Repeated protrusion of fluorescent pyrene nanorods on the surface of crosslinked poly(allylamine hydrochloride) microcapsules†
Zhipeng
Wang
,
Yang
Xie
and
Changyou
Gao
*
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: cygao@mail.hz.zj.cn; Fax: +86-571-87951108; Tel: +86-571-87951108
First published on 17th October 2012
Abstract
The ability to prepare micro/nano materials with a stable structure is highly demanded in advanced sciences and technologies. In this work, poly(allylamine hydrochloride)-graft-pyrene microcapsules with a stable structure were obtained by glutaraldehyde crosslinking, which could then be employed as microreactors to protrude 1-pyrenecarboxaldehyde nanorods at low pH. The protrusion length of nanorods (NR) was tuned by the pH value, e.g. shorter NRs and a larger covering density or longer NRs and a lower covering density on the microcapsule surface at higher and lower pHs, respectively. The protruded pyrene NRs showed strong fluorescent emissions on the surface of microcapsules. The NRs could be repeatedly protruded by manipulating the reversible Schiff base formation and hydrolysis.
Shape and/or structure specific colloidal particles, such as elliptical, discoidal, and needle-shaped particles, and/or hierarchically combined micro/nano structures, have aroused keen and broad interest from materials science to biological medicine areas.1–5 These kinds of colloidal particles not only possess specific or even outstanding properties but also appropriately fulfill the practical applications compared to normal spherical particles. For instance, Doshi et al., reported that the needle-shaped polystyrene nanoparticles could induce cell membrane disruption and increase cell mobility after co-incubation, while spheres and elliptical discs exhibited minimal interactions.5 The biconcaved microcapsules behaved like red blood cells, showing reversible deformability under physiological flow in capillaries, which mimics the blood vessel capillary environment.6 These results have significant implications in developing new methods of drug delivery. Furthermore, the colloids with hierarchically combined micro/nano structures could provide multicompartments in one-piece devices for multiple drugs loading and release.7,8 Meanwhile, such structures could be potentially developed to materials with anisotropic properties and function isolation. However, only a few examples have been demonstrated on the preparation and manipulation of the properties and functions of shape-specific colloidal particles. The hierarchically combined micro/nano structures are rarely reported up to the present.
Very recently, we discovered an intriguing phenomenon of protrusion of one-dimensional pyrene (Py) nanorods (NRs) from poly(allylamine hydrochloride) (PAH)-graft-Py microcapsules.9 In this process, an –NH2 group containing polyelectrolyte, PAH, was doped into CaCO3 particles during mineralization, and further reacted with 1-pyrenecarboxaldehyde (Py-CHO) by Schiff base formation. Removal of the CaCO3 template yielded hollow microcapsules (MCs) composed of Py-modified PAH (PAH-Py). The NRs are composed of 1-pyrenecarboxaldehyde (Py-CHO) (Py-CHO NR) and can be generally obtained through π–π stacking self-assembly during hydrolysis of Schiff base bonds between PAH and Py-CHO in weak acid solution. Since Py and its self-assembly structures show great fluorescent properties,10,11 and polyelectrolyte MCs have been proven to be a promising material for drug delivery, microreactors, and bio-sensors,12–18 our capsules with protruding Py NRs on the surface could be expected with more specific properties and functions for practical applications. However, during the NR protruding process we found, unfortunately, that the MCs cannot hold their structure thermodynamically and eventually disappear because of cleavage of the pendent hydrophobic pyrene groups, which physically crosslink the hydrophilic PAH backbones. Therefore, measures should be taken to obtain the composite MC-NRs in a more controllable way.
Crosslinking of the PAH backbones would be a straightforward method to stabilize the capsule structure.19–22 However, it is not known if the crosslinking will cause hindrance of the NR protrusion from the MCs. To elucidate these issues, glutaraldehyde (GA) crosslinking of the PAH-g-Py will be performed before removal of the CaCO3 template in this study (Scheme 1). The GA crosslinking results in a very stable structure against extreme pHs, although theoretically similar Schiff base bonds should be formed.19 The mechanism is rather complicated and not fully understood. Basically, the mono-GA yields a labile Schiff base, while the conjugated aldehyde moieties in a polymeric format in the GA solution produce more stable products by cyclization, dehydration, and internal redox reactions.20–22 Nonetheless, by this additional crosslinking, composite MC-NRs with a stable structure are expected following the NRs protrusion processes. In addition, crosslinking of the PAH in the MCs also provides a stable structure for the reversibility of the Schiff base reaction so that the repeated protrusion of Py-CHO NRs from the MCs could be reasonably expected, which opens new opportunities for the design of novel micro/nano structures and materials for nanoscience, and biological and other advanced technologies.
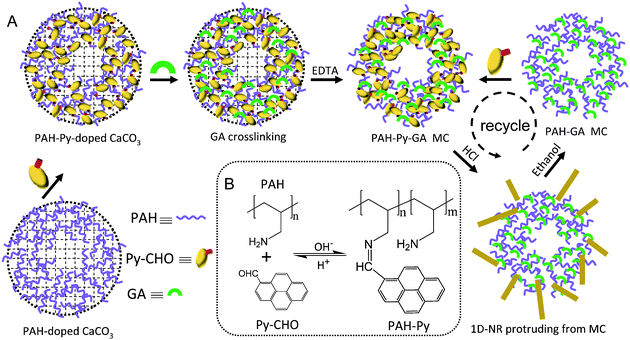 | ||
| Scheme 1 (A) Schematic illustration of PAH-Py-GA MC fabrication and Py-CHO NR protrusion from the MC at low pH. (B) Chemical structures of Py-CHO, PAH, and PAH-Py, and reversible Schiff base formation and hydrolysis at different pH values. | ||
The experiments started with incubation of the PAH-doped CaCO3 particles with a diameter of 5.5 ± 1.2 μm in Py-CHO ethanol solution, so that the Schiff base reaction between PAH and Py-CHO could take place sufficiently.9 After removal of excess Py-CHO, the PAH-Py-doped CaCO3 particles were dispersed in GA solution (1% in weight). The PAH-Py-GA MCs, which had a diameter of 7.7 ± 1.4 μm, were obtained after core removal with EDTA solution. They collapsed only in the middle parts due to the very thick shells, resulting in a concave morphology (Fig. 1A), which is very similar to that of the PAH-Py MCs.9 Although no additional peaks could be determined by Fourier transform infrared (FTIR) spectroscopy, the relative intensity of CH2 peaks at 2923 and 2854 cm−1 increased after GA crosslinking (Fig. S1, ESI†), which is attributed to the methylene groups of GA and thereby confirms occurrence of the reaction. This was verified further by the color change of the MCs from yellow to brown. The chemical reactions of GA crosslinking are so complicated that no agreement on the main reaction mechanism has been reached up to now. Aldehydes are expected to form Schiff bases with amine groups. However, the Schiff bases are unstable under acidic conditions and tend to break down to generate aldehydes and amines. In our experiments, the GA-crosslinked microcapsules have shown exceptional stability at low pH, such as pH 2, 1.5, and 1. So a simple Schiff base could be ruled out. The polymeric form of GA may contribute to the stability of the cross-linking,20–22 because the formed Schiff base will be stabilized by conjugated carbon double bonds.
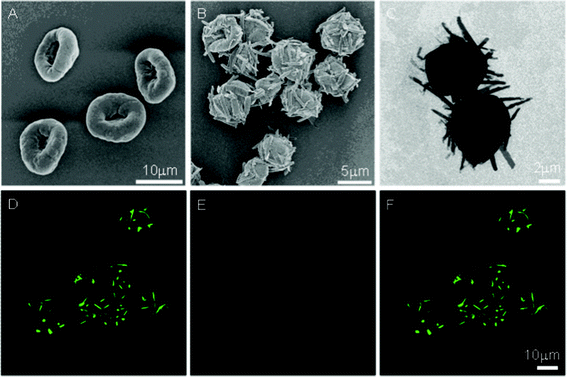 | ||
| Fig. 1 PAH-Py-GA MCs before (A, SEM image) and after treatment (B, SEM; C, TEM; and D–F CLSM images) at pH 1.5 for 4 h. (F) The merged images of (D) NRs (Py-CHO, excited with 405 nm laser) channel and (E) capsule (PAH-GA) channel. | ||
When the suspension of PAH-Py-GA MCs was incubated in a pH 1.5 HCl solution, protonation of the Schiff base bonds and amino groups in PAH-Py took place similarly to those without GA crosslinking. Along with the hydrolysis of the Schiff base and self-assembly of Py-CHO, NRs were protruded from the MC surface, as shown in Fig. 1B, C. The GA-crosslinked MCs maintained their macroscopic contour, while the uncrosslinked PAH-Py MCs gradually swelled, disintegrated, and finally disappeared, resulting in only NRs (Fig. S2, ESI†). Thus, the GA-crosslinking does stabilize the MC structure, so that the MC-NRs can be obtained in a more thermodynamically controllable way. The protruded NRs are 2–5 μm in length and 300–400 nm in diameter, which is shorter and wider than those fabricated from the uncrosslinked MCs (7–8 μm in length and ∼200 nm in diameter) at the same conditions (incubated at pH 1.5 for 4 h).9 Movement of the crosslinked PAH chains is restricted to some extent, weakening the effect in guiding the self-assembly of Py-CHO NRs. The protrusion of NRs from the crosslinked MCs was further identified by confocal laser scanning microscopy (CLSM) (Fig. 1D–F), in which the green (Fig. 1D, λex = 405 nm) and red (Fig. 1E, λex = 543 nm) colors represent the NRs and MCs, respectively. While the pyrene is a well-known fluorophore, the fluorescence emission of MCs originates from the conjugated structures of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds as a result of GA crosslinking. The merged image (Fig. 1F) further confirms the tight anchorage of the NRs on the MC surface. Z direction scanning of CLSM revealed that the Py-CHO NRs were dispersed in 3 dimensions on the MCs (Fig. S3, ESI†).
N bonds as a result of GA crosslinking. The merged image (Fig. 1F) further confirms the tight anchorage of the NRs on the MC surface. Z direction scanning of CLSM revealed that the Py-CHO NRs were dispersed in 3 dimensions on the MCs (Fig. S3, ESI†).
The protruding process of Py-CHO NRs from the PAH-Py-GA MCs was monitored by CLSM (Fig. 2A) and fluorescence spectroscopy (Fig. 3) when the MCs were incubated in a pH 2 HCl solution. As shown in Fig. 2A, the Py moiety revealed average but relatively weak fluorescence due to the quenching effect of protonation of the Schiff base in PAH-Py at the beginning of acid treatment, which was correlated with the fluorescence spectra at 0 min (red curve in Fig. 3). After 10 min, some green spots appeared at the periphery of the MCs, indicating the budding of NRs. The fluorescent peak of Py recovered slightly at 562 nm. With the prolonging of incubation time, more and more NRs were being protruded from the MC surface to eventually form the MC-NR structure. Meanwhile, the Py fluorescent peak shifted from 562 nm to 505 nm, accompanied by 86% recovering intensity of the original value. Moreover, the monomer peaks at the region between 350 nm and 400 nm also increased with the NR protruding process, indicating that a few Py molecules failed to assemble into the NRs but dissolved in the aqueous solution. It has to be mentioned that the PAH-GA crosslinked MCs remained intact during the whole process.
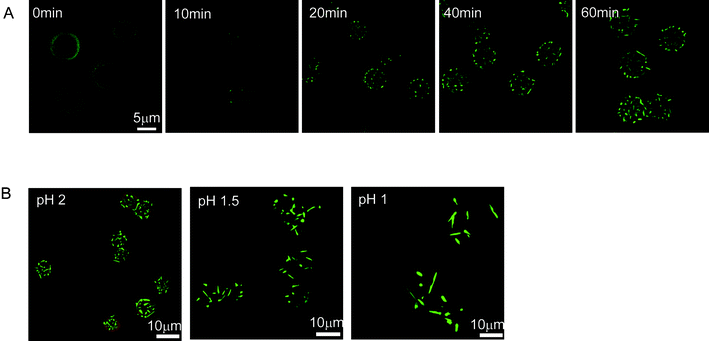 | ||
| Fig. 2 (A) Monitoring the NR protruding process from PAH-Py-GA MCs in pH 2 HCl solution by CLSM. (B) CLSM images of NR-protruded MCs obtained at pH 2 for 1 h, pH 1.5 for 4 h, and pH 1 for 24 h. The text in the left-upper corners of (A) and (B) represent the incubation time and solution pH, respectively. In the merged images, the green and red colors represent the Py molecules or NRs and GA-crosslinked MCs, respectively. | ||
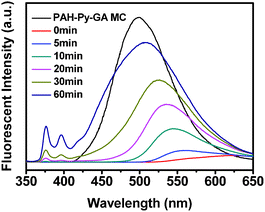 | ||
| Fig. 3 Fluorescence spectra of original PAH-Py-GA MCs and the NR protrusion process at pH 2. | ||
The Py-CHO NR length could be well manipulated by solution pH. Our previous study showed that the hydrolysis rate of the Schiff base of PAH-Py in pH 2 solution was 100 times higher than that in pH 0 solution, yielding short NRs and long nanotubes (Fig. S4, ESI†), respectively.9 Thus, the NRs growth rate could be tuned by solution pH. After incubation at different pHs for a sufficiently long time, different MC-NRs were obtained, as shown in Fig. 2B. Within 1 h, short (<2 μm) but very dense NRs were protruded on the pH 2 treated MCs. At pH 1.5 for 4 h, longer (3–5 μm) but fewer NRs were found on the MCs. Further observation revealed that at pH 2 or pH 1.5 the NRs could not grow further even after 24 h incubation and the morphologies maintained stable. By contrast, the NRs could grow above 8 μm with a still smaller number after incubation at pH 1 for 24 h. The NR protrusion length at different pH was further confirmed by SEM observation (Fig. S5, ESI†). The NRs were 3–4 times longer at pH 1 (average length 6.5 μm) than at pH 2 (average length 1.7 μm). Therefore, the NR protrusion length could be effectively controlled by the solution pH.
The Py-CHO NRs could be repeatedly protruded from the MCs by making use of the reversible covalent bond of the Schiff base in PAH-Py. The Schiff base hydrolyzes to amino and aldehyde groups under acidic conditions but forms reversibly under alkaline conditions. The NRs on the MCs (Fig. 4A) were confirmed by FTIR (Fig. S6, ESI†), in which the peak at 1680 cm−1 is assigned to an aldehyde of the Py moiety hydrolyzed from PAH-Py. After dissolution of Py-CHO molecules by ethanol, the NRs disappeared from the PAH-GA MCs (Fig. 4B), whose chemical structure change was verified by FTIR (absence of Py-CHO and enhanced intensity of free amino groups). The formation of the Schiff base between Py-CHO and PAH took place again after the PAH-GA MCs were incubated in the Py-CHO ethanol solution at pH 10 (Fig. S6, ESI†). The newly prepared PAH-GA-Py MCs had very similar morphology (Fig. 4C) to the PAH-Py-GA MCs (Fig. 4B), and produced Py-CHO NRs on the MCs after incubation in pH 2 solution (Fig. 4D). It is worth mentioning that this process could be recycled several times. As shown in Fig. 4E, the Py-CHO NRs were protruded from the PAH-Py-GA MCs for the 4th time and the structure of the MCs was still very robust. However, after many attempts at centrifugation during the repeated protrusion of NRs the microcapsules were aggregated, which influences the further use of the microcapsules as microreactors. The fluorescence intensity of the protruded NR also decreased with recycling times, which could be attributed to the progressively reduced amount of Py-CHO modified on the MCs (Fig. 5). Nevertheless, the PAH-Py-GA MCs have great potential applications as recyclable microreactors and micro/nano devices, with the improvement of technical problems. Furthermore, the protrusion of pyrene nanorods on the PAH microcapsules created an anisotropic chemical environment on these specific micro/nano structures, which would open a window for selective modification on either nanorods or microcapsules to greatly enhance the chemical anisotropy.
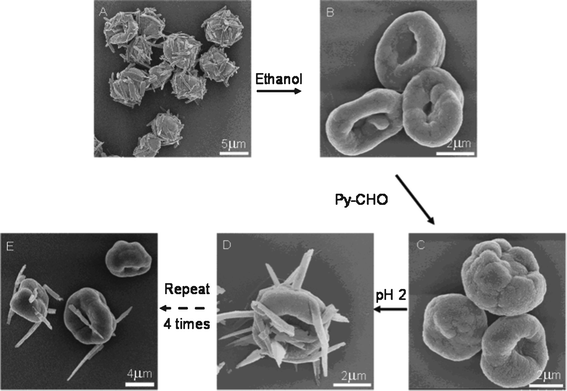 | ||
| Fig. 4 SEM images showing the repeated protrusion of NRs from the MCs. After the PAH-Py-GA MCs protruded with Py-CHO NRs obtained at pH 2 (A) were washed with ethanol to dissolve the NRs (B), the MCs were reacted with Py-CHO again (C), which protruded the Py-CHO NRs after treatment at pH 2 (D), and Py-CHO NRs protruded the 4th time from the microcapsules. | ||
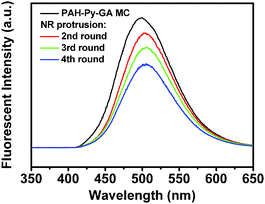 | ||
| Fig. 5 Fluorescence spectra of original PAH-Py-GA MC (1st) and after NR protrusion for different lengths of time. | ||
In conclusion, the Py-CHO NRs could be protruded from GA-crosslinked PAH-Py MCs, which could survive from incubation in low pH solutions. The NR protrusion length was controlled by the pH value of the incubation solution. Higher pH produced NRs with a shorter length but a larger covering density. By manipulating the reversible Schiff base formation and hydrolysis the NRs could be repeatedly protruded from the PAH-Py MCs. Strong fluorescent emission of Py remained on the surface of the MCs. This composite micro/nano material with a stable structure opens a new opportunity to develop advanced smart nanodevices.
Acknowledgements
This work is financially supported by the Natural Science Foundation of China (Nos. 51120135001 and 50873087), the Zhejiang Provincial Natural Science Foundation of China (No. Z4090177), the Ministry of Science and Technology of China for the Indo-China Cooperation (No. 2010DFA51510), and Open Project of State Key Laboratory of Supramolecular Structure and Materials (sklssm201224).References
- D. M. Vriezema, M. C. Aragonès, J. A. A. W. Elemans, J. J. L. M. Cornelissen, A. E. Rowan and R. J. M. Nolte, Chem. Rev., 2005, 105, 1445–1489 CrossRef CAS.
- M. Caldorera-Moore, N. Guimard, L. Shi and K. Roy, Expert Opin. Drug Delivery, 2010, 7, 479 CrossRef CAS.
- D. Peer, J. M. Karp and S. Hong, Nat. Nanotechnol., 2007, 2, 751 CrossRef CAS.
- S. Venkataraman, J. L. Hedrick, Z. Y. Ong, C. Yang, P. L. Rachel Ee, P. T. Hammond and Y. Y. Yang, Adv. Drug Delivery Rev., 2011, 63, 1228 CrossRef CAS.
- N. Doshi and S. Mitragotri, J. R. Soc. Interface, 2010, 7, 403 CrossRef.
- N. Doshi, A. S. Zahr, S. Bhaskar, J. Lahann and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21495 CrossRef CAS.
- R. Chandrawati, M. P. van Koeverden, H. Lomas and F. Caruso, J. Phys. Chem. Lett., 2011, 2, 2639 CrossRef CAS.
- S. F. M. van Dongen, H. M. de Hoog, R. J. R. W. Peters, M. Nallani, R. J. M. Nolte and C. M. van Hest Jan, Chem. Rev., 2009, 109, 6212 CrossRef CAS.
- Z. P. Wang, H. Moehwald and C.Y. Gao, ACS Nano, 2011, 5, 3930 CrossRef CAS.
- F. M. Winnik, Chem. Rev., 1993, 93, 587 CrossRef CAS.
- K. Kalyanasundaram and J. K. Thomas, J. Phys. Chem., 1977, 81, 2176 CrossRef CAS.
- A. S. Angelatos, K. Katagiri and F. Caruso, Soft Matter, 2006, 2, 18 RSC.
- L. Yang and P. Alexandridis, Curr. Opin. Colloid Interface Sci., 2000, 5, 132 CrossRef CAS.
- D. G. Shchukin and H. Möhwald, Small, 2007, 3, 926 CrossRef CAS.
- A. A. Antipov, G. B. Sukhorukov, S. Leporatti, I. L. Radtchenko, E. Donath and H. Möhwald, Colloids Surf., A, 2002, 198, 535 CrossRef.
- Z. P. Wang, Z. Q. Feng and C. Y. Gao, Chem. Mater., 2008, 20, 4194 CrossRef CAS.
- A. G. Skirtach, P. Karageorgiev, M. F. Bedard, G. B. Sukhorukov and H. Möhwald, J. Am. Chem. Soc., 2008, 130, 11572 CrossRef CAS.
- K. Köhler and G. B. Sukhorukov, Adv. Funct. Mater., 2007, 17, 2053 CrossRef.
- W. J. Tong, C. Y. Gao and H. Möhwald, Chem. Mater., 2005, 17, 4610 CrossRef CAS.
- W. J. Tong, C. Y. Gao and H. Möhwald, Macromol. Rapid Commun., 2006, 27, 2078 CrossRef CAS.
- P. M. Hardy, G. J. Hughes and H. N. Rydon, J. Chem. Soc., Perkin Trans. 1, 1979, 2282 RSC.
- P. M. Hardy, A. C. Nicholls and H. N. Rydon, J. Chem. Soc., Perkin Trans. 1, 1976, 958 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Description of experimental techniques used and supporting figures. See DOI: 10.1039/c2ra20672b |
| This journal is © The Royal Society of Chemistry 2012 |
