Synthesis of fluorine-doped multi-layered graphene sheets by arc-discharge
Baoshou
Shen
ab,
Jiangtao
Chen
a,
Xingbin
Yan
*a and
Qunji
Xue
a
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, China. Fax: +86 931 4968055; Tel: +86 931 4968055E-mail: xbyan@licp.cas.cn
bSchool of Science, Lanzhou University of Technology, Lanzhou, 730050, China
First published on 13th June 2012
Abstract
Fluorine-doped graphene sheets (F-doped GSs) were synthesized by arc discharge. The products were characterized by scanning and transmission electron microcopies, X-ray diffraction, Raman and X-ray photoelectron spectroscopies. The F-doped GSs contain about 10 wt% F. They are mainly multi-layered, with a much larger size than pure GSs, and are super-hydrophobic.
Graphene, a two-dimensional single atom thick layer of carbon, has extraordinary physical properties. Due to its extensive potential applications in many areas, a large scale production of graphene is urgently needed and has attracted considerable attention of researchers. Up to now, many methods have been developed to prepare graphene, mainly including micromechanical cleavage, chemical vapor deposition, epitaxial growth, chemical exfoliation, chemical conversion and arc discharge. Among them, arc discharge is a fast and easy technique to prepare few layer graphene sheets (GSs).1–3 Also, GSs and carbon nanotubes can be simultaneously synthesized and separated in the magnetic field-enhanced arc discharge.4 Furthermore, nitrogen- and boron-doped GSs have been prepared by arc discharge.5–7 They can display p- and n-type semiconducting behaviors by adjusting doping concentration, and can improve the field emission properties of pure GSs.5,6 There are many other methods for doping graphene, such as nitrogen-doped graphene: this has been prepared via a one-step hydrothermal reaction.8 In addition, Rao and co-workers have reported that few-layer graphene can be reversibly chlorinated or brominated by irradiation with UV light. 9
Graphite fluoride is a covalently-bond compound. It displays good thermal conductivity and stability, low shear strength, and good electrical insulation. Graphite fluoride has extensive applications in primary lithium cells and solid lubricants. Recently, graphene fluoride, one of the thinnest binary compounds is expected to retain the interesting electrochemical and electronic properties of the graphite fluoride. It may be useful in new technological applications pertinent to batteries or wide-band gap semiconductors.10,11 Fluorinated graphene has been synthesized using liquid-phase exfoliation of graphite fluoride12 and fluorination of graphene by xenon difluoride.13 In addition, it is an excellent insulator with high thermal and chemical stability.14 However, the above preparation methods are time consuming, and cannot be used to prepare fluorinated graphene in large scale. In this paper, we first report a rapid and simple technique, using a direct current arc discharge method, to prepare fluorine (F)-doped multi-layered GSs.
The arc-discharge process for preparing GSs was carried out in a water-cooled chamber and graphite rods with purity of 99.99% were used as the cathode and anode. After the pressure in the chamber reached 1 Pa, the chamber was filled by hydrogen (200 Torr) and helium (200 Torr). During the discharge, the current was maintained at 140 A. For preparing F-doped GSs, a hollow graphite rod filled with powdery graphite fluoride (content of fluorine: 60 wt%) was used as the anode. The as-obtained powders were collected only in the inner and top wall of the chamber in order to exclude relatively heavy products (such as unexfoliated graphite or graphite fluoride) dropped to the bottom of the chamber during the arc discharge process. The morphologies of the products were studied by field emission scanning electron microscopy (FE-SEM, JSM-6701F) and transmission electron microscopy (HRTEM, F-30). The thickness and lateral size of products were determined using an atomic force microscope (AFM, Nanoscope III, Digital Instruments Co.) in tapping mode under ambient conditions. In order to investigate the structure and composition of the samples, X-ray diffraction (XRD, Philips X'Pert Pro.) was carried out, and Raman spectra were recorded using a micro-Raman spectroscopy (JY-HR800, the excitation wavelength of 532 nm). X-Ray photoelectron spectroscope (XPS, Thermon Scientific, UK) was employed to analyze the chemical species. The hydrophobicity of the samples was investigated using a Krüss DSA 100 apparatus. Before the measurements, powdery samples were dispersed in the ethanol using an ultrasonic bath to get a suspension (1 mg ml−1). Then, the upper homogeneous suspension was sprayed onto polished copper substrates to form films. The further detailed experiments were described in our previous report.15 The average contact angle (CA) and sliding angle (SA) values were obtained by measuring the same film at five different positions. The volume of the individual water droplet in all measurements was 5 μl.
Fig. 1 shows a schematic representation of the formation mechanism of F-doped GSs in arc discharge. In the arc discharge process, high temperatures are generated in the arc area, leading to the evaporation of precursor graphite or graphite fluoride. During the subsequent growth of GSs, partial dissociative F atoms and/or ions would react with carbon species as a result of the formation of F-doped GSs.
 | ||
| Fig. 1 Schematic representation of formation mechanism of F-doped GSs in arc discharge. | ||
Fig. 2 shows the morphologies of the F-doped GSs and pure GSs prepared by arc discharge. As shown in Fig. 2 (a), network-like thin sheets have been formed, indicating an efficient exfoliation of the graphite fluoride and the formation of F-doped GSs. In comparison, the GSs with high purity (shown in Fig. 2 (b)) have relatively small-sizes and no impurities such as carbon nanotubes were found. TEM images further verify the distinct difference in the morphology, as shown in Fig. 2 (c) and Fig. 2 (d). Moreover, pure GSs show more wrinkles compared with F-doped GSs. Both F-doped GSs and GSs are transparent, revealing their thinness. High resolution TEM images are shown in Fig. 2 (e) and Fig. 2 (f). It is clearly seen that both F-doped GSs and GSs are mainly multi-layers. The basal plane of the F-doped GS is not straight, and there are wrinkles or ripples on the edges of the F-doped GS, as shown in Fig. 2 (e). Whereas, the basal planes of the pure GS are straight and parallel to each other. A similar phenomenon where the original parallel basal planes become distorted was observed in nitrogen doped graphene.16Fig. 2 (g) and Fig. 2 (h) show the planar spacing line profiles from the marked areas in Fig. 2 (e) and Fig. 2 (f). It can be calculated that the interplanar spacing of F-doped GS (∼0.397 nm) is larger than that of pure GS (∼0.343 nm). It indicates that F atoms have been incorporated in the graphene layers. However, it should be mentioned that the inherent mechanism of the increase of the size for F-doped GSs is still not clear.
 | ||
| Fig. 2 FESEM images for F-doped GSs (a) and pure GSs (b); TEM images for F-doped GSs (c) and pure GSs (d); high resolution TEM images for F-doped GSs (e) and pure GSs (f); (g) and (h) display planar spacing line profiles from marked areas of F-doped GS and GS, respectively. | ||
Representative AFM images of F-doped GSs and pure GSs with the height profiles are shown in Fig. 3. As shown in Fig. 3 (a), the thickness of F-doped GSs is around 2.8 nm, corresponding to multi-layers, and the lateral dimension of F-doped GSs is several microns, which is consistent with the SEM and TEM results. By comparison, as shown in Fig. 3 (b), it is obviously seen that GSs are also multi-layers. However, the lateral size of pure GSs is less than 50 nm, smaller than that of F-doped GSs.
 | ||
| Fig. 3 AFM images for F-doped GSs (a) and pure GSs (b); top: AFM topography images of them. Bottom: height profiles obtained by taking a horizontal cross-section as indicated by the black line with red arrows. | ||
The XRD patterns of graphite fluoride, F-doped GSs and GSs powders are shown in Fig. 4 (a). It is found that the precursor graphite fluoride could be converted to graphene doped with F atoms through the arc discharge process, which is reflected from the disappearing of the diffraction peaks of graphite fluoride. F-doped and undoped GSs both exhibit sharp diffraction peaks corresponding to the (002) plane, indicating the good crystallization properties. For F-doped GSs, the slight shift to a low angle of the (002) peak suggests that the expansion of the interplanar spacing is due to the incorporation of fluorine. Moreover, through calculating from the XRD data, the interplanar spacing of F-doped GSs and GSs are 0.338 and 0.336 nm, respectively. The interspacing calculated from XRD data is smaller than those calculated by TEM data. This is because XRD patterns give the information of the aggregate and the products maybe contain less unexfoliated precursor. Raman spectra of F-doped and undoped GSs are shown in Fig. 4 (b). In both samples, three typical peaks located at 1341, 1572 and 2685 cm−1 are indexed to the D, G and 2D bands, respectively. The intensity ratio of the G band to the D band reflects the crystal quality of GSs. Higher ratio indicates higher crystal quality. It can be inferred that pure GSs show higher crystal quality compared with F-doped GSs, which agrees with the XRD result. The electronic interaction, layer numbers, disorder and crystal quality determine the full-width at half-maximum (FWHM) of 2D peak and the ratio of the G band to the 2D band.16,17 When the GSs are doped by F, this ratio decreases and the FWHM of the D and 2D peaks increases, suggesting that the disorders (or defects) are introduced.
 | ||
| Fig. 4 (a) XRD patterns of powdery graphite fluorite, F-doped GSs and GSs; (b) Raman spectra of F-doped GSs and GSs. | ||
Fig. 5 (a) and Fig. 5 (b) display the typical XPS spectra of the C 1s and F 1s core levels for the F-doped GSs. It can be calculated that F-doped GSs contain about 10 wt% F. The peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond located at 284.3 eV is dominant in the sample. A weak peak centered at 289.2 eV is attributed to the semi-ionic C–F bond (290.2 ± 0.4 eV).18 In addition, as shown from Fig. 5 (b), an obvious F 1s core level peak appears at 688.0 eV. Therefore, the XPS spectra indicate that the F atoms doped in GSs have the ionic characteristic and this may modify the electrical and optical properties of the pristine GSs.
C bond located at 284.3 eV is dominant in the sample. A weak peak centered at 289.2 eV is attributed to the semi-ionic C–F bond (290.2 ± 0.4 eV).18 In addition, as shown from Fig. 5 (b), an obvious F 1s core level peak appears at 688.0 eV. Therefore, the XPS spectra indicate that the F atoms doped in GSs have the ionic characteristic and this may modify the electrical and optical properties of the pristine GSs.
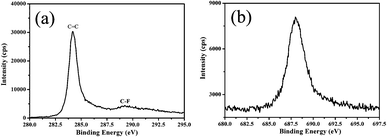 | ||
| Fig. 5 XPS spectra of F-doped GSs: (a) C 1s and (b) F 1s core levels. | ||
Water CA is strongly dependent on the surface chemical state of a material. As shown in Fig. 6, both F-doped GS and GS films exhibit super-hydrophobic properties (CA ≥ 150°). Although the values of the CA are similar, the values of the SA (referred to CA hysteresis) are distinctly different. The SA of F-doped GS film is about 13° while that of pure GS film is about 38°. The less hysteresis, the smaller SA and the better superhydrophobic property. It has been reported that the hysteresis of a superhydrophobic surface depends upon two properties: (1) metastable state energy and (2) barrier energy for the drop to move from one metastable state to another metastable state.19 The values of the SA indicate that the droplet on the F-doped GS film rolls away more easily than that on the pure GS film, which is related to the chemical heterogeneity, contact line topology, roughness, and the wetted fraction of the surface of F-doped GSs.20,21
 | ||
| Fig. 6 Photographs of water droplet shapes on (a) F-doped GS film and (b) GS film. | ||
The arc discharge technique is a simple and efficient approach to prepare F-doped multi-layered GSs. Furthermore, the fluorine-doped graphene can be tailored by using the precursor graphite fluoride with different contents of fluorine. The doping of fluorine may change the properties of GSs. Thus, we believe that such F-doped GSs would have various potential applications, such as batteries, low surface energy coatings and lubricants.
Acknowledgements
The authors acknowledge financial support from the Natural Science Foundation of China (51002161 and 51005225) and the Top Hundred Talents Program of Chinese Academy of Sciences.References
- K. S. Subrahmanyam, L. S. Panchakarla, A. Govindaraj and C. N. R. Rao, J. Phys. Chem. C, 2009, 113, 4257 CAS.
- Y. P. Wu, B. Wang, Y. F. Ma, Y. Huang, N. Li, F. Zhang and Y. S. Chen, Nano Res., 2010, 3, 661 CrossRef CAS.
- I. Levchenko, O. Volotskova, A. Shashurin, Y. Raitses, K. Ostrikov and M. Keidar, Carbon, 2010, 48, 4556 CrossRef.
- O. Volotskova, I. Levchenko, A. Shashurin, Y. Raitses, K. Ostrikov and M. Keidar, Nanoscale, 2010, 2, 2281 RSC.
- U. A. Palnitkar, R. V. Kashid, M. A. More, D. S. Joag, L. S. Panchakarla and C. N. R. Rao, Appl. Phys. Lett., 2010, 97, 063102 CrossRef.
- L. S. Panchakarla, K. S. Subrahmanyam, S. K. Saha, A. Govindaraj, H. R. Krishnamurthy, U. V. Waghmare and C. N. R. Rao, Adv. Mater., 2009, 21, 4726 CAS.
- N. Li, Z. Wang, K. Zhao, Z. Shi, Z. Gu and S. Xu, Carbon, 2010, 48, 255 CrossRef CAS.
- L. Sun, L. Wang, C. Tian, T. Tan, Y. Xie, K. Shi, M. Li and H. Fu, RSC Adv., 201210.1039/C2RA01367C Search PubMed.
- K. Gopalakrishnan, K. S. Subrahmanyam, P. Kumar, A. Govindaraj and C. N. R. Rao, RSC Adv., 2012, 2, 1605 RSC.
- J. C. Charlier, X. Gonze and J. P. Michenaud, Phys. Rev. B, 1993, 47, 16162 CrossRef CAS.
- H. Touhara and F. Okino, Carbon, 2000, 38, 241 CrossRef CAS.
- R. Zbořil, F. Karlický, A. B. Bourlinos, T. A. Steriotis, A. K. Stubos, V. Georgakilas, K. Šafářová, D. Jančík, C. Trapalis and M. Otyepka, Small, 2010, 6, 2885 CrossRef.
- J. T. Robinson, J. S. Burgess, C. E. Junkermeier, S. C. Badescu, T. L. Reinecke, F. K. Perkins, M. K. Zalalutdniov, J. W. Baldwin, J. C. Culbertson, P. E. Sheehan and E. S. Snow, Nano Lett., 2010, 10, 3001 CrossRef CAS.
- R. R. Nair, W. Ren, R. Jalil, I. Riaz, V. G. Kravets, L. Britnell, P. Blake, F. Schedin, A. S. Mayorov, S. Yuan, M. I. Katsnelson, H. M. Cheng, W. Strupinski, L. G. Bulusheva, A. V. Okotrub, I. V. Grigorieva1, A. N. Grigorenko, K. S. Novoselov and A. K. Geim, Small, 2010, 6, 2877 CrossRef CAS.
- B. S. Shen, J. J. Ding, X. B. Yan, W. J. Feng, J. Li and Q. J. Xue, Appl. Surf. Sci., 2012, 258, 4523 CrossRef CAS.
- N. Soin, S. S. Roy, S. Roy, K. S. Hazra, D. S. Misra, T. H. Lim, C. J. Hetherington and J. A. McLaughlin, J. Phys. Chem. C, 2011, 115, 5366 CAS.
- M. Okano, R. Matsunaga, K. Matsuda, S. Masubuchi, T. Machida and Y. Kanemitsu, Appl. Phys. Lett., 2011, 99, 151916 CrossRef.
- T. Nakajima, M. Koh, V. Gupta and K. Lutar, Electrochim. Acta, 2000, 45, 1655 CrossRef CAS.
- B. Balu, V. Breedveld and D. W. Hess, Langmuir, 2008, 24, 4785 CrossRef CAS.
- L. C. Gao and T. J. McCarthy, Langmuir, 2006, 22, 6234 CrossRef CAS.
- J. P. Youngblood and T. J. McCarthy, Macromolecules, 1999, 32, 6800 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
