Efficient and environmentally-benign three-component synthesis of quinolines and bis-quinolines catalyzed by recyclable potassium dodecatungstocobaltate trihydrate under microwave irradiation†
Salma
Anvar
a,
Iraj
Mohammadpoor-Baltork
*a,
Shahram
Tangestaninejad
*a,
Majid
Moghadam
a,
Valiollah
Mirkhani
a,
Ahmad Reza
Khosropour
a and
Reza
Kia
b
aCatalysis Division, Department of Chemistry, University of Isfahan, Isfahan 81746-73441, Iran. E-mail: imbaltork@sci.ui.ac.ir; stanges@sci.ui.ac.ir; Fax: +98(311)6689732; Tel: +98(311) 7932705
bDepartment of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran
First published on 25th July 2012
Abstract
Microwave-assisted one-pot three-component reaction between aromatic amines, aromatic aldehydes and phenylacetylene is accomplished efficiently in the presence of catalytic amounts of potassium dodecatungstocobaltate trihydrate (K5CoW12O40·3H2O) to afford the corresponding quinolines and bis-quinolines in excellent yields. Selective conversion of aromatic aldehyde and also arylacetylene to their corresponding quinolines in the presence of aliphatic aldehyde and alkylacetylene, respectively, can be considered as noteworthy advantages of this method and makes it a useful and attractive process for the synthesis of quinoline derivatives. The catalyst could be reused for several cycles without any significant loss of its catalytic activity.
Introduction
Multi-component reactions (MCR) are some of the most efficient and convenient synthetic tools for the construction of a wide variety of complex molecules. Generally, these reactions occur between three or more starting materials in one-pot to give the desired products with high atom economy and selectivity. Moreover, the use of multi-component reactions under solvent-free conditions provides even more benign processes.1Quinoline derivatives have long been the focus of considerable attention due to their abundance in numerous natural products as well as their extensive applications in biology and pharmacology.2 A great number of compounds containing quinoline moieties are reported to display antimalarial,3 antiasthmatic,4 antidiabetic,5 antibacterial,6 antiviral,7 and antitumor8 activities. These heterocycles are also useful synthetic building blocks in organic chemistry.9
Because of their wide range of applications in pharmaceutical and synthetic chemistry, various methods for the synthesis of quinoline derivatives have been reported in the literature.10–12 However, some of these synthetic methods suffer from disadvantages such as long reaction times, low yields and tedious work-up processes. Further, the use of toxic organic solvents, formation of side products and the use of corrosive, expensive and non-reusable catalysts limit the usefulness of some of these methods, especially for large scale operation and lead to serious environmental and safety problems. Consequently, the design of an efficient and green approach using a reusable catalyst for the synthesis of quinoline derivatives is in great demand.
Polyoxometalates (POMs) as metal–oxygen cluster anions, are remarkable for their molecular and electronic structural diversity and their significance in quite diverse disciplines, e.g., catalysis, medicine, and materials science. Catalysis by heteropoly acids (HPAs) and related POM compounds has several advantages such as low toxicity, non-corrosive, ease of handling, low cost, stability, reusability, and fewer disposal problems which make them economically and environmentally attractive.13 In addition, they are efficient oxidants and exhibit fast reversible multielectron redox transformations under mild conditions.14
Since publication of Chester's work in 1970, CoIIIW125− and related POM anions have been used as well-defined outer-sphere electron-transfer agents. On the other hand, the potential utility of numerous POM anions as electron-transfer agents in the selective catalytic oxidations of inorganic and organic substrates of practical importance has been recognized.15
The environmental problems mainly associated with the handling and disposal of inorganic acids, and their potential hazards, have attracted chemists attention to the development of alternative processes using novel catalysts. Among them, heteropoly compounds are useful and versatile catalysts for a number of important synthetic transformations.16,17
In continuation of our research directed to develop new and novel synthetic routes for important heterocyclic compounds and use of green chemistry techniques in organic synthesis,18 we would like to report a microwave-assisted one-pot three-component synthesis of 2,4-disubstituted quinolines and bis-quinolines through the reaction between aromatic amines, aromatic aldehydes and phenylacetylene catalyzed by K5CoW12O40·3H2O under solvent-free conditions (Scheme 1). To the best of our knowledge, this is the first report on the use of POM as catalyst for the one-pot three-component synthesis of quinolines and bis-quinolines under microwave irradiation.
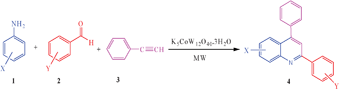 | ||
| Scheme 1 One-pot three-component synthesis of quinolines. | ||
At first, 4-nitroaniline was treated with 4-methylbenzaldehyde and phenylacetylene in the presence of different heteropoly acids and/or POMs under microwave irradiation19 (Table 1, entries 1–9); K5CoW12O40·3H2O was found to be the most effective catalyst to carry out this conversion (Table 1, entry 9). To examine the effect of the catalyst, the reaction was performed in the absence of K5CoW12O40·3H2O, but no desired product was obtained under these conditions (Table 1, entry 10). Then different reaction conditions such as the amount of catalyst, temperature, reaction time and the microwave power were checked (Table 1, entries 11–18). The results revealed that the best yield of the desired quinoline 4b was obtained by carrying out the reaction with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 molar ratios of 4-nitroaniline, 4-methylbenzaldehyde, phenylacetylene and K5CoW12O40·3H2O using a power of 800 W at 90 °C within 10 min under solvent-free conditions. No further yield increase was evidenced by increasing these parameters, while by reducing these parameters the yield of the product was reduced. It was also found that a significantly lower yield (35%) of 4b was obtained using conventional heating rather than the MW activated method even after 4 h. (Table 1, entry 19). Therefore, MW-assisted synthesis was more advantageous over conventional heating with regard to the reaction time and product yield.
0.11 molar ratios of 4-nitroaniline, 4-methylbenzaldehyde, phenylacetylene and K5CoW12O40·3H2O using a power of 800 W at 90 °C within 10 min under solvent-free conditions. No further yield increase was evidenced by increasing these parameters, while by reducing these parameters the yield of the product was reduced. It was also found that a significantly lower yield (35%) of 4b was obtained using conventional heating rather than the MW activated method even after 4 h. (Table 1, entry 19). Therefore, MW-assisted synthesis was more advantageous over conventional heating with regard to the reaction time and product yield.
| Entry | Catalyst (mol%) | T/°C | Time (min) | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: amine (1 mmol), aldehyde (1 mmol), phenylacetylene (1 mmol), MW irradiation (800 W). b Isolated yield. c Reaction was carried out with microwave power of 700 W. d Reaction was carried out with microwave power of 900 W. e Reaction was carried out under conventional heating conditions. | ||||
| 1 | AlPW12O40 (11) | 90 | 10 | 35 |
| 2 | AlPMo12O40 (11) | 90 | 10 | 30 |
| 3 | H3PW12O40 (11) | 90 | 10 | 70 |
| 4 | H3PMo12O40 (11) | 90 | 10 | 55 |
| 5 | H4SiW12O40 (11) | 90 | 10 | 43 |
| 6 | TBA5[PW11NiO39]·3H2O (11) | 90 | 10 | 7 |
| 7 | TBA5[PW11CuO39]·3H2O (11) | 90 | 10 | 12 |
| 8 | (NH4)8CeW10O36·20H2O (11) | 90 | 10 | 72 |
| 9 | K5WCo12O40·3H2O (11) | 90 | 10 | 92 |
| 10 | No catalyst | 90 | 120 | 0 |
| 11 | K5WCo12O40·3H2O (10) | 90 | 10 | 82 |
| 12 | K5WCo12O40·3H2O (12) | 90 | 10 | 92 |
| 13 | K5WCo12O40·3H2O (11) | 90 | 15 | 92 |
| 14 | K5WCo12O40·3H2O (11) | 90 | 7 | 70 |
| 15 | K5WCo12O40·3H2O (11) | 80 | 10 | 72 |
| 16 | K5WCo12O40·3H2O (11) | 100 | 10 | 92 |
| 17c | K5WCo12O40·3H2O (11) | 90 | 10 | 75 |
| 18d | K5WCo12O40·3H2O (11) | 90 | 10 | 92 |
| 19e | K5WCo12O40·3H2O(11) | 90 | 240 | 35 |
In order to evaluate the versatility of this catalytic system, the reaction of various anilines and aldehydes with phenylacetylene was investigated in the presence of catalytic amounts of K5CoW12O40·3H2O under the optimized conditions.20 As shown in Table 2, anilines and aldehydes containing both electron-donating and electron-withdrawing groups took part in the reaction smoothly to give the desired quinolines in excellent yields.
| Entry | Amine | Aldehyde | Quinoline | Time (min) | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: amine (1 mmol), aldehyde (1 mmol), phenylacetylene (1 mmol), catalyst (11 mol%), MW irradiation (800 W). b Isolated yield. | |||||
| 1 |

|

|

|
10 | 90 |
| 2 |

|

|

|
10 | 92 |
| 3 |

|

|
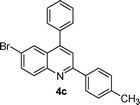
|
5 | 95 |
| 4 |

|

|

|
10 | 93 |
| 5 |

|

|
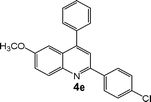
|
15 | 92 |
| 6 |

|

|
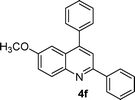
|
15 | 90 |
| 7 |

|
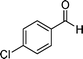
|

|
10 | 94 |
| 8 |
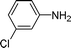
|
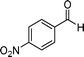
|

|
15 | 87 |
| 9 |
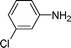
|

|
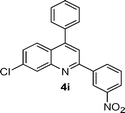
|
10 | 90 |
| 10 |

|

|

|
10 | 94 |
| 11 |

|
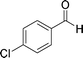
|

|
10 | 92 |
| 12 |

|

|

|
15 | 90 |
| 13 |
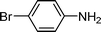
|

|
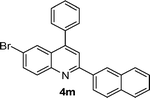
|
10 | 95 |
| 14 |

|

|

|
10 | 92 |
| 15 |

|

|
|
10 | 96 |
| 16 |

|

|

|
5 | 95 |
| 17 |

|

|

|
15 | 95 |
| 18 |

|

|

|
15 | 98 |
It is worth mentioning that diamines such as 1,4-phenylenediamine and dialdehydes such as terephthalaldehyde underwent the reaction under the same conditions and generated the corresponding bis-quinolines in high yields (Table 3). The synthesis of bis-quinolines through a one-pot three-component reaction has not been previously reported.
| Entry | Amine | Aldehyde | Bis-quinoline | Time (min) | Yield (%)a |
|---|---|---|---|---|---|
| a Isolated yield. b Diamine (1 mmol), aldehyde (2 mmol), phenylacetylene (2 mmol), catalyst (22 mol%), MW irradiation (800 W). c Amine (2 mmol), dialdehyde (1 mmol), phenylacetylene (2 mmol), catalyst (22 mol%), MW irradiation (800 W). | |||||
| 1b |

|

|

|
15 | 95 |
| 2b |

|

|

|
15 | 80 |
| 3c |

|

|

|
20 | 90 |
| 4c |

|

|
|
15 | 98 |
| 5c |

|

|

|
15 | 84 |
In contrast, an alkylacetylene such as 1-hexyne did not give the desired product under the same reaction conditions. In addition, an aliphatic aldehyde like heptanal did not undergo such a reaction. These observations prompted us to explore the chemoselectivity of the presented method in the synthesis of quinoline derivatives. When an equimolar mixture of phenylacetylene and 1-hexyne was treated with 4-nitroaniline and 4-methylbenzaldehyde in the presence of the catalyst under microwave irradiation, only phenylacetylene was chemoselectively transformed to its corresponding quinoline with excellent yield (Scheme 2). Furthermore, 4-methylbenzaldehyde was converted to its quinoline in the presence of heptanal with excellent chemoselectively under the same reaction conditions (Scheme 3). Such selectivities are of practical importance and can be considered as one of the significant aspects of this methodology.
 | ||
| Scheme 2 Competitive reaction of arylacetylene in the presence of alkylacetylene for the synthesis of quinoline derivatives. | ||
 | ||
| Scheme 3 Competitive reaction of arylaldehyde in the presence of aliphatic aldehyde for the synthesis of quinoline derivatives. | ||
Recently, we have reported the synthesis of 2,4-disubstituted quinolines by the reaction of 2-aminoaryl ketones and arylacetylenes catalyzed by K5CoW12O40·3H2O in high yields.18d A limitation of our previous work is that a wide variety of 2-aminoaryl ketones are not easily accessible. In contrast, a wide variety of anilines and aldehydes (both substitutionally and structurally) are used in the present work, which can afford different quinoline derivatives. Moreover, ready availability of the diamines and dialdehydes enables preparation of bis-quinolines, that were not synthesizable in our pervious work due to the unavailability of the necessary bis(2-aminoaryl ketone) precursors. Therefore, this one-pot three-component reaction is superior to our previously reported method for the preparation of quinolines and bis-quinolines.
The structure of the products was characterized by their IR, mass, 1H NMR and 13C NMR spectra and elemental analysis.
To investigate the electronic effects of different substituents on the crystal packing, the solid state structures of 4p (Fig. 1, CCDC 841685) and 4q (Fig. 2, CCDC 841686), which both contain an anthracene ring, were also determined by X-ray crystallography. The crystal packing of 4p (Fig. 3) is stabilized by the intermolecular C–H⋯O hydrogen bonds which connect the molecules into individual centrosymmetric dimers. The interesting features of the crystal packing of 4q (Fig. 4) are the short intermolecular C⋯Cl [C11⋯Cl1i = 3.376(4) Å, (i) symmetry code: 1 + x, y, z] contacts which are shorter than the sum of the van der Waals radii of these atoms [3.45 Å; C = 1.70 and Cl = 1.75 Å]21 and also the weak intermolecular C–H⋯Cl interactions which connect the molecules like a tetramer, propagating along the a-axis of the unit cell. In spite of the presence of different aromatic rings, there are no π–π interactions in both 4p and 4q due to the long distances between the centroids of the rings.
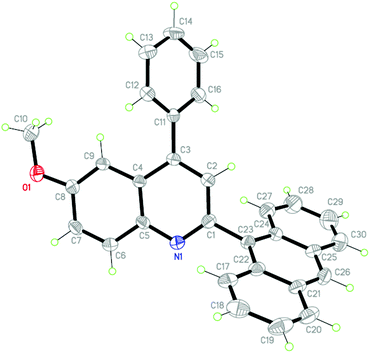 | ||
| Fig. 1 The molecular structure of 4p, showing 30% probability displacement ellipsoids and the atomic numbering. | ||
 | ||
| Fig. 2 The molecular structure of 4q, showing 30% probability displacement ellipsoids and the atomic numbering. | ||
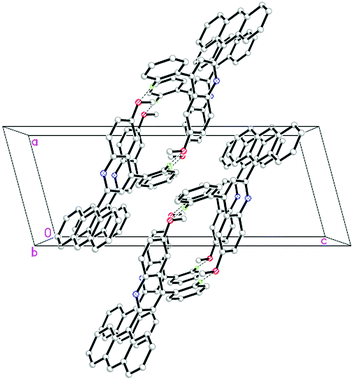 | ||
| Fig. 3 The crystal packing of 4p, showing individual dimer formation through the intermolecular C–H⋯O hydrogen bonds. | ||
 | ||
| Fig. 4 The crystal packing of 4q, showing tetramer formation through the intermolecular short C–Cl contacts and the weak intermolecular C–H⋯Cl interactions. | ||
To check the reaction pathway of the K5CoW12O40·3H2O catalyzed three-component synthesis of quinolines, different experiments were performed. First, a model reaction was carried out in the presence of acrylamide as a radical scavenger under MW irradiation; no desired product was obtained under these conditions. This is a good indication for the presence of radical species in the reaction. On the other hand, the intermediate N-arylimine prepared by the reaction of amine with aldehyde, was treated with phenylacetylene in the presence of K5CoW12O40·3H2O under MW irradiation. Under these conditions, the corresponding quinolines were obtained in excellent yield. On the basis of these experimental results and together with the previously reported works,15,22 it is reasonable to assume that K5CoW12O40·3H2O acts as a one-electron transfer catalyst. Accordingly, a plausible mechanism as shown in Scheme 4 is proposed. At first the intermediate imine 6 is formed by the condensation of amine 1 with aldehyde 2. Then transferring an electron from phenylacetylene 3 to Co(III) results in a radical cation intermediate 7 and Co(II) species. (On the basis of observed regiochemistry in the products, it seems that positive charge at benzylic carbon and single electron at primary carbon of radical cation is more suitable. It is also important to note that the observed regiochemistry in products is confirmed unambiguously by X-ray analysis of products 4p and 4q.) Nucleophilic attack of imine 6 on radical cation 7 provides 8. One-electron transfer from Co(II) to 8 and subsequent prototropic shift from nitrogen to carbon gives 9. The intermediate 9 is transformed to dihydroquinoline 10via an electrocyclic reaction followed by prototropic shift from carbon to nitrogen. Finally, aromatization of 10 under ambient atmosphere in the presence of catalyst afforded the corresponding quinoline 4 and releases the Co(III) species for the next catalytic cycle.
 | ||
| Scheme 4 Plausible reaction pathway for the three-component synthesis of quinolines. | ||
In order to show the actual role of acrylamide, which acts as a radical scavenger or undergoes a Michael addition with aniline, the reaction of acrylamide with aniline in the presence of the catalyst was investigated under MW irradiation and no Michael adduct was obtained under these conditions. After addition of aldehyde to the reaction mixture, the imine was formed. While upon addition of phenylacetylene to this reaction mixture, no quinoline was produced. It is also noteworthy that, no desired quinoline was obtained when 2,6-di-tert-butylphenol was applied as radical scavenger in the model reaction. All these findings showed that the proposed mechanism is reasonable.
The recyclability and reusability of the catalyst are very important for commercial and industrial applications and are also highly preferable for a green process. In this respect, the reusability of the catalyst was investigated in the reaction of 4-nitroaniline, 4-methylbenzaldehyde and phenylacetylene. After completion of the reaction, the mixture was cooled to room temperature and diluted with cold EtOH. The catalyst was easily recovered by simple filtration and then reused for subsequent reactions. As shown in Fig. 5, the catalyst can be reused at least five times without any significant loss in chemical yield of the product.
 | ||
| Fig. 5 Recycling of the catalyst in the synthesis of 4b. | ||
On the other hand, the possibility of the presence of metal in the final product was checked to ensure that the final product is free of metal. In this manner, the filtrates were used for determination of the amounts of W or Co which may leached from catalyst by ICP. No metal was detected in the final product which confirms the green nature of the present method.
In conclusion, an efficient, selective and green one-pot synthesis of quinolines and bis-quinolines has been developed under microwave irradiation and solvent-free conditions. High yield, neat conditions, short reaction times, environmentally-friendly experimental procedures, and the avoidance of toxic organic solvents are the notable advantages of this method. Besides, the catalyst recovery and reusability is another feature of this procedure. Thus, this work represents a valuable complement to the existing procedures for the synthesis of quinoline derivatives.
Acknowledgements
The authors are grateful to the Center of Excellence of Chemistry and also the Research Council of the University of Isfahan for financial support of this work.References
- (a) J. Zhu and H. Bienayme, Multicomponent Reactions, Wiley-VCH: Weinheim, 2005 Search PubMed; (b) V. V. Kouznetsov, Tetrahedron, 2009, 65, 2721 CrossRef CAS.
- P. M. S. Chauhan and S. K. Srivastava, Curr. Med. Chem., 2001, 8, 1535 CAS.
- A. Sparatore, N. Basilico, M. Casagrande, S. Parapini, D. Taramelli, R. Brun, S. Wittlin and F. Sparatore, Bioorg. Med. Chem. Lett., 2008, 18, 3737 CrossRef CAS.
- D. Doube, M. Blouin, C. Brideau, C. Chan, C. Desmarais, D. Ethier, J. P. Falgueyret, R. W. Friesen, M. Girard, Y. Girard, J. Guay, P. Tagari and R. N. Young, Bioorg. Med. Chem. Lett., 1998, 8, 1255 CrossRef.
- D. Edmont, R. Rocher, C. Plisson and J. Chenault, Bioorg. Med. Chem. Lett., 2000, 10, 1831 CrossRef CAS.
- K. Yearick, K. Ekoue-Kovi, D. P. Iwaniuk, J. K. Natarajan, J. Alumasa, A. C. de Dios, P. D. Roepe and C. Wolf, J. Med. Chem., 2008, 51, 1995 CrossRef CAS.
- D. Narsinh and S. Anamik, Ind. J. Pharm. Sci., 2001, 63, 211 Search PubMed.
- A. G. Myers, N. J. Tom, M. E. Fraley, S. B. Cohen and D. J. Madar, J. Am. Chem. Soc., 1997, 119, 6072 CrossRef CAS.
- Y. L. Chen, K. C. Fang, J.-Y. Sheu, S. L. Hsu and C. Tzeng, J. Med. Chem., 2001, 44, 2374 CrossRef CAS.
- (a) O. D. De Paolis, L. Teixeira and B. Török, Tetrahedron Lett., 2009, 50, 2939 CrossRef CAS; (b) J. Quiroga, J. Trilleras, B. Insuasty, R. Abonia, M. Negueras, A. Marchal and J. Cobo, Tetrahedron Lett., 2010, 51, 1107 CrossRef CAS; (c) N. D. Heindel, T. A. Brodof and J. E. Kogelschatz, J. Heterocycl. Chem., 1996, 3, 222 CrossRef; (d) K. A. Reynolds, D. J. Young and W. A. Loughlin, Synthesis, 2010, 3645 CAS; (e) S. Rotzoll, B. Willy, J. Schoenhaber, T. J. Mueller and F. Rominger, Eur. J. Org. Chem., 2010, 3516 CrossRef CAS; (f) R. Leardini, D. Nanni, A. Tundo, G. Zanardi and F. Ruggieri, J. Org. Chem., 1992, 57, 1843 CrossRef.
- (a) A. Arcadi, M. Chiarini, S. Di Giuseppe and F. Marinelli, Synlett, 2003, 203 CrossRef CAS; (b) B. Das, K. Damodar, N. Chowdhury and R. A. Kumar, J. Mol. Catal. A: Chem., 2007, 274, 148 CrossRef CAS; (c) A.-H. Li, D. J. Beard, H. Coate, A. Honda, M. Kadalbajoo, A. Kleinberg, R. Laufer, K. M. Mulvihill, A. Nigro, P. Rastogi, D. Sherman, K. W. Siu, A.G. Steinig, T. Wang, D. Werner, A. P. Crew and M. J. Mulvihill, Synthesis, 2010, 1678 CrossRef CAS; (d) K. C. Lekhok, D. Prajapati and R. C. Boruah, Synlett, 2008, 655 CAS; (e) R. Sarma and D. Prajapati, Synlett, 2008, 3001 CAS; (f) R. P. Korivi and C. Cheng, J. Org. Chem., 2006, 71, 7079 CrossRef CAS.
- (a) H. M. Mack, E. A. Davis, B. Kadkhodayan, R. A. Taylor, D. C. Duncan and C. F. Beam, J. Heterocycl. Chem., 1987, 24, 1733 CrossRef CAS; (b) A. Kulkarni and B. Török, Green Chem., 2010, 12, 875 RSC; (c) S. K. Guchhait, K. Jadeja and C. Madaan, Tetrahedron Lett., 2009, 50, 6861 CrossRef CAS; (d) A. Kumar and V. K. Rao, Synlett, 2011, 2157 CrossRef CAS; (e) M. S. Reddy, N. Thirupathi and Y. Kiran, RSC Adv., 2012, 2, 3986 RSC.
- (a) N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS; (b) I. V. Kozhevnikov, J. Mol. Catal. A: Chem., 2007, 262, 86 CrossRef CAS; (c) H. Firouzabadi and A. A. Jafari, J. Iran. Chem. Soc., 2005, 2, 85 CrossRef CAS.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171 CrossRef CAS.
- (a) A. W. Chester, J. Org. Chem., 1970, 35, 1797 CrossRef CAS; (b) I. A. Weinstock, Chem. Rev., 1998, 98, 113 CrossRef CAS.
- (a) W. Zhao, Y. Zhang, B. Ma, Y. Ding and W. Qiu, Catal. Commun., 2010, 11, 527 CrossRef CAS; (b) S. Tangestaninejad, V. Mirkhani, M. Moghadam, I. Mohammadpoor-Baltork, E. Shams and H. Salavati, Ultrason. Sonochem., 2008, 15, 438 CrossRef CAS; (c) I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad, V. Mirkhani, K. Mohammadiannejad-Abbasabadi and H. R. Khavasi, Eur. J. Org. Chem., 2011, 1357 CrossRef CAS; (d) M. H. Habibi, S. Tangestaninejad, I. Mohammadpoor-Baltork, V. Mirkhani and B. Yadollahi, Tetrahedron Lett., 2001, 42, 2851 CrossRef CAS; (e) V. Kozhevnikov, Catalysis by Polyoxometalates. Catalysis for Fine Chemical Synthesis, Vol. 2, Wiley: UK, Chichester, 2002 Search PubMed.
- (a) L. Nagarapu, S. Apuri and S. Kantevari, J. Mol. Catal. A: Chem., 2007, 266, 104 CrossRef CAS; (b) D. Sloboda-Rozner and R. Neumann, Green Chem., 2006, 8, 679 RSC; (c) L. Nagarapu, M. Baseeruddin, S. Apuri and S. Kantevari, Catal. Commun., 2007, 8, 1729 CrossRef CAS; (d) E. Rafiee and S. Eavani, Green Chem., 2011, 13, 2116 RSC.
- (a) I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad, V. Mirkhani and S. F. Hojati, Polyhedron, 2008, 27, 750 CrossRef CAS; (b) Z. Khorsandi, A. R. Khosropour, V. Mirkhani, I. Mohammadpoor-Baltork, M. Moghadam and S. Tangestaninejad, Tetrahedron Lett., 2011, 52, 1213 CrossRef CAS; (c) I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad, V. Mirkhani and Z. Eskandari, J. Heterocycl. Chem., 2011, 48, 479 CrossRef CAS; (d) I. Mohammadpoor-Baltork, S. Tangestaninejad, M. Moghadam, V. Mirkhani, S. Anvar and A. Mirjafari, Synlett, 2010, 3104 CrossRef CAS; (e) S. Safaei, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, S. Tangestaninejad, V. Mirkhani and R. Kia, RSC Adv., 2012, 2, 5610 RSC.
- The microwave system used in these experiments includes the following items: Micro-SYNTH labstation, equipped with a glass door, a dual magnetron system with pyramid-shaped diffuser, 1000 W delivered power, exhaust system, magnetic stirrer, “quality pressure” sensor for flammable organic solvents, and a ATCFO fibre optic system for automatic temperature control.
- Microwave-assisted synthesis of quinolines; General procedure: A mixture of arylamine 1 (1 mmol) and arylaldehyde 2 (1 mmol) was irradiated for 30 s and then phenylacetylene 3 (1 mmol) and K5WCo12O40·3H2O (11 mol%) were added. The resulting mixture was subjected to microwave irradiation under solvent-free conditions for the appropriate time according to Table 2. The progress of the reaction was monitored by TLC (eluent: n-hexane–ethyl acetate: 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After completion of the reaction, the mixture was cooled to room temperature and EtOH (20 ml) was added. The residue was filtered, the filtrate was evaporated and the crude product was purified by recrystallization from EtOH to give the pure product in 87–98% yields..
3). After completion of the reaction, the mixture was cooled to room temperature and EtOH (20 ml) was added. The residue was filtered, the filtrate was evaporated and the crude product was purified by recrystallization from EtOH to give the pure product in 87–98% yields.. - A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- (a) E. Rafiee, S. Tangestaninejad, M. H. Habibi and V. Mirkhani, Bioorg. Med. Chem. Lett., 2004, 14, 3611 CrossRef CAS; (b) S. K. Saha, M. Ali and P. Banerjee, Coord. Chem. Rev., 1993, 122, 41 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference numbers 841685 and 841686. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra20639k |
| This journal is © The Royal Society of Chemistry 2012 |
