Hollow magnetic Janus microspheres templated from double Pickering emulsions†
Yin
Ning
a,
Chaoyang
Wang
*a,
To
Ngai
*b,
Yu
Yang
a and
Zhen
Tong
a
aResearch Institute of Materials Science, South China University of Technology, Guangzhou, China. E-mail: zhywang@scut.edu.cn; Fax: +86-20-8711 2886-605; Tel: +86-20-2223 6269
bDepartment of Chemistry, The Chinese University of Hong Kong, Hong Kong, China. E-mail: tongai@cuhk.edu.hk; Fax: +852-2603 5057; Tel: +852-2696 1222
First published on 25th April 2012
Abstract
We report a versatile method to fabricate magnetic Janus microspheres based on Pickering-type double emulsions. This method allows synthesis of anisotropic magnetic microspheres with multi-hollow structure concentrated on one side of the spheres in a high yield.
Multi-hollow microspheres, magnetic materials and anisotropic microparticles have attracted increasing attention due to their special properties and structures that endow themselves with potential applications, covering encapsulation,1 targeted drug delivery,2 solid surfactants,3 supramolecular assemblies,4 and medical imaging.5 These kinds of materials can be prepared by templating Pickering emulsions, that are emulsions stabilized by solid particles instead of common surfactants or block copolymers.6 Various multi-hollow spheres, for instance, have been reported using double Pickering emulsions as templates, which thereby is considered to be a facile and fascinating method to prepare hollow microspheres.7 On the other hand, magnetic microspheres have been fabricated through directly employing magnetic particles (such as Fe2O3, Fe3O4) as emulsifiers to stabilize Pickering emulsions so as to endow them with magnetic properties.8 Recently, Janus particles with controllable geometry from Pickering emulsions have also been reported, in which solid particles are first absorbed to the oil-in-water emulsion interface and subsequently chemically modified on the side of the solid particles facing the aqueous phase.9
In spite of many synthetic strategies that have been developed to fabricate novel materials with special structures and/or comprehensive natures, to our knowledge approaches to produce multi-hollow magnetic Janus microspheres are scarce. Recently, Yang et al.10 showed the fabrication of multi-hollow magnetic polystyrene microspheres via double emulsions, but in which a mixture of surfactants was used to stabilize the emulsions. Dyab et al.11 successfully prepared anisotropic magnetic microspheres based on emulsions stabilized by a polymerisable anionic surfactant, but the continuous phase required gelation in order to stop the coalescence of the dispersed drops upon polymerization. Rahman et al.12 reported seed emulsion polymerization technique for the fabrication of Janus magnetic microparticles containing inorganic iron oxide nanoparticles and organic polymer starting from an oil-in-water magnetic emulsion rather than a double emulsion.
Herein, we present a facile approach to the preparation of Janus microspheres with a multi-hollow structure (possessing magnetite nanoparticles) located within one side of the solid microsphere. Scheme 1 shows the process that has been used for the fabrication of Janus microparticles. Firstly, a stable aqueous Fe3O4 dispersion in oil-in-water (WF/O/W) double Pickering emulsion was successfully generated by using hydrophobic amorphous fumed silica (H30) and our recently synthesized hydrophilic mesoporous silica nanoparticles (MSNs; Fig. S1, ESI†) to stabilize the primary inner WF/O droplets and outer O/W droplets, respectively. Note that aqueous Fe3O4 dispersion (Fig. S2, ESI†) is intensively chosen as the inner water phase (WF), which adds the magnetic property to the inner emulsions. After that, the polymerization of the middle oil phase of the double emulsions was started while applying an external magnetic field on the desired position of the sample. The Janus microspheres with the multi-hollow structures concentrating on one side of the sphere were obtained after completing the polymerization and washed as well as redispersed in an aqueous phase.
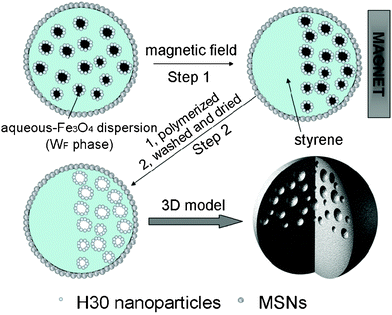 | ||
| Scheme 1 Schematic representation of our method for the preparation of Janus microspheres from a WF/O/W emulsion template. | ||
In this work, we focused on studying two key prerequisites in order to produce the anisotropic multi-hollow magnetic particles. Firstly, the inner droplets should be drivable when they are magnetizing. To satisfy this, the inner magnetic WF/O emulsions need to contain enough Fe3O4 nanoparticles. At the mean time, the viscosity of the inner WF/O emulsions should be properly tuned. Secondly, the stability of the double Pickering emulsions needs to be maintained under the conditions of both polymerization temperature and applying external magnetic force.
We performed various series of emulsion preparations in the presence of different amounts of Fe3O4 nanoparticles, contents of H30 and volume ratios of inner water phase to middle oil phase in order to have the best recipe for the manufacture of the resulting Janus microsphere (Table S1, SI†). Note that the amount of MSNs and the volume ratio of (WF/O)/W (kept at 4 wt‰ and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively) are not discussed in this study due to their lesser effect on the preparation of the target products. In our first series, we fixed the amounts of H30 (3 wt‰) and volume ratio of the inner water phase to middle oil phase (1
5, respectively) are not discussed in this study due to their lesser effect on the preparation of the target products. In our first series, we fixed the amounts of H30 (3 wt‰) and volume ratio of the inner water phase to middle oil phase (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), then we varied the amounts of Fe3O4 nanoparticles, that is, 0, 10, 20, 30 and 40 mg mL−1, to influence the formation of simple and double emulsions. We altered the corresponding parameters to study each variable in the following series.
4), then we varied the amounts of Fe3O4 nanoparticles, that is, 0, 10, 20, 30 and 40 mg mL−1, to influence the formation of simple and double emulsions. We altered the corresponding parameters to study each variable in the following series.
Single emulsions cannot be effectively attracted to one side of the bottle when Fe3O4 concentration was lower than 20 mg mL−1, indicating that the magnetic force is not strong enough to drive WF/O droplets due to the inadequate Fe3O4 content (a–e, Fig. S3, ESI†). On the other hand, double emulsions become unstable when Fe3O4 concentration was increased up to 40 mg mL−1. Just as shown in the insets of Fig. S3 (from (A) to (E)), double emulsions were suspended and the colour of the subnatants turned increasingly darker with the growing Fe3O4 content, presumably due to breaking-up of the emulsions. It is worth pointing out that the amount of H30 also plays an important role in manipulating the size of inner droplets and their magnetability. The average size of inner droplets decreases from ∼15 μm to ∼2 μm when the H30 amount increases from 2 wt‰ to 7 wt‰ (a–d, Fig. S4, ESI†), but at the same time, the viscosity of the inner WF/O increases accordingly, resulting in the non-drivability of the droplets under the applied magnetic field (see the insets of c and d, Fig. S4, ESI†). The emulsions become unstable when the amount of H30 was decreased to 2 wt‰, which could be easily found from the colour changes of the subnatants (shown in the insets of A–D, Fig. S4, ESI†), causing by breaking-up of double emulsions. In principle, the content of the inner droplets in each double emulsion grows correspondingly to the increasing volume ratio of inner water phase to middle oil phase. However, the volume ratio variation indirectly makes the change of relative content of H30, resulting in breaking-up of inner droplets (Fig. S5, ESI†).
Based on the aforementioned observation, the best recipe, that is, 30 mg ml−1 Fe3O4, 3 wt‰ H30, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 WF/O volume ratio, has been proposed to manufacture the anisotropic microspheres. The synthesis method is simple, but it is crucial to choose the proper recipe. Fig. 1 presents a series of microspheres that are synthesized under different conditions. It is very clear to see that there are distinct differences between non-Janus microspheres and Janus microspheres. For non-Janus microspheres, opaque small spheres are uniformly distributed within the big sphere, whilst for Janus ones, opaque small spheres are side-concentrated inside the big sphere (Fig. 1a∼d). As expected, the target microspheres cannot be produced neither by introducing the Fe3O4 nanoparticles nor by applying a magnetic field. As can be seen from Fig. 1a, b, A and B, small voids are disorderly distributed within the big microspheres with the absence of Fe3O4 nanoparticles and magnetic fields, respectively. Meanwhile, at 7 wt‰ H30, smaller voids are also uniformly distributed inside the microspheres (Fig. 1c and C) in spite of the presence of a magnetic field. This is likely because the viscosity of primary droplets is too high such that the WF/O droplets are immoveable even under the magnetic field. Fig. 1d and D provide the direct evidence that the small pores, which seem like solid black spheres in Fig. 1d for the optical reasons, are side-concentrated in a big solid sphere. It is noteworthy that the size distribution discrepancy observed by comparison of optic photographs and SEM images is caused by a random slice during the sample preparation in SEM experiments. Only the multi-hollow part of the microsphere (labeled with a red arrow) can be observed if the microsphere was cut following the red line (see Fig. 1d), while asymmetric structure (blue arrows) can be surveyed when following the blue lines (shown in Fig. 1d). It is interesting to note that the small pores are not in contact but keep a certain distance between each other under different conditions. A precise theoretical explanation of this phenomenon is not clear, but, at least, it indirectly indicates that there must be some kind of repulsive force among the inner droplets to keep them apart even under the external magnetic interaction, and it also explains why the microspheres are just half-filled with small voids when WF/O volume ratio is fixed at 1
4 WF/O volume ratio, has been proposed to manufacture the anisotropic microspheres. The synthesis method is simple, but it is crucial to choose the proper recipe. Fig. 1 presents a series of microspheres that are synthesized under different conditions. It is very clear to see that there are distinct differences between non-Janus microspheres and Janus microspheres. For non-Janus microspheres, opaque small spheres are uniformly distributed within the big sphere, whilst for Janus ones, opaque small spheres are side-concentrated inside the big sphere (Fig. 1a∼d). As expected, the target microspheres cannot be produced neither by introducing the Fe3O4 nanoparticles nor by applying a magnetic field. As can be seen from Fig. 1a, b, A and B, small voids are disorderly distributed within the big microspheres with the absence of Fe3O4 nanoparticles and magnetic fields, respectively. Meanwhile, at 7 wt‰ H30, smaller voids are also uniformly distributed inside the microspheres (Fig. 1c and C) in spite of the presence of a magnetic field. This is likely because the viscosity of primary droplets is too high such that the WF/O droplets are immoveable even under the magnetic field. Fig. 1d and D provide the direct evidence that the small pores, which seem like solid black spheres in Fig. 1d for the optical reasons, are side-concentrated in a big solid sphere. It is noteworthy that the size distribution discrepancy observed by comparison of optic photographs and SEM images is caused by a random slice during the sample preparation in SEM experiments. Only the multi-hollow part of the microsphere (labeled with a red arrow) can be observed if the microsphere was cut following the red line (see Fig. 1d), while asymmetric structure (blue arrows) can be surveyed when following the blue lines (shown in Fig. 1d). It is interesting to note that the small pores are not in contact but keep a certain distance between each other under different conditions. A precise theoretical explanation of this phenomenon is not clear, but, at least, it indirectly indicates that there must be some kind of repulsive force among the inner droplets to keep them apart even under the external magnetic interaction, and it also explains why the microspheres are just half-filled with small voids when WF/O volume ratio is fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
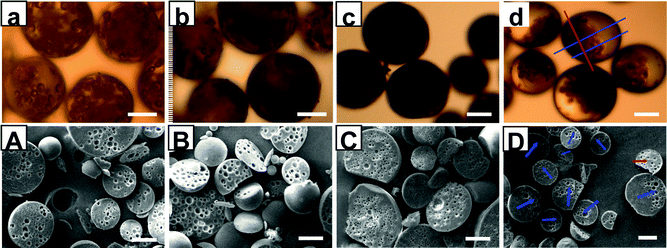 | ||
Fig. 1 (a) and (A), (b) and (B), and (c) and (C) show the optical micrographs and SEM images of the microspheres obtained under in the absence of Fe3O4 nanoparticles, without magnetic force during polymerization, and with a high H30 concentration of 7 wt‰, respectively. (d) and (D) present the microspheres produced under the standard conditions (entry No. 4, Table S1, SI†). The observed morphologies of the microspheres (arrows in (D)) depend on the sites at which the microspheres were cut (lines in (d)). WF/O volume ratio in all above samples was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and all scale bars represent 50 μm. 4 and all scale bars represent 50 μm. | ||
Fig. 2a shows that the Janus microspheres in the ethanol solution can be magnetized even with the saturation magnetization values (Ms) reduced to 0.32 emu g−1 (Fig. 6S, ESI†). The observed decrease in Ms reflected the standard practice of normalizing the magnetization by sample volume. As is well-known, Ms was directly proportional to the volume fraction of the particles and the saturation moment of a single particle.13 It could be considered that the saturation magnetization of the microspheres depended mainly on the volume fraction of the Fe3O4 particles, due to the non-magnetic polystyrene contribution to the total magnetization, resulting in the decrease of the saturation magnetization. The asymmetric distribution of Fe within the microspheres was confirmed by energy-dispersive spectroscopy (SEM-EDS) analysis. Fig. 2c and d show that the inner-wall of hollow spheres are composed of C, O, Si and Fe while other side contains of C, O and Si, indicating that Fe3O4 nanoparticles did not migrate to the oil phase during the polymerization process. Moreover, H30 nanoparticles were not completely absorbed onto the WF/O interface but partly dispersed within the oil phase owing to its hydrophobicity.
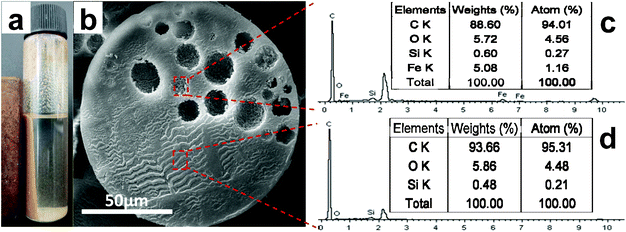 | ||
| Fig. 2 (a) presents a digital photograph of the as-prepared Janus microspheres dispersed in ethanol solution being attracted under a permanent magnet; (b) shows details of the inner structure of a Janus microsphere; elemental analysis of the sample, both at the inner-wall of hollow spheres (c) and solid sites (d), indicates the asymmetric distribution of Fe element. | ||
In conclusion, hollow magnetic Janus microspheres were successfully fabricated by a facile route based on Pickering-type double emulsion templates. The Janus multihollow microspheres may have potential applications in biomedical applications, catalysis, and drug delivery. By introducing a water-soluble monomer into the inner water phase, it is possible to prepare side-distributed multiple core/shell composites under a similar procedure. More importantly, employing oil-in-water-in-oil emulsions with modified Fe3O4 in the inner oil phase would provide us with the flexibility to obtain magnetically and multi-hollowly anisotropic microgels, and such experiments are in progress.
The financial support of this work by National Basic Research Program of China (973 Program, 2012CB821500), NSFC (50973034), the Fundamental Research Funds for the Central Universities and the Planned Science and Technology Project of Guangdong Province (No.2010B010800017).
References
- Y. Zhao and L. Jiang, Adv. Mater., 2009, 21, 3621 CrossRef CAS; K. An and T. Hyeon, Nano Today, 2009, 4, 359 CrossRef; H. Tamber, P. Johansen, H. P. Merkle and B. Gander, Adv. Drug Delivery Rev., 2005, 57, 357 CrossRef.
- J. -H. Park, G. von Maltzahn, L. Zhang, M. P. Schwartz, E. Ruoslahti, S. N. Bhatia and M. J. Sailor, Adv. Mater., 2008, 20, 1630 CrossRef CAS; Y. M. Huh, Y. W. Jun, H. T. Song, S. Kim, J. S. Choi, J. H. Lee, S. Yoon, K. S. Kim, J. S. Shin, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 12387 CrossRef.
- Y. K. Takahara, S. Ikeda, S. Ishino, K. Tachi, K. Ikeue, T. Sakata, T. Hasegawa, H. Mori, M. Matsumura and B. Ohtani, J. Am. Chem. Soc., 2005, 127, 6271 CrossRef CAS; A. Walther, M. Hoffman and A. H. E. Muller, Angew. Chem., Int. Ed., 2008, 47, 711 CrossRef.
- L. Hong, A. Cacciuto, E. Luijten and S. Granick, Nano Lett., 2006, 6, 2510 CrossRef CAS.
- K.-H. Roh, D. C. Martin and J. Lahaan, Nat. Mater., 2005, 4, 759 CrossRef CAS; K.-H. Roh, M. Yoshida and J. Lahaan, Materialwiss. Werkstofftech., 2007, 38, 1008 CrossRef.
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156 CrossRef CAS; S. U. Pickering, J. Chem. Soc. Trans., 1907, 91, 2001 RSC; S. Fujii, Y. L. Cai, Jonathan V. M. Weaver and S. P. Armes, J. Am. Chem. Soc., 2005, 127, 7304 CrossRef.
- T. Chen, P. J. Colver and S. A. F. Bon, Adv. Mater., 2007, 19, 2286 CrossRef CAS; Q. X. Gao, C. Y. Wang, H. X. Liu, Y. H. Chen and Z. Tong, Polym. Chem., 2010, 1, 75 RSC; H. Maeda, M. Okada, S. Fujii, Y. Nakamura and T. Furuzono, Langmuir, 2010, 26, 13727 CrossRef; Q. Yuan, L. B. Yang, M. Z. Wang, H. Wang, X. P. Ge and X. W. Ge, Langmuir, 2009, 25, 2729 CrossRef; A. Walsh, K. L. Thompson and S. P. Armes, Langmuir, 2010, 26, 18039 CrossRef; J. N. Zhang, X. W. Ge, M. Z. Wang, J. J. Yang, Q. Y. Wu, M. Y. Wu, N. N. Liu and Z. L. Jin, Chem. Commun., 2010, 46, 4318 RSC; A. S. Miguel, J. Scrimgeour, J. E. Curtis and S. H. Behrens, Soft Matter, 2010, 6, 3163 RSC; D. Lee and D. A. Weitz, Adv. Mater., 2008, 20, 3498 CrossRef; D. Lee and D. A. Weitz, Small, 2009, 5, 1932 CrossRef; N. Ahmed, M. Michelin-Jamois, H. Fessi and A. Elaissari, Soft Matter, 2012, 8, 2554 RSC.
- S. Sacanna and A. P. Philipse, Adv. Mater., 2007, 19, 3824 CrossRef CAS; Q. Xiao, X. K. Tan, L. L. Ji and J. Xue, Synth. Met., 2007, 157, 784 CrossRef; H. X. Liu, C. Y. Wang, Q. X. Gao, X. X. Liu and Z. Tong, Acta Biomater., 2010, 6, 275 CrossRef; J. Zhou, X. Y. Qiao, B. P. Binks, K. Sun, M. W. Bai, Y. L. Li and Y. Liu, Langmuir, 2011, 27, 3308 CrossRef.
- L. Hong, S. Jiang and S. Granick, Langmuir, 2006, 22, 9495 CrossRef CAS; D. Suzuki, S. Tsuji and H. Kawaguchi, J. Am. Chem. Soc., 2007, 129, 8088 CrossRef; B. Liu, W. Wei, X. Z. Qu and Z. Z. Yang, Angew. Chem., Int. Ed., 2008, 120, 4037 CrossRef; D. Li, Y. J. He and S. Wang, J. Phys. Chem. C, 2009, 113, 12927 Search PubMed; A. Perro, F. Meunier, V. Schmitt and S. Ravaine, Colloids Surf., A, 2009, 332, 57 CrossRef; N. P. Pardhy and B. M. Budhlall, Langmuir, 2010, 26, 13130 CrossRef.
- S. Yang, H. R. Liu and Z. C. Zhang, Langmuir, 2008, 24, 10395 CrossRef CAS.
- A. K. F. Dyab, M. Ozmen, M. Ersoz and V. N. Paunov, J. Mater. Chem., 2009, 19, 3475 RSC.
- M. M. Rahman, F. Montagne, H. Fessi and A. Elaissari, Soft Matter, 2011, 7, 1483 RSC.
- J. C. Bacri, R. Perzynski, D. Salin, V. Cabuil and R. Massart, J. Magn. Magn. Mater., 1986, 62, 36 CrossRef CAS; F. Sauzedde, A. Elaissari and C. Pichot, Colloid Polym. Sci., 1999, 277, 846 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental part, characteristics of MSNs and Fe3O4 nanoparticles, optical micrographs of emulsions, and magnetic properties, sizes and size distributions of the Janus microspheres. See DOI: 10.1039/c2ra20547e |
| This journal is © The Royal Society of Chemistry 2012 |
