Design, testing and characterization of innovative TiN–TiO2 surfaces inactivating bacteria under low intensity visible light
S.
Rtimi
a,
O.
Baghriche
a,
C.
Pulgarin
*a,
R.
Sanjines
b and
J.
Kiwi
*c
aEcole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-GPAO, Station 6, CH-1015, Lausanne, Switzerland. E-mail: cesar.pulgarin@epfl.ch
bEcole Polytechnique Fédérale de Lausanne, EPFL-SB-IPMC-LNNME, Bat PH, Station 3, CH-1015, Lausanne, Switzerland
cEcole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LPI, Bat Chimie, Station 6, CH-1015, Lausanne, Switzerland. E-mail: john.kiwi@epfl.ch
First published on 15th August 2012
Abstract
Ti was sputtered in a plasma chamber under a N2 atmosphere, depositing TiN films on polyester fibers. These films show a significant adsorption in the visible spectral region. A TiN layer 50 nm thick sputtered for 3 min under low intensity/actinic visible light led to the fastest bacterial inactivation (120 min). These innovative TiN nanoparticulate films were characterized by XPS, DRS and TEM.
Introduction
Sputtered TiN films are widely used as protective layers with high chemical resistance to corrosion/oxidation in the electrical and machinery/tool industry.1 P. Kelly has recently reported TiN and other composite nitride structures with Ag are able to inactivate Gram-negative and Gram-positive bacteria in the dark.2 Ag and Cu have also been sputtered on TiN rig metals in an Ar–N2 atmosphere.3 Our laboratory has very recently reported the antibacterial activity of sputtered ZrN nanoparticulate films on polyester in the dark. A 15 min sputtered ZrN-sample led to bacterial inactivation within ∼8 h.4In the last decade, there has been increased interest in innovative antibacterial coatings due to the increasing resistance of pathogenic bacteria to antibiotics. Ag-films present acceptable adhesion and adequate bacterial inactivation kinetics along with favourable Ag-nanoparticle cell compatibility.5,6 But when washing textiles, some Ag leaches out and becomes an environmental problem.7,8 To avoid this environmental problem we investigated the bacterial inactivation by TiN films in this study since they do not contain Ag. Different antibacterial surfaces have been reported on textiles, glass, prostheses and catheters.9–13 Our laboratory has achieved bacterial inactivation on TiO2,14–16 Cu and Ag films.17–20
The objectives of this study are: a) to present the first report on the bactericide action of TiN-nanoparticle films activated by light in the visible range b) to explain the unexpected finding related the light induced activity of TiN films by XPS and c) to report on the dependence of the E. coli inactivation kinetics on the intensity/dose of the commercial actinic lights. TiN films on hospital textiles as suggested in this study should negate the spreading of toxic bacteria when irradiated under visible or actinic light.
Experimental section
TiN was deposited onto polyester at room temperature. The polyester samples were 2 × 2 cm2 in size. Before the deposition of the films, the residual pressure Pr in the sputtering chamber was typically Pr ≤ 10−4 Pa. The substrate-to-target distance was fixed at 9.5 cm. The TiN thin films were deposited by reactive DC magnetron sputtering (DC) using a 5 cm diameter Ti target 99.99 atomic% (Kurt J. Lesker, East Sussex, UK) in an Ar + N2 atmosphere. The total working pressure PT = (PAr+PN2) was fixed at 0.5 Pa and the ratio PN2/PT = 4.5%. The applied sputtering current on the Ti target was fixed at 250 mA providing a power of 112 Watt (U = −450 V) and a current density of 12.7 mA cm−2.The TiN film thickness was determined with a profilometer (Alphastep500, TENCOR). A film thickness of 50 nm was found for TiN-polyester after 3 min. Knowing the atomic distances of ∼0.3 nm we can estimate that one layer is 1015 atoms cm−2. The thickness of each layer is ∼0.2 nm thick,21 then it follows that for a layer 50 nm thick, the amount sputtered is 2.5 × 1017 atoms cm−2.
The polyester used corresponds to the EMPA test cloth sample No 407 Dacron polyethylene-terephthalate, type 54 spun, plain weave ISO 105-F04 used for color fastness determinations. The Ti sputtered on the polyester was evaluated by X-ray fluorescence (RFX, PANalytical PW2400).
The samples of Escherichia coli (E. coli K12) were obtained from the Zellkulturen GmbH (DSMZ) Braunschweig, Germany. The bacterial survival determination method has been recently reported.18,19 To verify that no re-growth of E. coli occurs after total inactivation, the TiN film was incubated for 24 h at 37 °C. Then bacterial suspensions of 100 microliters were deposited on 3 Petri dishes to obtain replica samples and the samples were incubated at 37 °C for 24 h. No bacterial re-growth was observed.
The irradiation of the polyester samples was carried out in a cavity provided with tubular Osram Lumilux 18W/827 actinic lamps. These lamps have a visible emission spectrum between 400 and 700 nm with an integral output of 1.1 mW cm−2 resembling the light distribution found in solar irradiation. The bacterial inactivation kinetics is reported for diverse light intensities for both lamps.
XPS was carried out on an AXIS NOVA photoelectron spectrometer (Kratos Analytical, Manchester, UK). The surface atomic concentration was determined from the peak areas using sensitivity factors.22 The spectrum background was subtracted according to Shirley.23 The XPS peaks of the Ti-species were analyzed by spectra deconvolution software (CasaXPS-Vision 2, Kratos Analytical UK).
Diffuse reflectance spectroscopy was carried out using a Perkin Elmer Lambda 900 UV-VIS-NIR spectrometer provided for with a PELA-1000 accessory within the wavelength range of 200–800 nm and a resolution of one nm. The absorption of the samples was plotted in Kubelka-Munk (KM) arbitrary units vs. wavelength.
A Philips CM-12 (field emission gun, 300 kV, 0.17 nm resolution) microscope at 120 kV was used to measure the grain size of the TiN-films. The textiles were embedded in epoxy resin 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 359 Fluka and the fabrics were cross-sectioned with an ultramicrotome (Ultracut E) and at a knife angle at 35°.
359 Fluka and the fabrics were cross-sectioned with an ultramicrotome (Ultracut E) and at a knife angle at 35°.
Results and discussion
Fig. 1a shows the inactivation of E. coli in the dark and under light by TiN sputtered polyester samples. E. coli inactivation occurred within 120–140 min. The formation of TiO2 can be understood in terms of: a) the partial oxidation of TiN takes place in the presence of an oxygen source due to residual H2O vapor in the sputtering chamber at the residual pressure Pr = 10−4 Pa. This pressure is representative of about 1015 molecules cm−2, there are sufficient O-radicals available to induce partial oxidation of TiN films19,21 and b) the films readily oxidize after deposition when exposed to air and during the sterilization process when autoclaving at 121 °C. If TiN films present an inherent photocatalytic activity, the photocatalysis by TiO2 is not essential for bacterial inactivation. | ||
| Fig. 1 E. coli survival on TiN-polyester sputtered for different times: (1) polyester alone, (2) TiN, 3 min in dark, (3) TiN, 1 min, (4) TiN, 1.5 min, (5) TiN, 10 min, (6) TiN, 5 min and (7) TiN, 3 min, irradiated with an Osram light (400–700 nm) L18W/827 (4 mW/cm2). Fig. 1b shows the E. coli survival percentage for TiN samples sputtered for different times, leading to a bacterial reduction of 3log10. | ||
Fig. 1a shows a progressively faster bacterial inactivation as the sputtering time increases from 1 to 3 min (traces 2, 3, 7). The amount of generated charges increases with a growing number of TiN layers up to a certain limit. At longer sputtering times, the bacterial inactivation in traces 5 and 6 (10 and 5 min respectively) slows down compared to the results presented in trace 7 (3 min). The increase in the TiN thickness hinders the free charge diffusion of carriers due to bulk inward diffusion.9,17 These charge carriers are responsible for electrostatic attraction with the bacteria. After 3 min sputtering time, the TiN coated polyester presents the highest amount of active sites/carriers in the TiN held in exposed positions leading to the shortest bacterial inactivation time. Fig. 1b shows the percentage decrease vs. time of the E. coli concentration for a bacterial reduction of 3log10. Fig. 1b, trace 1 shows that a 3 min TiN sputtered sample is able to inactivate 99.9% E. coli within 30 min in a kinetically fast process.
Fig. 2 shows the bacterial inactivation kinetics mediated by TiN-polyester samples under light irradiation from a visible light source, Osram 18W/827. It is readily seen that the bacterial inactivation is strongly dependent on the applied light dose in the reactor cavity. The inset of Fig. 2 shows the spectral distribution of the Osram light source used.
 | ||
| Fig. 2 E. coli survival on TiN polyester sputtered for 3 min and irradiated with a Lumilux Osram actinic light source (400–700 nm) 18W/827. | ||
Fig. 3a presents the evidence for TiO2 (Ti 2p3/2 doublet) formation on the polyester when sputtering TiN for 3 min. The peaks in Fig. 3a assigned to TiN and Ti2O3 (Ti3+ in the net sense)22 have been deconvoluted by the program cited in the Experimental section. The TiN species shows a peak at 455.62 eV, the Ti3+ doublet is seen at 456.22 eV the Ti4+ doublet at 458.43 eV. Fig. 3b) presents the XPS deconvoluted spectra for the TiN sample at the end of the bacterial inactivation process (120 min). The TiO2 (Ti2p3/2 doublet) BE is seen to shift to 459.01 eV. Shifts ≥ 0.2 eV are due to changes in the oxidation states of the species.23 This shift is due to a redox process taking place during bacterial inactivation involving the couple Ti4+/Ti3+ when oxidizing bacteria. The shift of the Ti2O3/Ti3+ doublet to 457.13 eV at 120 min reflects an increase in the reduced Ti3+-species at the end of the bacterial inactivation period. The surface atomic concentration of a TiN (3 min) sample at time zero was determined by XPS as: O1s 10.2%; Ti2p 44.7%; N1s 13.75% and C1s 22.31%.
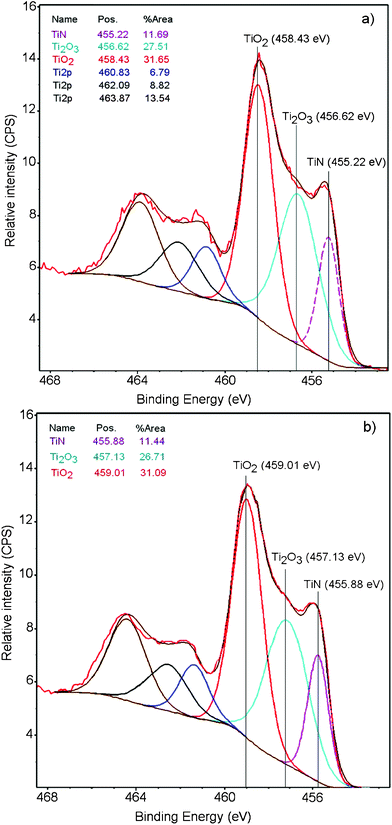 | ||
| Fig. 3 X-ray photoelectron spectroscopy (XPS) of the TiN (3 min) in contact with bacteria for 3 s: a) at time = 0 min and b) at time = 120 min, showing the shift in the deconvoluted peaks after bacterial inactivation. | ||
Fig. 4 shows the diffuse reflection spectroscopy (DRS) of TiN sputtered for 1 and 3 min and for the polyester sample. The absorption of the TiN extends between 400 and 700 nm and a 0.34% Ti loading for the 3 min sputtered sample was determined by X-ray fluorescence spectroscopy (XRF).
 | ||
| Fig. 4 DRS of the TiN sputtered on polyester for: (1) TiN 1 min; (2) 3 min, and (3) polyester alone. | ||
Fig. 5 presents the TEM of: a) a polyester sample and b) a TiN sample sputtered on polyester for 3 min, presenting a width of 30–50 nm—equivalent to 150–250 TiN layers. By scanning transmission electron microscopy in high angular dark field imaging for TiN we found the N, Ti and O-atoms aligned in a continuous layer (data not shown). By X-ray diffraction (XRD) we attempted to determine the TiO2 crystallographic phase sputtered on polyester. This was not possible, due to the small amount of TiO2 sputtered on the polyester within 3 min.
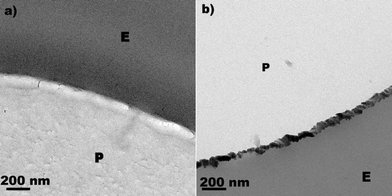 | ||
| Fig. 5 Transmission electron microscopy (TEM) of a) polyester sample alone (E: epoxide, P: polyester, b) for a TiN (3 min) polyester sputtered sample. | ||
Conclusions
This study presents the first report of TiN-surfaces as an effective bactericide photocatalyst when exposed to low intensity visible light. A 3 min TiN sputtered sample is able to inactivate 99.9% of E. coli in short times of up to 30 min. The magnitude of the optical absorption of TiN films runs parallel to the E. coli inactivation kinetics. Fig. 3 presents evidence for TiO2 formation on the polyester when sputtering TiN on polyester and also for redox processes involving the Ti4+/Ti3+ couple within the bacterial inactivation time.Acknowledgements
We thank the COST Action MP0804 Highly Ionized Impulse Plasma Processes (HIPIMS) and the EPFL for support of this work.References
- F. Magnus, O. B. Sveinsson, S. Olafson and J. T. Gudmundsson, J. Appl. Phys., 2011, 110, 083306 CrossRef.
- P. J. Kelly, H. Li, P. S. Benson, K. A. Whitehead, J. Verran, R. D. Arnell and I. Iordanova, Surf. Coat. Technol., 2010, 205, 1606–1610 CrossRef CAS.
- P. J. Kelly, H. Li, K. A. Whitehead, J. Verran, R. D. Arnell and I. iordanova, Surf. Coat. Technol., 2009, 204, 1137–1141 CrossRef CAS.
- O. Baghriche, J. Kiwi, C. Pulgarin and R. Sanjinés, J. Photochem. Photobiol., A, 2012, 229, 39–45 CrossRef CAS.
- Thüringer Surface and Biomaterial Kolloquium, 13/15 September, Zeulenroda, Germany Search PubMed.
- J. Liao, M. Anchun, Z. Zhu and Y. Quan, Int. J. Nanomedicine, 2010, 13, 337–342 Search PubMed.
- D. Hegemann, M. Amberg, A. Ritter and M. Heugeberg, Mater. Technol, 2009, 24, 41–45 CAS.
- D. Hegemannn, M. Hossain and M. Balazs, Prog. Org. Coat., 2007, 58, 237–240 CrossRef.
- A. Fujishima, T. Tao and D. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1–21 CrossRef CAS.
- K. Page, M. Wilson and I. P. Parkin, J. Mater. Chem., 2009, 19, 3819–3831 RSC.
- S. Noimark, Ch. Dunnill, M. Wilson and I. P. Parkin, Chem. Soc. Rev., 2009, 38, 3435–3448 RSC.
- A. H. Foster, D. W. Sheel, P. Sheel, P. Evans, S. Varghese, N. Rutschke and H. M. Yates, J. Photochem. Photobiol., A, 2010, 216, 283–289 CrossRef.
- P. S. M. Dunlop, C. P. Sheeran, J. A. Byrne, M. A. S. McMahon, M. A. Boyle and K. G. McGuigan, J. Photochem. Photobiol., A, 2010, 216, 303–3010 CrossRef CAS.
- V. Nadtochenko, A. Rincon, S. Stanka and J. Kiwi, J. Photochem. Photobiol., A, 2005, 169, 131–137 CrossRef CAS.
- R. Bacsa, J. Kiwi, T. Ohno, P. Albers and V. Nadtochenko, J. Phys. Chem. B, 2005, 109, 5994–6003 CrossRef CAS.
- J. Kiwi and V. Nadtochenko, Langmuir, 2005, 21, 4631–4641 CrossRef CAS.
- E. Kusiak-Nejman, A.W. Morawski, A. P. Ehiasarian, O. Baghriche, C. Pulgarin, E. Mielczarski, J. Mielczarski, A. Kulik and J. Kiwi, J. Phys. Chem. C, 2011, 115, 21113–21119 CAS.
- O. Baghriche, A. P. Ehiasarian, E. Kusiak-Nejman, A.W. Morawski, C. Pulgarin, R. Sanjines and J. Kiwi, J. Photochem. Photobiol., A, 2012, 227, 11–17 CrossRef CAS.
- O. Baghriche, A. P. Ehiasarian, E. Kusiak-Nejman, A. W. Morawski, C. Pulgarin, R. Sanjines and J. Kiwi, Thin Solid Films, 2012, 520, 3567–3573 CrossRef CAS.
- M. I. Mejía, G. Restrepo, J. M. Marín, R. Sanjines, C. Pulgarín, E. Mielczarski, J. Mielczarski and J. Kiwi, ACS Appl. Mater. Interfaces, 2010, 2, 230–235 Search PubMed.
- J. B. Mathews, Epitaxial Growth Part B, IBM Thomas Watson Research Center, Academic Press, New York, 1975, p. 382–436 Search PubMed.
- C. D. Wagner, M. W. Riggs, E. L. Davis and G. E. Müllenberg (Eds), Handbook of X-ray Photoelectron spectroscopy, Perkin-Elmer Corporation Physical Electronics Division, Minnesota, 1979 Search PubMed.
- A. D. Shirley, Corrections of electrostatic charged species in SP-spectroscopy, Phys. Rev., 1972, B5, 4709–4716 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
