Hydrocarbon blend membranes with suppressed chemical crossover for redox flow batteries†
Xinsheng
Zhao
,
Yongzhu
Fu
,
Wei
Li
and
Arumugam
Manthiram
*
Materials Science and Engineering Program and Texas Materials Institute, University of Texas at Austin, Austin, TX 78712, United States. E-mail: rmanth@mail.utexas.edu (A. Manthiram); Fax: +1-512-471-7681; Tel: +1-512-471-1791
First published on 13th April 2012
Abstract
A hydrocarbon blend membrane consisting of an acidic polymer (sulfonated poly (ether ether ketone), SPEEK) and a basic polymer (polysulfone-2-amide-benzimidazole, PSf-ABIm) has been prepared and assessed in vanadium redox flow batteries (VRBs). The vanadium ion (VO)2+ permeability through the blend membrane is about 50 times lower than that of Nafion 117 and 4 times lower than that of the plain SPEEK. The Coulombic efficiency and energy efficiency of the single cell with the blend membrane are higher than those with Nafion and plain SPEEK membranes. The blend membrane also shows better chemical stability than plain SPEEK. The results demonstrate that blend membranes of this type are promising for VRBs.
Vanadium redox flow batteries (VRBs) were initially fabricated and operated by Skyllas-Kazacos et al.,1,2 in the 1980s, utilizing the V(II)/V(III) and V(IV)/V(V) redox couples in sulfuric acid solution as the anolyte and catholyte, respectively. They are attractive electrical energy storage systems for the utilization of renewable energies like solar and wind due to their significant advantages such as high energy efficiency, deep discharge ability, low self-discharge, long cycle life, and most importantly, independent power and energy ratings. A key component of VRBs is the ion exchange membrane (IEM), which separates the anolyte and catholyte and transports protons during charge/discharge. IEMs should have low vanadium ion permeation to minimize the cross-mixing of active species, high proton conductivity, and good chemical and mechanical stabilities. Nafion® membranes are the most commonly used in VRBs due to their high proton conductivity and good chemical stability,3 but the high permeability of vanadium ions results in a low Coulombic efficiency; also, the high cost of Nafion hampers the commercialization of VRBs. Therefore, development of new low-cost membranes with high selectivity is critical for VRBs, as well as other redox flow batteries, to be successful and competitive for large-scale electrical energy storage.
A number of polymers and modified Nafion membranes have been developed to lower vanadium ion permeability and improve Coulombic efficiency.4 Among them, sulfonated hydrocarbon polymers such as polysulfone and poly(ether ether ketone) are appealing due to their low cost, especially, and lower permeability for vanadium ions than Nafion membranes.5,6 However, lowering the vanadium ion permeability further is needed for VRBs to be commercially viable. Our group has developed a series of blend membranes consisting of an acidic polymer such as sulfonated poly(ether ether ketone) (SPEEK) and a basic polymer containing N-heterocycles such as benzimidazole tethered polysulfone (PSf-BIm) and 2-amide-benzimidazole tethered polysulfone (PSf-ABIm) for direct methanol fuel cells.7–9 These aromatic polymers have good mechanical and chemical stabilities and also exhibit excellent compatibility due to their similar backbones. The insertion of N-heterocycle groups into the sulfonic acid group domains of SPEEK through acid–base interactions improves the proton conduction and reduces methanol crossover. Analogous to methanol crossover through IEMs, vanadium ion permeation occurs in VRBs when protons are transferred via a vehicle-type mechanism (water as the carrier) during charge/discharge. The blend membranes with the N-heterocycles inserted into the ionic channels could suppress the vanadium ion crossover in VRBs. Accordingly, we presented here the evaluation in VRBs of a blend membrane consisting of the acidic polymer SPEEK and 5 wt.% basic polymer PSf-ABIm, designated as SPEEK/PSf-ABIm.
The membrane properties, chemical stability and VRB single cell performance were examined (see the ESI†).
Table 1 presents some properties of Nafion 117, plain SPEEK, and SPEEK/PSf-ABIm blend membranes. Although the plain SPEEK has a higher IEC (1.62 mmol g−1) than the Nafion 117 membrane (0.91 mmol g−1), the area resistances of the SPEEK and blend membranes are higher than that of Nafion 117 due to their low acidity and microstructure discrepancy.10,11 The blend membrane shows a slightly higher area resistance than the plain SPEEK. The addition of PSf-ABIm decreases the IEC of the blend membrane due to the protonation of the N atoms in 2-amide-benzimidazole by the sulfonic acid groups.8 The blend membrane has a lower water uptake than the plain SPEEK, implying low swelling, which is consistent with its low IEC value. The water uptake is closely related to the water transport and permeability through the membrane. The SPEEK and blend membranes have a higher Young's modulus than Nafion 117, indicating the excellent stiffness of the aromatic-based polymers.
Fig. 1 shows the schematic structures of the plain SPEEK and SPEEK/PSf-ABIm blend membranes. The insertion of the pendant 2-amide-benzimidazole groups into the hydrophilic domains could suppress vanadium ion migration. Fig. 2 gives the variation of the permeated vanadium ion concentration with time. The concentrations of vanadium ions through the plain SPEEK and blend membranes are below 10 mM after 16 h in contrast to over 60 mM for Nafion 117. Moreover, the vanadium ion concentration through the blend membrane is even lower than that through the plain SPEEK. The permeability of vanadium ions through the membranes was calculated according to Fick's law of diffusion.12 As seen in Table 1, the permeability of vanadium ions through the blend membrane is only 1.12 × 10−7 cm min−1, which is 4 times lower than that of the plain SPEEK (4.68 × 10−7 cm min−1) and almost 50 times lower than that of Nafion 117 (54.3 × 10−7 cm min−1). It has been reported that the separation between the hydrophobic and hydrophilic groups in SPEEK is much smaller that in Nafion,11 resulting in a stronger confinement of (VO)2+ and water in the narrow channels. As illustrated in Fig. 1, adding 2-amide-benzimidazole groups into the ionic domains of SPEEK further blocks the crossover of (VO)2+ ions. The decrease rate of open circuit voltage (OCV) is usually used to measure the degree of self-discharge arising from vanadium ion permeation. The single cell was circulated with the electrolytes and self-discharge started at the state of charge (SOC) of 50%. Fig. 3 shows the changes of OCV with time for the VRB single cell with different membranes. It can be seen that the OCV decreases gradually and exhibits an almost linear relationship with time until the OCV reaches 1.3 V. For Nafion 117, the OCV decreases to 1.3 V in about 6 h, and then degrades rapidly to < 0.9 V within 1 h. In contrast, the plain SPEEK experiences a slower decreasing process. The OCV reaches 1.3 V in about 7 h, followed by a long slow decreasing process. The OCV of the blend membrane decreases to 1.3 V in a period of 8 h, which is longer than that of Nafion 117 and the plain SPEEK, indicating its lower vanadium ion permeability. This result is consistent with the permeability data shown in Table 1. Fig. 4a shows the charge/discharge curves of the cells with these membranes at a current density of 20 mA cm−2. The single cell with the Nafion 117 membrane shows higher mean charge voltage than those with the plain SPEEK and SPEEK/PSf-ABIm blend membranes because of its lower area resistance. However, the discharge duration of the single cells with the plain SPEEK and SPEEK/PSf-ABIm blend membranes lasts longer than that with Nafion 117. This can be attributed to the reduction in the cross-mixing of vanadium ions. The blend membrane shows even better performance than plain SPEEK. The average Coulombic efficiency (CE), voltage efficiency (VE), and energy efficiency (EE) are shown in Fig. 4b. Although the lower area resistance of Nafion 117 leads to higher VE, the lower permeability of vanadium ions with the plain SPEEK and blend membranes results in higher CE and EE. The cell with the Nafion 117 membrane exhibits a higher VE of 94.7% than those with the plain SPEEK (93.2%) and SPEEK/PSf-ABIm (90.6%) membranes. However, the cells with the SPEEK/PSf-ABIm and plain SPEEK membranes exhibit higher CE of 96.7% and 94.0%, respectively, than the Nafion 117 membrane (82.3%). Similarly, the cells with the plain SPEEK and SPEEK/PSf-ABIm membranes exhibit higher energy efficiencies of, respectively, 87.6 and 87.7%, compared to that with the Nafion 117 membrane (77.9%).
 | ||
| Fig. 1 Schematic diagram of (a) SPEEK and (b) SPEEK/PSf-ABIm blend membranes. | ||
 | ||
| Fig. 2 Comparison of vanadium ion permeability across Nafion 117, SPEEK, and SPEEK/PSf-ABIm blend membranes. | ||
 | ||
| Fig. 3 Change of OCV of the VRB cells as a function of time using Nafion 117, SPEEK and SPEEK/PSf-ABIm blend membranes. | ||
 | ||
| Fig. 4 (a) Charge/discharge curves at a current density of 20 mA cm−2, and (b) Coulombic, voltage, and energy efficiencies of VRB single cells with Nafion 117, SPEEK, and SPEEK/PSf-ABIm blend membranes. | ||
Finally, ex-situ stability testing was carried out by soaking the SPEEK and blend membranes in a charged (VO2)+ electrolyte to evaluate their chemical stability. SEM images of the surface of the membranes were taken after 2 days and 100 days. As seen in Fig. 5a and 5b, the blend membrane does not show any pinholes, unlike the plain SPEEK membrane (circled in red), after 2 days. After 100 days, many craters appeared on the surface of the plain SPEEK membrane (Fig. 5c), while there are only a few minor pinholes on the blend membrane (Fig. 5d). Moreover, the plain SPEEK membrane shattered in the vanadium electrolyte due to structural degradation, while the blend membrane still remained intact. The N-heterocycle group may delocalize the charge on the sulfonic acid groups, thereby resisting the chemical attack by the (VO2)+ ions and improving the chemical stability.
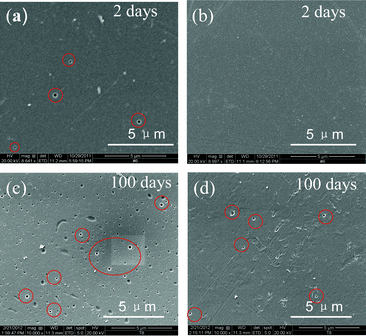 | ||
| Fig. 5 SEM images of (a, c) SPEEK and (b, d) SPEEK/PSf-ABIm membranes after being immersed in (VO2)+ electrolyte for 2 days and 100 days. | ||
In summary, the SPEEK/PSf-ABIm blend membrane shows much better ion selectivity and chemical stability compared to the plain SPEEK membrane. The VRB single cell employing the blend membrane exhibits higher Coulombic efficiency (96.7%) and energy efficiency (87.7%) than those with Nafion 117 and plain SPEEK. The good performance along with low-cost and excellent chemical stability make the blend membrane a feasible separator for flow battery systems.
Acknowledgements
This work was supported by the Department of Energy Office of Basic Energy Science grant No. DE-SC0005397.References
- M. Skyllas-kazacos and F. Grossmith, J. Electrochem. Soc., 1987, 134, 2950–2953 CrossRef CAS.
- M. Skyllas-kazacos, M. Rychcik, R. G. Robins, A. G. Fane and M. A. Green, J. Electrochem. Soc., 1986, 133, 1057–1058 CrossRef CAS.
- T. Mohammadi and M. Skyllas-kazacos, J. Appl. Electrochem., 1997, 27, 153–160 CrossRef CAS.
- X. F. Li, H. M. Zhang, Z. S. Mai, H. Z. Zhang and I. Vankelecom, Energy Environ. Sci., 2011, 4, 1147–1160 CAS.
- S. Kim, J. Yan, B. Schwenzer, J. L. Zhang, L. Y. Li, J. Liu, Z. G. Yang and M. A. Hickner, Electrochem. Commun., 2010, 12, 1650–1653 CrossRef CAS.
- Q. T. Luo, H. M. Zhang, J. Chen, D. J. You, C. X. Sun and Y. Zhang, J. Membr. Sci., 2008, 325, 553–558 CrossRef CAS.
- Y. Z. Fu, A. Manthiram and M. D. Guiver, Electrochem. Solid-State Lett., 2007, 10, B70–B73 CrossRef CAS.
- Y. Z. Fu, A. Manthiram and M. D. Guiver, Electrochem. Commun., 2007, 9, 905–910 CrossRef CAS.
- W. Li, Y. Z. Fu, A. Manthiram and M. D. Guiver, J. Electrochem. Soc., 2009, 156, B258–B263 CrossRef CAS.
- K. D. Kreuer, J. Membr. Sci., 2001, 185, 29–39 CrossRef CAS.
- B. Yang and A. Manthiram, J. Power Sources, 2006, 153, 29–35 CrossRef CAS.
- X. G Teng, Y. T. Zhao, J. Y. Xia, Z. H. Wu, X. P. Qiu and L. Q. Chen, J. Membr. Sci., 2009, 341, 149–154 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Membrane properties, chemical stability and VRB single cell performance are included. See DOI: 10.1039/c2ra20668d |
| This journal is © The Royal Society of Chemistry 2012 |
