Formation of a renewable amide, 3-acetamido-5-acetylfuran, via direct conversion of N-acetyl-D-glucosamine†
Marcus W.
Drover
,
Khaled W.
Omari
,
Jennifer N.
Murphy
and
Francesca M.
Kerton
*
Department of Chemistry, Memorial University of Newfoundland, St. John's, NL, Canada. E-mail: fkerton@mun.ca; Fax: +1 709 8643702; Tel: +1 709 8648089
First published on 5th April 2012
Abstract
An effective method for transforming an amino-sugar into an N-substituted furan in an ionic liquid is reported. B(OH)3 significantly improves the yield (60%, 3 min MW heating).
The transformation of biomass into useful chemicals has become an important area of research, as a way to reduce global dependence on fossil fuel resources.1 Conversion of carbohydrates and their derivatives into useful materials is one such area, e.g. production of 5-hydroxymethylfurfural (5-HMF) from glucose and fructose. Of course, as carbohydrates typically contain C, H and O only, products from these processes are typically small molecule oxygenated species. We wondered whether transformations similar to those performed on more typical carbohydrates would be able to produce N-containing molecules from amino-sugars such as N-acetyl-D-glucosamine (NAG). NAG is the monomer unit of the polysaccharide chitin from which it can be obtained. Chitin is naturally abundant and can be found in the shells of crustaceans (e.g. waste from the fishing industry), and exoskeletons of insects. The recently formed American Chemical Society Green Chemistry Institute Formulator's Roundtable has highlighted greener small amines, including those sources from renewable feedstocks, as highly desirable for the consumer products industries.
Ionic liquids (ILs) have been used quite widely in the dehydration of carbohydrates.2 ILs can be considered ‘green’ solvents under certain conditions, as they are normally non-volatile, non-flammable and potentially re-usable reaction media.3 They can also act as catalysts in reactions. Several are known to dissolve cellulose and other sugars, which makes them ideal reaction media for studying the reactivity of hexoses. Previously, 3-acetamido-5-acetylfuran (3A5AF) has been obtained as one of the major products from the thermal degradation of NAG albeit in only 2% yield.4,5 The work reported herein represents a feasible ionic liquid/solution phase method for the direct conversion of NAG to 3A5AF, Fig. 1. From this foundation, the chemistry and transformations of 3A5AF can be studied and potentially lead to new renewable amines in the future. Recent research by others on the formation of “renewable” amines has employed ammonia as the source of nitrogen.6 The work presented here represents a source of biologically-fixed nitrogen in the product and as far as we are aware, this is the first report of its kind.
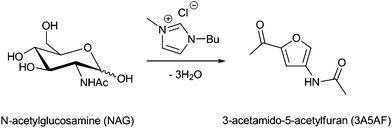 | ||
| Fig. 1 Direct conversion of NAG to 3A5AF using a combination of an imidazolium ionic liquid and microwave heating. | ||
In a typical reaction, 100 mg of NAG and 750 mg of IL were mixed and warmed for 1 min until a homogeneous solution formed. The reaction mixture was then microwave-heated for the appropriate amount of time, an aliquot extracted with ethyl acetate and analyzed via GC-MS, using an internal standard and calibration curve (Fig. S3, ESI†). The dehydration process was first studied at 120 °C, under additive-free conditions, Table 1. Six different ILs were used with various alkyl chains and anions. These included 1-ethyl-3-methylimidazolium bromide ([EMim]Br) and acetate ([EMim]OAc), and 1-butyl-3-methylimidazolium chloride ([BMim]Cl), bromide ([BMim]Br), and acetate ([BMim]OAc), and 1,2-dimethyl-3-butylimidazolium chloride [BMMim]Cl. NAG was found to be readily soluble in all the ionic liquids studied, under the experimental conditions employed. The reaction of NAG in [BMim]Cl at 120 °C gave 14.1% 3A5AF following 3 min of microwave (MW) heating. A 25.5% yield was obtained at 180 °C under the same conditions, but prolonged heating (longer than 3 min) or higher temperatures were found to decrease the yield of product, most likely through decomposition of 3A5AF via accelerated side reactions.
| Entry | Solvent | T/°C | Yield 3A5AF (%)b |
|---|---|---|---|
| a Reaction Conditions : solvent (750 mg), NAG (100 mg, 0.452 mmol), 3 min. b Determined by GC-MS. c Analyzed by 1H NMR. | |||
| 1 | [BMim]Cl | 120 | 14.1 |
| 2 | [BMim]Cl | 180 | 25.5 |
| 3 | [BMim]Br | 120 | 4.7 |
| 4 | [BMim]OAc | 120 | trace |
| 5 | [BMim]BF4 | 120 | trace |
| 6 | [EMim]Br | 120 | trace |
| 7 | [EMim]OAc | 120 | trace |
| 8 | [BMMim]Cl | 180 | 25.3 |
| 9 | 20 mol % [BMim]Cl in DMSO-d6 | 180 | tracec |
The anion within the ionic liquid was found to have a profound effect on reactivity. The incorporation of a chloride counterion (within the IL) was found necessary to form significant quantities of 3A5AF with trace or low yields obtained when bromide or acetate ILs were used (Table 1, entries 1, 3–5). This has been observed in the dehydration of glucose/fructose to 5-HMF using imidazolium ILs, where high conversions were obtained in the presence of a loosely bound chloride ion.7 Chloride ion concentration has also been shown to increase conversions in aqueous transformations of cellulose and hexoses.8,9 Alkyl chain length on the cation was found to slightly affect yield. In the case of entries 3 and 6, it was found that [BMim]Br was more able to facilitate the dehydration than the ethyl equivalent. [BMim]Cl and [BMMim]Cl (entries 2 and 8) showed equal activity towards 3A5AF formation. In previous research, using fructose as the feedstock, 0% yield of 5-HMF was obtained in [BMMim]Cl whereas 63% yield was obtained in [BMim]Cl.10 It has been proposed that the acidic protons on the imidazolium ring help to catalyze the dehydration reaction. In our studies using [BMMim]Cl, substitution of a methyl group at the C2 position removes the most acidic proton of the imidazolium cation. Therefore, the protons at the C4 and C5 positions must play a larger role in this conversion process for NAG compared with fructose. This difference may be due to the basic nitrogen atom within NAG and its absence in fructose. As ionic liquids are expensive and can be toxic, we also wished to explore whether the reaction could be carried out using catalytic amounts of [BMim]Cl partnered with a co-solvent, entry 9, but unfortunately only a trace amount of product was detected.
To further the study, additives were screened in the hope of increasing product yield, Table 2. The addition of water (entry 2) did not affect the yield of product. This is important for biomass transformations where feedstocks are unlikely to be 100% dry. GC-traces of the EtOAc extracts from the reactions showed the presence of 1-methylimidazole and 1-butylimidazole, presumably from the decomposition of the IL under reaction conditions. If additional 1-methylimidazole is added at the beginning of the reaction, the yield of 3A5AF is dramatically reduced (entry 3). Use of a more inert reaction medium would therefore be highly desirable, as the presence of IL decomposition products are likely inhibiting the reaction. Future research will focus on using more thermally stable or supported ionic liquids in this reaction. However, it should be noted that 3A5AF could be isolated in an analytically pure form using flash chromatography (see ESI†).
| Entry | Additive | T/°C | Yield 3A5AF (%)b |
|---|---|---|---|
a Reaction conditions: [BMim]Cl (750 mg, 0.573 mmol), NAG (100 mg, 0.456 mmol), 10 mol% additive, 3 min (MW).
b Determined by GC-MS.
c Using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 B(OH)3 1 B(OH)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NAG.
d Heated by oil-bath at 180 °C for 1 h.
e 1,8-Diazabicycloundec-7-ene.
f 1,4-Diazabicyclo[2.2.2]octane. NAG.
d Heated by oil-bath at 180 °C for 1 h.
e 1,8-Diazabicycloundec-7-ene.
f 1,4-Diazabicyclo[2.2.2]octane.
|
|||
| 1 | none | 180 | 25.5 |
| 2 | water | 120 | 28.7 |
| 3 | 1-methylimidazole | 120 | 2.9 |
| 4 | B(OH)3 | 180 | 60.0c,d |
| 5 | NH4OH | 180 | 30.9 |
| 6 | NH4Cl | 180 | 25.3 |
| 7 | HCl | 180 | 24.1 |
| 8 | ZrO2/SO42− | 180 | 10.3 |
| 9 | DBUe | 180 | 16.5 |
| 10 | DABCOf | 180 | 16.6 |
| 11 | K2CO3·1.5H2O | 180 | 24.3 |
| 12 | NaOH | 180 | 11.5 |
| 13 | CrCl2 | 120 | 12.1 |
| 14 | CrCl3 | 120 | 12.4 |
| 15 | SnCl4·5H2O | 180 | 17.8 |
| 16 | NaCl | 180 | 38.3d |
A wide range of basic and acidic additives were studied (entries 4–12), and with the exception of boric acid, yields of 3A5AF of between 10 and 30% were obtained. Metal salts proved ineffective at increasing the yield of 3A5AF under the reaction conditions employed. This result was surprising given the high catalytic activity of chromium(II)/(III) chlorides in the dehydration of fructose.11 This difference might be due to the presence of nitrogen in the substrate, which would coordinate strongly with the transition metal and inhibit turnover within the catalytic cycle.
B(OH)3 afforded the highest yield of 3A5AF (Entry 4). Of particular relevance to this work, Riisager and co-workers reported B(OH)3 mediated dehydration of glucose to 5-HMF using ionic liquids.9,12 A yield of up to 42% from glucose and as much as 66% from sucrose was obtained. B(OH)3 has also been used as a selectivity inducer in glycerol hydrogenolysis, via formation of an intermediate borate ester.13 B(OH)3 acts as a Lewis acid in aqueous solution, resulting in the formation of a tetrahydroxyborate complex, which, upon the addition of a hexose (e.g. glucose or NAG), forms a doubly coordinated borate–hexose complex.12 The formation of this complex helps to shift the hexose–aldose equilibrium towards the right, resulting in the release of acidic protons which aid in the dehydration process. In order to study the effect of B(OH)3 loading, three reactions were screened using 10, 100 and 200 mol% boric acid, yielding 33.5, 44.5 and 60.0% 3A5AF respectively. For comparative purposes, the reaction using 200 mol% B(OH)3 was repeated using conventional heating (180 °C for 1 h) and 60.0% 3A5AF was obtained. After purification, isolated yields of 57–58% could be obtained. Overall, as the amount of boric acid was increased, the yield of 3A5AF increased, presumably due to both formation of a borate–hexose complex and also increased acidity of the reaction mixture. It is also interesting that a larger quantity of boric acid is optimum for this reaction compared with glucose. Again, this is likely due to the presence of the basic nitrogen atom in the substrate.
The mechanism for this reaction (Fig. 2) likely has much in common with previously studied fructose and glucose dehydration processes. For example, dissolution of N-acetyl-D-glucosamine in an IL leads to a disruption of the hydrogen bonds between sugar molecules. Chloride ions are thought to be important in this process and addition of NaCl to the reaction mixture led to a moderate increase in yield of 3A5AF (entry 16). Acidic protons present on the imidazolium ring (or the added B(OH)3) are proposed to interact with the hydroxyl oxygen of the sugar to give a complex, which increases the concentration of the open chain aldose form of the sugar. Next, nucleophilic attack by a hydroxyl group affords the 5-membered heterocycle, which undergoes subsequent dehydration and keto-enol tautomerization to give the product. In order to help probe the mechanism and the sugar dissolution, the reaction was followed by 1H NMR and the shifts of the three imidazolium protons studied. In the case of H2, H4 and H5 a gradual shift to higher frequency was indeed noted as the reaction progressed. We suggest that this observation is linked to increased hydrogen bonding with H-bond acceptors (sugar hydroxyl groups) resulting in the deshielding of the acidic imidazolium protons. However, it could also be due to H-bonding with the water released during the reaction. Kinetic studies were performed to assess the activation energy and pre-exponential factor associated with the decomposition of NAG in the absence of an additive. As such, reactions were performed at 140, 160, 180 and 200 °C and the concentration of NAG monitored using 1H NMR. In a typical reaction, [BMim]Cl (1.00 g, 5.75 mmol) and 33 wt% NAG (424 mg 1.92 mmol) were mixed. The sample was heated using an oil bath and an aliquot taken (20–50 mg) at the desired time. To this sample, was added 3.00 μL acetophenone (internal standard) and 600 μL DMSO-d6. 1H NMR spectra were obtained, and the amount of NAG measured using the added internal standard (Fig. S6, ESI†). The decomposition reaction data at 140, 160, 180 and 200 °C were fitted to first order rate plots, yielding linear correlation coefficients (R2) close to unity (Fig. S8, ESI†). Through a plot of ln (kobs) vs. 1/T, Fig. 3, the energy of activation and pre-exponential factor were determined to be 82.8 kJ mol−1 and 1.34 × 108 min−1, respectively. Qi and Watanabe et al. calculated the activation energy and pre-exponential factor to be 114.6 kJ mol−1 and 3.54 × 1014 min−1, respectively, for the conversion of glucose to 5-HMF using CrCl3 in [BMim]Cl under microwave irradiation.14 Although the two processes differ it is important that the activation energy values are of roughly equal magnitude, as they both involve the dehydration of a hexose molecule. However, the pre-exponential factor determined from this work is six orders of magnitude lower than the value reported for glucose. This is to be expected, as a microwave-heated reaction should have a larger pre-exponential factor compared with a conventionally heated one because of an increased number of collisions among reactant molecules.15 Further kinetic studies are needed to understand the role of chloride anions and boric acid on the process described herein.
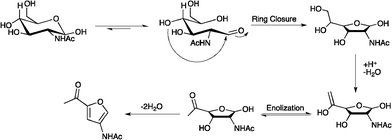 | ||
| Fig. 2 Possible mechanism for the formation of 3A5AF from NAG. | ||
![Arrhenius plot for the conversion of NAG into 3A5AF in [BMim]Cl.](/image/article/2012/RA/c2ra20578e/c2ra20578e-f3.gif) | ||
| Fig. 3 Arrhenius plot for the conversion of NAG into 3A5AF in [BMim]Cl. | ||
In summary, we have shown that the N-substituted furan, 3-acetamido-5-acetylfuran, can be obtained in good yield from the dehydration of N-acetyl-D-glucosamine in an imidazolium based ionic liquid. These data contrast with recently published work from our group where levulinic acid was obtained as the primary product through transformations of aminocarbohydrates (glucosamine, chitosan and chitin) in aqueous media.16 Although, some clues concerning the mechanism have been obtained, more detailed studies are underway in our group. Initial studies suggest that there are both similarities and significant differences between this process and previously reported reactions using fructose and glucose. We intend to investigate 3A5AF as a source of renewable amines, and as a high-value precursor to proximicins (biologically active compound) of which it is a structural motif.17
We thank NSERC of Canada (Discovery and RTI grants—FMK; USRA—MWD), the Canada Foundation for Innovation (Leaders Opportunity Fund Award), the Provincial Government of Newfoundland and Labrador and Memorial University (SWASP—JNM) for funding.
References
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- (a) For example: L. Changzhi, Z. Zhang and Z. K. Zhao, Tetrahedron Lett., 2009, 50, 5403 CrossRef; (b) L. Changzhi, Z. K. Zhao, H. Cai, A. Wang and T. Zhang, Biomass Bioenergy, 2011, 35, 2013 CrossRef.
- F. M. Kerton, Alternative Solvents for Green Chemistry, RSC Publishing, Cambridge, 2009 Search PubMed.
- J. Chen, M. Wang and C. Ho, J. Agric. Food Chem., 1998, 46, 3207 CrossRef CAS.
- R. A. Franich and S. J. Goodin, J. Anal. Appl. Pyrolysis, 1984, 7, 91 CrossRef CAS.
- T. Buntara, S. Noel, P. H. Phua, I. Melián-Cabrera, J. G. de Vries and H. J. Heeres, Angew. Chem., Int. Ed., 2011, 50, 7083 CrossRef CAS.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979 CrossRef CAS.
- J. Potvin, E. Sorlien, J. Hegner, B. DeBoef and B. L. Lucht, Tetrahedron Lett., 2011, 52, 5891 CrossRef CAS.
- T. S. Hansen, J. Mielby and A. Riisager, Green Chem., 2011, 13, 109 RSC.
- Q. Cao, Z. Guo, S. Yao, J. Guan, X. Wang, X. Mu and D. Zhang, Carbohydr. Res., 2011, 346, 956 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597 CrossRef CAS.
- T. Ståhlberg, S. Rodriguez-Rodriguez, P. Fristrup and A. Riisager, Chem.–Eur. J., 2011, 17, 1456 CrossRef.
- J. ten Dam, F. Kapteijn, K. Djanashvili and U. Hanefeld, Catal. Commun., 2011, 13, 1 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr., ChemSusChem, 2010, 3, 1071 CrossRef CAS.
- M. Hosseini, N. Stiasni, V. Barbieri and C. O. Kappe, J. Org. Chem., 2007, 72, 1417 CrossRef CAS.
- K. W. Omari, J. E. Besaw and F. M. Kerton, Green Chem., 2012 10.1039/C2GC35048C.
- (a) F. E. Wolter, K. Schneider, B. P. Davies, E. R. Socher, G. Nicholson, O. Seitz and R. D. Sussmuth, Org. Lett., 2009, 11, 2804 CrossRef CAS; (b) K. Schneider, S. Keller, F. E. Wolter, L. Roglin, W. Beil, O. Seitz, G. Nicholson, C. Bruntner, J. Riedlinger, H. Fiedler and R. D. Sussmuth, Angew. Chem., Int. Ed., 2008, 47, 3258 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: General information, experimental methods, GC-calibration curve, sample GC trace, mass spectrum, IR spectra, NMR spectra and kinetics data. See DOI: 10.1039/c2ra20578e/ |
| This journal is © The Royal Society of Chemistry 2012 |
