Resorcinol-formaldehyde coated XAD resin beads for removal of cesium ions from radioactive waste: synthesis, sorption and kinetic studies
Charu
Dwivedi
a,
Amar
Kumar
b,
Juby K.
Ajish
a,
Krishan Kant
Singh
a,
Manmohan
Kumar
*a,
Piaray Kishen
Wattal
b and
Parma Nand
Bajaj
a
aRadiation & Photochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India. E-mail: manmoku@barc.gov.in; Fax: (+)91-22-25505151; Tel: (+) 91-22-25593994
bProcess Development Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India
First published on 7th March 2012
Abstract
A novel synthetic method was developed to synthesize resorcinol-formaldehyde (RF) resin in spherical form, of required mesh size, using XAD-4 as template beads. The synthesized RF-coated XAD (RF-XAD) beads were characterized, using different techniques. Suitable size and mechanical stability, along with their spherical shape, make these beads most appropriate for column operation. The efficiency of these beads was evaluated for removal of cesium from alkaline medium, in batch conditions, using a radioanalytical technique. The effect of sodium ion concentration, the initial cesium ion concentration and the contact time were also investigated. It was observed that the Kd value for Cs+ ions decreases with increase in Na+ ion concentration. The equilibrium data were fitted into different isotherm models, and were found to be represented well by the Langmuir isotherm equation, with a monolayer sorption capacity of 287 mg g−1. Kinetic modeling analysis, using pseudo first-order, pseudo second-order and intraparticle diffusion equations, shows that the pseudo second-order equation is the most appropriate model for the description of the sorption of cesium ions onto the RF-XAD beads. The rate constants were determined at different initial concentrations. The process mechanism was found to be complex, consisting of both surface sorption and pore diffusion.
Introduction
During the last several decades, considerable efforts have been directed towards removal and recovery of fission products from nuclear waste streams. Among these fission products, 137Cs is of special concern, because, along with 90Sr, it constitutes a major source of heat in the waste. This radionuclide has a long half-life and is a biological hazard.1 So, its removal from the waste streams, before discharge to the environment, is necessary. A variety of methods, based on liquid/liquid extraction, solid/liquid extraction, ion-exchange processes, etc., have been proposed for the removal/recovery of 137Cs from the nuclear waste. Among these, the ion exchange method has several advantages. For example, the process is simple, compact, flexible, efficient enough to achieve decontamination factors of several orders of magnitude, and does not require any hazardous organic solvents.2 In the literature, a considerable number of reports are available on the use of various organic and inorganic sorbents, which have strong affinity for one, or more, radionuclides over a wide pH range. These include aluminosilicates,3 phosphates,4 ferrocyanides and hydrous oxides of multivalent cations,5–7 pillared clays, ammonium molybdophosphate (AMP), and resorcinol formaldehyde resin. Among these, only RF resin has been considered as a satisfactory option for removal of Cs+ ions from highly alkaline media because of low-cost, safety, availability, selectivity, easy operation and efficiency considerations.8 Moreover, other organic ligands and extractants do not work in the presence of high concentrations of salts and harsh alkaline conditions, whereas RF resin has an exceptionally high affinity for Cs+ ions, which can be attributed to the presence of the –OH group, which ionizes under highly alkaline conditions. Conventionally, RF resin is prepared by bulk polymerization reaction between resorcinol and formaldehyde in alkaline medium, to get big chunks of the polymeric resin, and then, these chunks are grounded and sieved, to get the desirable mesh size.9 But, these grounded gel particles are of irregular shape, have broad particle size distribution, and exhibit poor column hydraulic behavior. These problems can be solved by using spherical resin material. We have developed a new method for the synthesis of spherical RF resin (RF-XAD), using pre-formed commercially available divinylbenzene cross-linked polystyrene microspheres (XAD-4) of the desired size as a base material. These RF-coated beads are characterized, using a TGA technique, SEM, BET surface area analysis and a universal testing machine. The sorption and kinetic studies for Cs+ ions have been carried out in detail, using a radiotracer technique (134Cs radiotracer). The experimental data are analyzed, using various sorption isotherm models and kinetic models.Experimental
Materials
Resorcinol, formaldehyde (37% in methanol, AR grade), sodium hydroxide (99%), sodium nitrate (99%), cesium nitrate (99%) and Amberlite XAD-4 were obtained from Merck. All the other solvents and the chemicals used were of analytical grade, procured from local suppliers. Water obtained from a Millipore-Q water purification system, with conductivity 0.3 μS cm−1, or lower, was used in all the experiments.Synthesis of RF-XAD resin beads
Resorcinol formaldehyde pre-condensate was prepared by reacting resorcinol and formaldehyde in aqueous media, using NaOH as a catalyst. An aqueous solution, with resorcinol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formaldehyde
formaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst in the molar ratio of 1
catalyst in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, was prepared, and allowed to react for some time till it attained a suitable viscosity. Then, XAD beads were equilibrated with this RF pre-condensate solution for 2 hours, the equilibrated beads were separated from the rest of the RF pre-condensate solution, and the solution sorbed onto the beads was allowed to cure completely. After complete curing at 105 °C for 4 h, brown-colored RF-coated XAD beads were obtained. These beads were in the sodium form, and hence, were dark-colored. The synthesized resin beads were converted into the hydrogen form, by mixing these with 0.5 M HNO3 in a 3 to 1 ratio of liquid-to-resin volume for 1 hour, with occasional gentle shaking. After that, the resin beads were separated and washed with water thoroughly, to remove any residual acid, till the pH of the washing became near neutral. This acid treatment turned the resin into a light-colored hydrogen form. This hydrogen form of the resin was air-dried, and stored. It was used as such, without any further pre-treatment, in all the sorption experiments.
6, was prepared, and allowed to react for some time till it attained a suitable viscosity. Then, XAD beads were equilibrated with this RF pre-condensate solution for 2 hours, the equilibrated beads were separated from the rest of the RF pre-condensate solution, and the solution sorbed onto the beads was allowed to cure completely. After complete curing at 105 °C for 4 h, brown-colored RF-coated XAD beads were obtained. These beads were in the sodium form, and hence, were dark-colored. The synthesized resin beads were converted into the hydrogen form, by mixing these with 0.5 M HNO3 in a 3 to 1 ratio of liquid-to-resin volume for 1 hour, with occasional gentle shaking. After that, the resin beads were separated and washed with water thoroughly, to remove any residual acid, till the pH of the washing became near neutral. This acid treatment turned the resin into a light-colored hydrogen form. This hydrogen form of the resin was air-dried, and stored. It was used as such, without any further pre-treatment, in all the sorption experiments.
Techniques
Optical microscope (OM) images of the RF-XAD beads were recorded in a digital Blue QX5 computer microscope at 10× magnification. Scanning electron microscopy (SEM) was employed to observe the microscopic morphology of the synthesized RF-XAD beads on a CamScan 3200 LV SEM from the United Kingdom. The RF resins were mounted on the aluminum stub using carbon tape and the beads were coated with gold to avoid charging of the sample, using a Quarum (Q150R ES) sputter coater. The image was recorded using an SEI detector. Thermogravimetry (TG) was performed on a Mettler Toledo (TG/DSC STARe System). The mechanical strength of the synthesized RF-XAD beads was tested using a universal testing machine (LRX plus). The Brunauer–Emmett–Teller (BET) surface area of the RF-XAD beads was determined by N2 adsorption–desorption measurements, using a ‘SORPTOMATIC 1990’ analyzer from CE Instruments Italy. Samples were out-gassed at 100 °C in vacuum before the measurement.Sorption studies
134Cs radiotracer (T1/2 = 2.06 years; specific activity = 8.44 Ci mL−1) was procured from the Board of Radiation and Isotope Technology (BRIT), Mumbai, India. The solution was further diluted to the required concentrations, as and when required. The batch capacities of the resin were determined by shaking 0.1 g of RF-XAD resins with 10 mL of the CsNO3 solution of appropriate concentration, containing cesium-134 as a radiotracer. The solutions were stirred well, using a mechanical shaker, for 4 hours, which was found to be sufficient for attaining equilibrium. However, for studying the kinetics of sorption, the equilibration time was varied from 0 to 6.5 hours. All the test solutions containing cesium ions were prepared in 0.1 N NaOH solutions, to maintain similar alkalinity of the solutions. After the equilibration, a small portion of the aqueous phase (1 mL) was separated, and taken for counting gamma activity. The gamma activity measurements were carried out in a well-type NaI (T1) [ECIL] detector, connected to a single-channel analyzer.Equilibrium sorption capacity, qe, was calculated, using eqn (1):

| (1) |
Where C0 is the initial cesium ion concentration, Ce is the equilibrium cesium ion concentration, V is volume of the solution equilibrated with m weight of the sorbent.
Results
Characterization of the RF resin beads
 | ||
| Fig. 1 TGA profile of (a) granular RF particles, (b) XAD beads and (c) RF-XAD beads. | ||
BET surface area
The specific surface area and pore volume of the beads were determined by the BET N2 adsorption method. The nitrogen adsorption desorption isotherms were measured at 77 K and at relative partial pressure (i.e. P/P0) of N2 of 0.98, after degassing the samples at 100 °C for 5 h. The surface area was found to be 170 m2 g−1 and the pore volume was found to be 0.19 cm3 g−1. The pore radius was determined to be ∼20 Å confirming the mesoporous nature of the synthesized beads. The surface area of the blank Amberlite XAD-4 beads, as per specifications, is 750 m2 g−1 with an average pore diameter of 100 Å. The reduction in the surface area and shrinkage of the pore diameter of the template beads suggest the presence of the RF inside the pores also.Mechanical strength testing
Mechanical strength of the synthesized RF-XAD beads was tested and compared with that of the conventional grounded RF gel particles and blank XAD beads, using a universal testing machine, by applying the load in the range of 0–250 N, using a 500 N load cell. XAD blank bead showed the first breakage at the applied load of 2 N and the second breakage at 4 N; at this stage the XAD bead was completely compressed, and no machine extension was observed for even higher applied loads. Spherical RF-XAD beads showed the first break at a load of 13–15 N for different samples tested. This increase in mechanical strength of RF-XAD beads suggests that the RF is present not only on the surface of the XAD template beads, but also it has penetrated in the pores of the XAD beads and solidified. This observation supports the results from the BET surface area analysis. Under similar experimental conditions, the first breakage for the grounded RF gel was observed at 2.5–5 N, and different breakages were seen in the range of 2.5–15 N, depending on the shape and size of the granular RF particles. The irregular-sized grounded gel particles have some sharp and weak edges, which are the most susceptible for breaking under pressure, and results in the generation of a fine powder which leads to column choking. The synthesized RF-XAD beads are spherical in shape and have better mechanical strength as compared to that of the currently in use grounded RF resin gel particles, making them more suitable for column operation.Optical microscope and SEM studies
Fig. 2 represents the optical microscope and SEM images of the XAD and RF-XAD beads, and confirms the coating of RF on the pre-formed XAD beads. The SEM image [Fig. 2(c)] of the outer surface of these RF-XAD beads shows porosity. This porous nature of the beads, which is also corroborated from the results of the TGA study, is a desirable feature, as it will contribute to better sorption behavior. The SEM image of the cross-section of RF XAD beads does not show any distinct boundary or contact between RF coating and the XAD template, indicating that the RF has penetrated inside the XAD-4 beads. This supports the observations from mechanical strength testing studies. | ||
| Fig. 2 Optical microscope image of (a) XAD beads, and (b) RF-XAD beads (10× magnification) and SEM image of (c) surface and (d) cross-section of RF-XAD bead (scale bar 500 nm). | ||
Ion-exchange capacity
About 0.5 g of the H+-form, air-dried RF-XAD resin beads were equilibrated with 100 mL of 0.1 M NaOH solution, containing 5% NaCl. From the amount of NaOH consumed, the total H+–Na+ ion-exchange capacity was found to be 2.35 milliequivalents g−1, i.e. 2.35 mmol g−1 of air-dried, H+-form RF-XAD resin. Capacity of the template XAD-4 beads was also tested using the 134Cs radiotracer and it was confirmed that blank XAD-4 beads do not pick up cesium ions from aqueous solution.Effect of Na+ ion concentration
The competitive effect of Na+ ions on the sorption of Cs+ ions onto the RF-XAD beads was determined, by varying Na+ ion concentration, at a constant Cs+ ion concentration of 0.02 M, at 300 K. The solutions were prepared in 0.1 M NaOH solution, and the concentration of Na+ ions was varied, using NaNO3. The batch distribution coefficient (Kd) was calculated, using eqn (2):
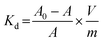
| (2) |
Where A0 and A are the initial and the final activities, respectively, of the solution, V is the volume of equilibrating solution (10 mL), and m is the weight of the resin taken (0.1 g). It is observed that the Kd value for Cs+ ions decreases quite rapidly with increase in Na+ ion concentration (Fig. 3), due to the competitive sorption of Na+ ions onto the available exchange sites.
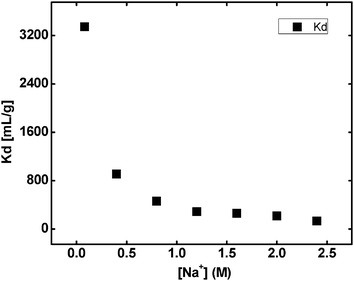 | ||
| Fig. 3 Effect of the Na+ ion concentration on the sorption of Cs+ ions onto the RF-XAD beads at 300 K. | ||
Sorption isotherms
 | ||
| Fig. 4 Effect of the initial cesium ion concentration on the sorption of Cs+ ions onto RF-XAD beads at 300 K. | ||
To evaluate the nature of the sorption, the data were fitted into Langmuir and Freundlich isotherm models. Since the quantity of the cesium sorbed by the sorbent is a function of both the cesium ion concentration and the temperature, the amount of cesium ions sorbed was determined as a function of the initial cesium ion concentration at a constant temperature and at the sorption equilibrium.
Langmuir adsorption isotherm
The Langmuir adsorption isotherm is the simplest isotherm model for sorption of a solute from a liquid solution, and it is valid for monolayer sorption onto a surface containing a finite number of identical active sites.10 It is described by the following equation:
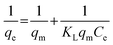
| (3) |
Where qe is the amount of the Cs+ ions sorbed at equilibrium onto the swollen beads, in mmol g−1, qm is the maximum capacity at complete monolayer coverage of the swollen beads (mmol g−1), and KL is the sorption coefficient. The plot of 1/qevs. 1/Ce is a straight line (Fig. 5). The values of qm and KL can be obtained from the intercept and the slope, respectively. The value of correlation coefficient R2 is 0.99815, which indicates a good agreement between the experimental data and the Langmuir sorption model. The maximum sorbing capacity of the RF-XAD beads, for Cs+ ions, is determined to be 2.17 mmol g−1i.e. 287 mg g−1 of the resin. The sorption coefficient, KL, which is related to the apparent energy of sorption for Cs+ ions onto the RF-XAD, is determined to be 0.066 mmol−1. The high sorption capacity of the synthesized beads can be attributed to the high surface area of the RF-XAD beads.
 | ||
| Fig. 5 Langmuir isotherm plot for sorption of Cs+ ions onto RF-XAD beads, at 300 K. | ||
The favorability of sorption of Cs+ ions onto the RF-XAD beads can be expressed in terms of a dimensionless constant, (RL), called separation factor, using the essential features of the Langmuir isotherm, as given below:

| (4) |
The calculated values of RL are less than one (in the range of 0.94 to 0.15), indicating that the RF-XAD beads are good sorbents for Cs+ ions, and the sorption process is favorable for efficient removal of Cs+ ions from alkaline aqueous waste solutions.
Freundlich isotherm
The Freundlich isotherm stipulates that the ratio of the solute sorbed to the solute concentration is a function of the solution concentration. This model allows the existence of a heterogeneous surface, and it assumes that the stronger binding sites are occupied first, and that the binding strength decreases with an increase in the degree of site occupation. The data were also analyzed using the linearized form of Freundlich isotherm, which is given by eqn (5):11

| (5) |
Where KF is the Freundlich constant, indicating sorption capacity, and n is the Freundlich parameter, which is indicative of heterogeneity of the sorbent surface. The plot of log qevs. log Ce is a straight line, as shown in Fig. 6, and from the slope and the intercept of this straight line, the values of 1/n and KF are determined.
 | ||
| Fig. 6 Freundlich isotherm plot for the sorption of Cs+ ions onto the RF-XAD beads, at 300 K. | ||
The value of the correlation coefficient R2 for this plot is 0.93023, which suggests that the agreement between the experimental data and the Freundlich isotherm model is not as good as that of the Langmuir adsorption model. The value of n is 1.378, which lies between 1 and 10, indicating favorable sorption of cesium ions onto RF-XAD beads. The different sorption parameters, obtained by fitting the experimental data into both the models, are given in Table 1.
| Isotherm model | Parameters | |
|---|---|---|
| Langmuir | q m | 2.17 mmol g−1 |
| K L | 0.66 L mmol−1 | |
| R 2 | 0.99815 | |
| Freundlich | K F | 136 mmol g−1 |
| n | 1.38 | |
| R 2 | 0.93023 | |
Sorption kinetics
The kinetics of sorption describes the rate of the metal ion uptake by ion exchange resins, and this rate governs the equilibrium time. The kinetics of a sorbate uptake is required for selecting optimum operating conditions for the full-scale batch process. Therefore, the effect of equilibration time on the sorption of cesium ions, from aqueous solutions, was also studied. The sorption increases with increase in contact time (Fig. 7). | ||
| Fig. 7 The effect of equilibration time on the sorption of Cs+ ions onto the RF-XAD beads, at three different initial concentrations. | ||
Uptake of Cs+ ions is rapid in the initial 2 hours, and the equilibrium is reached in ∼4 hours, indicating a quite fast sorption rate. The initial rapid sorption can be related to the abundant availability of the active sites in the initial stage. Later on, the process becomes relatively slower, and equilibrium conditions are reached within about 4 hours. At this point, the amount of the cesium ions desorbing from the sorbent is in dynamic equilibrium with the amount of the cesium ions being sorbed onto the sorbent. The time required to attain this equilibrium is termed as the equilibrium time, and the amount of cesium ions sorbed at the equilibrium time reflects the maximum sorption capacity of the sorbent under those operating conditions. As the equilibrium stage is attained in 4 hours, further batch experiments, for uptake determination, were carried out at 4 hours of equilibration time. The experimental qe values, at the three studied concentrations of 100, 500 and 1000 μM, are found to be 6.7, 23.8 and 36.8 μmol g−1, respectively.
Kinetics modeling
In order to investigate the sorption process of cesium ions onto RF-XAD beads, three kinetic models, namely pseudo first-order model, pseudo second-order model and intraparticle diffusion model, are used. Table 2 presents the results obtained on fitting experimental data into these models.| Kinetics model | Parameters | C 0 = 100 μM | C 0 = 500 μM | C 0 = 1000 μM |
|---|---|---|---|---|
| Pseudo-1st order | k 1 (h−1) | 0.51 | 0.44 | 0.46 |
| q e (μmol g−1) | 3.33 | 12.54 | 12.08 | |
| R 2 | 0.9653 | 0.9777 | 0.8084 | |
| Pseudo-2nd order | k 2 (g μmol−1 h−1) | 0.27 | 0.06 | 0.08 |
| q e (μmol g−1) | 7.14 | 25.55 | 38.05 | |
| R 2 | 0.9989 | 0.9973 | 0.9982 | |
| Intra-particle diffusion | K id (g μmol−1 h−0.5) | 2.2 | 8.09 | 10.68 |
| I (μmol g−1) | 2.36 | 7.13 | 16.90 | |
| R 2 | 0.9876 | 0.9995 | 0.9818 |

| (6) |
where qt (μmol g−1) is the amount of the cesium ions sorbed at time t, and k1 is the rate constant of the pseudo first-order sorption process (h−1). After integration and applying the boundary conditions, at t = 0, qt = 0, the integrated form of the eqn (6) becomes:

| (7) |
The values of k1 and qe can be determined from the slope and intercept, respectively, of the straight line plot of log[qe − qt] vs. t (Fig. 8).
 | ||
| Fig. 8 Pseudo-first order plots for Cs+ ion sorption onto the RF-XAD beads, at three different initial Cs+ ion concentrations. | ||
The data are fitted with correlation coefficient values in the range 0.81–0.98 (Table 2), which indicates that the rate of sorption of cesium ions onto the RF-XAD beads cannot be explained by the pseudo first-order kinetic model for the entire studied range of the initial cesium ion concentration. The qe values obtained from this model are significantly lower than the experimental qe values of 6.7, 23.8 and 36.8 μmol g−1, respectively, at the three studied initial concentrations of 100, 500 and 1000 μM.
Pseudo second-order model
The pseudo second-order kinetics equation is expressed as follows:13

| (8) |
Where k2 is the rate constant of the pseudo second-order sorption process (g μmol−1 h−1). After integration and applying the similar boundary conditions, eqn (8) can be written as:
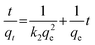
| (9) |
The above equation can be further simplified by substituting h in place of k2qe2 and the equation can be written as:

| (10) |
Where h (mg g−1 min−1) can be considered as the initial sorption rate, when t/qt → 0. From the slope and the intercept of the t/qt vs. t plot (Fig. 9), the values of qe, h and k2 can be determined.
 | ||
| Fig. 9 Pseudo second-order plots for the Cs+ ion sorption onto the RF-XAD beads at three different initial concentrations of Cs+ ions. | ||
The amount of the cesium ions sorbed onto the surface and the equilibrium cesium ion concentration affect the rate of the pseudo second-order reaction significantly. The rate is directly proportional to the number of the active sites on the surface of the sorbent. From Table 2, it is clear that k2 decreases with increase in the initial Cs+ ion concentration. The values of R2 for this model are higher than those for the pseudo first-order kinetic model for all the studied concentrations of cesium. And, the qe values calculated, using this model, are also in good agreement with the actual experimental values. Therefore, this model can be applied to the sorption process in the entire studied concentration range, and we can conclude that the pseudo-second order model explains the kinetics of the process in a better way.
Mechanism
The transport of the sorbate from the solution phase to the sorbent particles is a gradual process, and is complete after several steps.14 These steps are summarized below.•The transport of the sorbate from bulk solution to the outer surface of the sorbent by molecular diffusion, known as external or film diffusion.
•The transport of sorbate from the particle surface into the interior sites i.e. internal diffusion.
•The sorption of the solute particles from the active sites into the interior surfaces of the pores.
From Fig. 10, it is clear that, with increase in contact time, there is an increase in the sorption capacity of the RF-XAD beads for Cs+ ions, which indicates that the sorption process can be intra-particle diffusion controlled. Therefore, such a possibility was explored by using the intra-particle diffusion model. This model is represented by the following equation:15
| qt = Kidt1/2 + I | (11) |
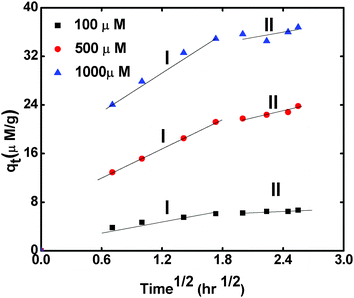 | ||
| Fig. 10 Intra-particle diffusion plots for the Cs+ ion sorption onto RF-XAD beads at three different initial concentrations of Cs+ ions. | ||
Where Kid is the intra-particle diffusion rate parameter and I (μmol g−1) is a constant that gives an idea about the thickness of the boundary layer. If the intra-particle diffusion occurs, then the qt vs. t1/2 plot will be linear, and if this straight line passes through the origin, then only intra-particle diffusion can be considered as the rate limiting step; otherwise, some other mechanism may also be involved simultaneously.
As shown in the Fig. 10, the sorption process can be divided into two stages. In the first stage (indicated by the steep slope portion of the curve), the external surface of the sorbent gets saturated in about 60 min; this stage is called the boundary layer diffusion effect.14 Then, the stage of intra-particle diffusion starts, indicated by the gentle slope portion of the curve, and continues up to 6.5 h. Generally, the slope of stage II is called the intra-particle diffusion rate constant Kid. The value of Kid is calculated from the slope of the second stage, and its value for the studied system increases with increase in the initial concentration from 100 to 1000 μmol L−1. The I value also increases with increase in the initial cesium ion concentration. In order to predict whether sorption proceeds via the film diffusion or intra-particle diffusion mechanism, the kinetic data were further analyzed, using the kinetic expression given by Boyd, eqn (12).16
| Bt = −0.4977 − ln[1 − F] | (12) |
Where F is the ratio qt/qe and Bt is a constant. On plotting the calculated values of Bt vs. t, as per Boyd's equation, the fitted lines, for all the concentrations studied, do not pass through the origin, indicating that the sorption process is mainly governed by the external mass transport, and intra-particle diffusion may not be the only rate controlling step in the removal of the sorbate (Fig. 11).
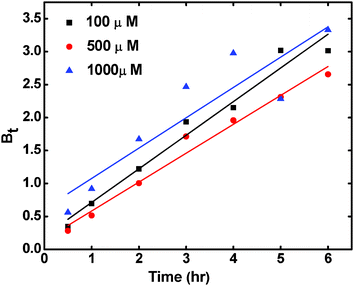 | ||
| Fig. 11 Boyd's plot for Cs+ ion sorption onto the RF-XAD beads at three different initial concentrations of Cs+ ions. | ||
Conclusions
The present study shows that the RF-XAD composite beads can be easily synthesized by coating RF onto XAD beads, and can be used as sorbent for the removal of cesium ions from nuclear waste solutions. The equilibrium data follow the Langmuir isotherm, confirming the monolayer coverage of cesium ions onto the composite beads. The maximum monolayer capacity is 287 mg g−1. Among the different kinetics equations applied, the kinetics data fit well into a pseudo second-order kinetics model. Analysis of the mechanistic steps involved in the sorption process reveals the complex nature of the sorption process. From the intra-particle diffusion model, it can be inferred that the process mechanism consists of both surface sorption and pore diffusion.Acknowledgements
The author, Charu Dwivedi, is grateful to BRNS, Department of Atomic Energy, for awarding a research fellowship. The authors are thankful to Dr. H. S. Sodaye for SEM experiments and Dr. S. K. Mukerjee and Dr. K. T. Pillai for BET surface area analysis. The authors also wish to acknowledge Dr. T. Mukherjee and Dr. S. K. Sarkar, for their encouragement during the course of the study.References
- K. Stark and H. B. L. Pettersson, Radiat. Environ. Biophys., 2008, 47, 481 CrossRef CAS.
- M. Panagiotis, Microporous Mesoporous Mater., 2011, 144, 15 CrossRef.
- H. Sepehrian, S. J. Ahmadi, S. Waqif-Husain, H. Faghihian and H. Alighanbari, J. Hazard. Mater., 2010, 176(1–3), 252–256 CrossRef CAS.
- A. Nilchi, M. G. Maragheh and A. R. Khanchi, Sep. Sci. Technol., 1999, 34, 1833 CrossRef CAS.
- T. Moller, R. Harjula and A. Paajanen, Sep. Sci. Technol., 2003, 38, 2995 CrossRef.
- S. P. Mishra and Vijaya, Sep. Purif. Technol., 2007, 54, 10 CrossRef.
- S. Inan and Y. Altas, Sep. Sci. Technol., 2010, 45, 269 CrossRef CAS.
- N. M. Hassan and K. Adu-Wusu, Solvent Extr. Ion Exch., 2005, 23, 375 CrossRef CAS.
- S. K. Samanta, M. Ramaswamy and B. M. Misra, Sep. Sci. Technol., 1992, 27, 255 CrossRef CAS.
- K. R. Hall, L. C. Eagleton, A. Acrivos and T. Vermeulen, Ind. Eng. Chem. Fundam., 1966, 5, 212 CAS.
- D. Das, N. Das and L. Mathew, J. Hazard. Mater., 2010, 184, 765 CrossRef CAS.
- Y. S. Ho, Scientometrics, 2004, 59, 171 CrossRef CAS.
- H. Yuh-Shan, Water Res., 2006, 40, 119 CrossRef.
- B. K. Singh and N. S. Rawat, J. Chem. Technol. Biotechnol., 1994, 61, 57 CrossRef CAS.
- F.-C. Wu, R.-L. Tseng and R.-S. Juang, Chem. Eng. J., 2009, 153, 1 CrossRef CAS.
- M. Sankar, G. Sekaran, S. Sadulla and T. Ramasami, J. Chem. Technol. Biotechnol., 1999, 74, 337 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
