5(8), 1261–1275 CrossRef CAS.
- T. Senthilnathan, Profile of ambient air nano-enviro air pollutant materials, Indian J. Environ. Protect., 2010, 30(7), 587–589 Search PubMed.
- T. R. Pisanic, S. Jin and V. I. Shubayev, Iron oxide magnetic nanoparticle nanotoxicity: incidence and mechanisms, Nanotoxicity, 2009, 397–425 Search PubMed.
- B. Fubini, I. Fenoglio, G. Martra, R. Ceschino, M. Tomatis, R. Cavalli and M. Trotta, An overview on the toxicity of inhaled nanoparticles, NATO Sci. Ser. II: Math., Phys. Chem., 2006, 228 Search PubMed (Surface Chemistry in Biomedical and Environmental Science), 241–252.
- X. Wu, Y. Tan, H. Mao and M. Zhang, Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells, Int. J. Nanomed., 2010, 385–399 Search PubMed.
- K. B. Gregory, G. V. Lowry, Z. Li, P. J. Alvarez, K. Greden, Engineering polymeric nanoparticle coatings for decreased toxicological impacts of nanoscale zerovalent iron. Abstracts of Papers, 239th ACS National Meeting, San Francisco, CA, United States, Mar. 21–25, 2010, ENVR-549.
- A. Bajaj, B. Samanta, H. Yan, D. J. Jerry and V. M. Rotello, Stability, toxicity and differential cellular uptake of protein passivated-Fe3O4 nanoparticles, J. Mater. Chem., 2009, 19(35), 6328–6331 RSC.
- S.-J. Choi, J.-M. Oh and J.-H. Choy, Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells, J. Inorg. Biochem., 2009, 103(3), 463–471 CrossRef CAS.
- L. C. Varanda, M. Imaizumi, F. J. Santos and M. Jafelicci Jr, Iron oxide versus Fe55Pt45/Fe3O4: improved magnetic properties of core/shell nanoparticles for biomedical applications, IEEE Trans. Magn., 2008, 44(11), 4448–4451 CrossRef CAS.
- M. Mahmoudi, A. Simchi and M. Imani, Recent advances in surface engineering of superparamagnetic iron oxide nanoparticles for biomedical applications, J. Iran. Chem. Soc., 2010, 7(S2), S1–S27 Search PubMed.
- N. Singh, G. J. S. Jenkins, R. Asadi and S. H. Doak, Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION), NanoReviews, 2010, 1 Search PubMed . http://www.nano-reviews.net/index.php/nano/article/view/5358/6032.
- S. Maiti, Nanotoxicity of gold and iron nanoparticles, J. Biomed. Nanotechnol., 2011, 7(1), 65 Search PubMed.
- M. Auffan, J. Rose, J.-Y. Bottero, G. V. Lowry, J.-P. Jolivet and M. R. Wiesner, Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective, Nat. Nanotechnol., 2009, 4(10), 634–641 CrossRef CAS.
- N. Eisenreich, M. Lerner and A. Vorozhtsov, Safety aspects and approaches to fire hazard classification of metal nanopowders, International Annual Conference of ICT, ed. N. N. Li, 2008, 39th, p55/1 Search PubMed.
- D. Napierska, V. Rabolli, L. Thomassen, D. Dinsdale, C. Princen, L. Gonzalez, K. L. Poels, M. Kirsch-Volders, D. F. Lison and J. A. Martens et al., Oxidative stress induced by pure and iron-doped amorphous silica nanoparticles in sub-toxic conditions, Chem. Res. Toxicol., 2012, 25(4), 828–837 Search PubMed.
- Z. Xie, J. Li, J. Qian, S. Huang, Z. Wan, L. Yu, C. Liu and Y. Lu, Preparation and cytotoxicity of SiO2/Nd-Fe-B core–shelled nanoparticles applied to surgical anastomosis, Zhongguo Zuzhi Gongcheng Yanjiu Yu Linchuang Kangfu, 2010, 14(42), 7829–7834 Search PubMed.
- M. Liao, H. He and J. Xiong, Effects of iron-doped TiO2 nanoparticles and constant magnetic field on HL60 leukemia cells activity, Jiguang Shengwu Xuebao, 2009, 18(3), 324–328 Search PubMed.
- P.-J. Chen, C.-H. Su, C.-Y. Tseng, S.-W. Tan and C.-H. Cheng, Toxicity assessments of nanoscale zerovalent iron and its oxidation products in medaka (Oryzias latipes) fish, Mar. Pollut. Bull., 2011, 63(5–12), 339–346 Search PubMed.
- M. T. Zhu, B. Wang, Y. Wang, L. Yuan, H. J. Wang, M. Wang, H. Ouyang, Z. F. Chai, W. Y. Feng and Y. L. Zhao, Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis, Toxicol. Lett., 2011, 203(2), 162–171 CrossRef CAS.
- K. Buyukhatipoglu, T. Miller and A. M. Clyne, In vitro toxicity and intracellular uptake of flame synthesized iron oxide nanoparticles: an alternative to wet synthesis methods, AIChE Annual Meeting, Conference Proceedings, Philadelphia, PA, United States, Nov. 16–21, 2008, 910/1-910/6 Search PubMed.
- L. E. Murr and K. M. Garza, Natural and anthropogenic environmental nanoparticulates: Their microstructural characterization and respiratory health implications, Atmos. Environ., 2009, 43(17), 2683–2692 CrossRef CAS.
- M. Mahmoudi, H. Hofmann, B. Rothen-Rutishauser and A. Petri-Fink, Assessing the In Vitro and In Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles, Chem. Rev., 2012, 112(4), 2323–2338 CrossRef CAS.
- W. Gao, C. Fan and Z. Chen, Evaluation of acute toxicity and anti-phagocytosis of tumor-targeting superparamagnetic iron oxide nanoparticles in mice, Zhongguo Yaofang, 2011, 22(25), 2344–2346 Search PubMed.
- M. Mahmoudi, S. Laurent, M. A. Shokrgozar and M. Hosseinkhani, Toxicity Evaluations of Superparamagnetic Iron Oxide Nanoparticles: Cell “Vision” versus Physicochemical Properties of Nanoparticles, ACS Nano, 2011, 5(9), 7263–7276 CrossRef CAS.
- M. Safi, M. Yan, M.-A. Guedeau-Boudeville, H. Conjeaud, V. Garnier-Thibaud, N. Boggetto, A. Baeza-Squiban, F. Niedergang, D. Averbeck and J.-F. Berret, Interactions between magnetic nanowires and living cells: uptake, toxicity and degradation, Condensed Matter, 2011, 1–21 Search PubMed , arXiv: 1108.2081v1.
- P. Dua, K. N. Chaudhari, C. H. Lee, N. K. Chaudhari, S. W. Hong, J.-S. Yu, S. Kim and D.-K. Lee, Evaluation of toxicity and gene expression changes triggered by oxide nanoparticles, Bull. Korean Chem. Soc., 2011, 32(6), 2051–2057 CrossRef CAS.
- P. Candeloro, L. Tirinato, N. Malara, A. Fregola, E. Casals, V. Puntes, G. Perozziello, F. Gentile, M. L. Coluccio and G. Das et al., Nanoparticle microinjection and Raman spectroscopy as tools for nanotoxicology studies, Analyst, 2011, 136(21), 4402–4408 RSC.
- A. Hanini, A. Schmitt, K. Kacem, F. Chau, S. Ammar and J. Gavard, Evaluation of iron oxide nanoparticle biocompatibility, Int. J. Nanomed., 2011, 6, 787–794 Search PubMed.
- A. Garcia, R. Espinosa, L. Delgado, E. Casals, E. Gonzalez, V. Puntes, C. Barata, X. Font and A. Sanchez, Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests, Desalination, 2011, 269(1–3), 136–141 CrossRef CAS.
- L. Wang, L. Wang, W. Ding and F. Zhang, Acute toxicity of ferric oxide and zinc oxide nanoparticles in rats, J. Nanosci. Nanotechnol., 2010, 10(12), 8617–8624 CrossRef CAS.
- J. M. Berg, A. Romoser, N. Banerjee, R. Zebda and C. M. Sayes, The relationship between pH and zeta potential of ∼30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations, Nanotoxicology, 2009, 3(4), 276–283 Search PubMed.
- M. Mahmoudi, A. Simchi, M. Imani, A. S. Milani and P. Stroeve, An in vitro study of bare and poly(ethylene glycol)-co-fumarate-coated superparamagnetic iron oxide nanoparticles: a new toxicity identification procedure, Nanotechnology, 2009, 20(22), 225104–225104/8 CrossRef.
- B. Szalay, E. Tatrai, G. Nyiro, T. Vezer and G. Dura, Potential toxic effects of iron oxide nanoparticles in in vivo and in vitro experiments, J. Appl. Toxicol., 2012, 32(6), 446–453 Search PubMed.
- S. Sulek, B. Mammadov, D. I. Mahcicek, H. Sozeri, E. Atalar, A. B. Tekinay and M. O. Guler, Peptide functionalized superparamagnetic iron oxide nanoparticles as MRI contrast agents, J. Mater. Chem., 2011, 21(39), 15157–15162 RSC.
- J. Yin, Q. Wu and D. Zhang, Study on L-02 cells toxicity effect of Mn0.5Zn0.5Fe2O4 nanoparticles in vitro, Dulixue Zazhi, 2011, 25(2), 111–114 Search PubMed.
- Z. Beji, A. Hanini, L. S. Smiri, J. Gavard, K. Kacem, F. Villain, J.-M. Greneche, F. Chau and S. Ammar, Magnetic properties of Zn-substituted MnFe2O4 nanoparticles synthesized in polyol as potential heating agents for hyperthermia. Evaluation of their toxicity on Endothelial cells, Chem. Mater., 2010, 22(19), 5420–5429 CrossRef CAS.
- P. W. Boyd, A. J. Watson, C. S. Law and E. R. Abraham et al., A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization, Nature, 2000, 407, 695–702 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
