Zirconium nitrate: a reusable water tolerant Lewis acid catalyst for the synthesis of N-substituted pyrroles in aqueous media
Alireza
Hasaninejad
*a,
Mohsen
Shekouhy
ab,
Mohammad
Reza Mohammadizadeh
a and
Abdolkarim
Zare
c
aDepartment of Chemistry, Faculty of Sciences, Persian Gulf University, Bushehr 75169, Iran. E-mail: ahassaninejad@yahoo.com; Fax: +98 (771) 4541494
bDepartment of Chemistry, Islamic Azad University, Shiraz Branch, Shiraz, Iran
cDepartment of Chemistry, Payame Noor University, PO BOX 19395-4697, Tehran, Iran
First published on 8th May 2012
Abstract
A simple, efficient procedure for the synthesis of 11-(1H-pyrrol-1-yl)-11H-indeno[1,2-b]quinoxaline and 3-(1H-pyrrol-1-yl)indolin-2-one derivatives in aqueous media is described. The condensation reaction between 4-hydroxyproline and isatin derivatives or 11H-indeno[1,2-b]quinoxalin-11-ones, using a catalytic amount of Zr(NO3)4 as a water tolerant Lewis acid catalyst affords, the title compounds in high to excellent yields and in short reaction times.
1. Introduction
Among numerous heterocycles, the pyrrole core has always been one of the most prominent, since it is a constituent of many important classes of natural products such as heme, chlorophyll and vitamin B12.1–3 They are also found in various bioactive drug molecules such as atrovastatin, anti-inflammatories, antitumor agents and immunosuppressants.3–7 They are very useful intermediates, not only for the synthesis of drugs, pigments and pharmaceuticals, but also for the development of organic functional materials.8,9 As a result, a large number of synthetic methods for the preparation of diversely substituted pyrroles have been developed.10 Conjugate addition reactions,11 transition metal-mediated reactions,12 reductive couplings,13 aza-Wittig reactions,14 and other multistep operations15 have been performed for the synthesis of pyrroles.Beside this, indenoquinoxaline16 and indole17 derivatives are important classes of nitrogen-containing heterocycles and have attracted attention because of their applications in dyes and pharmaceuticals. Moreover, they have been used as building blocks for the synthesis of organic semiconductors. There is a resultant pharmacological interest in compounds that belong to the quinoxaline, indole, and pyrrole families; the synthesis of 11-(1H-pyrrol-1-yl)-11H-indeno[1,2-b]quinoxaline and 3-(1H-pyrrol-1-yl)indolin-2-one derivatives via the condensation of 4-hydroxy proline with 11H-indeno[1,2-b]quinoxalin-11-one or isatin derivatives is more interesting. Some reagents have been reported for the synthesis of this class of compounds.18–22 Most of these methods suffer from drawbacks such as the use of hazardous organic solvents, toxic catalysts, tedious work up, a need for special apparatus and/or high cost. Hence, the development of a new efficient procedure based on a green chemistry protocol for the preparation of the above mentioned compounds is of prime interest.
Various kinds of Lewis acid have been developed and used in organic synthesis.23 These Lewis acids must generally be used under strictly anhydrous conditions. The presence of even a small amount of water stops the reaction, because most Lewis acids immediately react with water rather than the substrate.23 In recent years, Zr(IV) compounds have gained special attention as catalysts in organic synthesis and some of these are stable in aqueous media.24 Recently, Zolfigol et al. reported the Friedlander synthesis of quinoline derivatives in aqueous media, using Zr(NO3)4 as a catalyst, which demonstrates the water stability of this compound.25
In continuation of our interest in green chemistry protocols and the synthesis of aza-heterocyclic compounds,26 we herein report the application of Zr(NO3)4 as a water tolerant Lewis acid catalyst for the synthesis of 3-pyrrolyl-indolinones and pyrrolylindeno[1,2-b]quinoxalines via the condensation reaction between 4-hydroxyproline and isatin derivatives or 11H-indeno[1,2-b]quinoxalin-11-ones in aqueous media (Scheme 1).
![The condensation reaction between isatin and/or 11H-indeno[1,2-b]quinoxalin-11-one derivatives with 4-hydroxyproline in the presence of Zr(NO3)4 in EtOH–H2O 3 : 1 at 80 °C.](/image/article/2012/RA/c2ra20294h/c2ra20294h-s1.gif) | ||
Scheme 1 The condensation reaction between isatin and/or 11H-indeno[1,2-b]quinoxalin-11-one derivatives with 4-hydroxyproline in the presence of Zr(NO3)4 in EtOH–H2O 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 80 °C. 1 at 80 °C. | ||
2. Experimental
All chemicals were purchased from Merck or Fluka Chemical Companies. All compounds are known, and their structures were identified by comparing their melting points and 1H and 13C NMR data with those reported in the literature. The 1H NMR (500 MHz) and 13C NMR (125 MHz) were run on a Bruker Avance DPX-250, FT-NMR spectrometer (δ in ppm). Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.2.1. Preparation of zirconium nitrate
Zirconium nitrate was prepared based on the reported procedure:27 7 g zirconyl oxychloride was dissolved in 26 mL of a 5 M solution of nitric acid at room temperature. The resulting solution was slowly heated. It first became yellowish brown in color, and then over 90 min of gentle heating at the boiling point with constant stirring, the color disappeared as a result of the formation of zirconium nitrate. This colorless solution reduced in volume when a white precipitate was formed. The latter was separated and dried by heating in an oven at 110 °C for 12 h.2.2. General procedure for the synthesis of 11-(1H-pyrrol-1-yl)-11H-indeno[1,2-b]quinoxaline and 3-(1H-pyrrol-1-yl)indolin-2-one derivatives
Isatin derivatives or 11H-indeno[1,2-b]quinoxalin-11-one derivatives (2 mmol), 4-hydroxyproline (2 mmol) and Zr(NO3)4 (0.2 mmol) were added into a 15 mL round-bottomed flask containing EtOH–H2O 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 connected to a reflux condenser. The mixture was stirred at 80 °C for the appropriate time (Table 2). Afterward, the reaction mixture was cooled to room temperature, H2O (30 mL) was added to the reaction mixture, and the precipitated crude products were collected by filtration, dried and purified with column chromatography using n-hexane–EtOAc 3
1 connected to a reflux condenser. The mixture was stirred at 80 °C for the appropriate time (Table 2). Afterward, the reaction mixture was cooled to room temperature, H2O (30 mL) was added to the reaction mixture, and the precipitated crude products were collected by filtration, dried and purified with column chromatography using n-hexane–EtOAc 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the eluent. After isolation of the product, the filtrate was extracted with CHCl3 (2 × 15 mL). The aqueous layer (including Zr(NO3)4) was separated, and the solvent was evaporated to obtain about 5 mL aqueous solution of Zr(NO3)4. The recycled catalyst was used with fresh ethanol and substrates for the next run under identical reaction conditions.
1 as the eluent. After isolation of the product, the filtrate was extracted with CHCl3 (2 × 15 mL). The aqueous layer (including Zr(NO3)4) was separated, and the solvent was evaporated to obtain about 5 mL aqueous solution of Zr(NO3)4. The recycled catalyst was used with fresh ethanol and substrates for the next run under identical reaction conditions.
2.2. Selected spectral data of the products
3. Results and discussion
Our initial efforts focused on the search for a catalyst for the condensation reaction between 4-hydroxyproline (2 mmol) and isatin (2 mmol). The synthesis of compound 3a was selected as a model reaction in the presence of different catalytic systems and the results are summarized in Table 1.| Entry | Catalyst | Reaction conditions | Time (min) | Yield (%)a |
|---|---|---|---|---|
| a Isolated yield. b Obtained results in the presents of recovered ZrCl4 in 2nd run. c Obtained results in the presents of 40 mol% of catalyst. | ||||
| 1 | NH2SO3H (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 81 |
| 2 | Silica chloride (0.5 g) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 37 |
| 3 | P2O5/SiO2 (0.5 g) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | trace |
| 4 | FeCl3 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
360 | 85 |
| 5 | Bi(NO3)3 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 24 |
| 6 | Ni(NO3)2 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 59 |
| 7 | Co(NO3)2 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 42 |
| 8 | ZnO (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | trace |
| 9 | AlCl3 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
270 | 81 |
| 10 | CaCl2 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 32 |
| 11 | Silphox (0.5 g) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 29 |
| 12 | ZrOCl2 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | 63 |
| 13 | ZrCl4 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
45 (480)b | 87 (52)b |
| 14 | ZrO2 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | trace |
| 15 | HCl (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 (120)c | 48 (77)c |
| 16 | HNO3 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
240 (90)c | 50 (73)c |
| 17 | Zr(NO3)4 (10 mol%) | CH3Cl, reflux | 480 | 22 |
| 18 | Zr(NO3)4 (10 mol%) | EtOH, reflux | 240 | 88 |
| 19 | Zr(NO3)4 (10 mol%) | CH3CN, reflux | 360 | 76 |
| 20 | Zr(NO3)4 (10 mol%) | H2O, reflux | 480 | 59 |
| 21 | Zr(NO3)4 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
45 | 91 |
| 22 | Zr(NO3)4 (15 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
45 | 91 |
| 23 | Zr(NO3)4 (5 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
60 | 78 |
| 24 | — | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C 1), 80 °C |
480 | trace |
| 25 | Zr(NO3)4 (10 mol%) | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 60 °C 1), 60 °C |
480 | 73 |
After extensive screening, we found that the optimized best yields and time profiles were obtained with 10 mol % Zr(NO3)4 in EtOH–H2O 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 80 °C, which furnished the corresponding 3-(1H-pyrrol-1-yl)indolin-2-one 3a in 91% yield within 45 min. Increasing the amount of Zr(NO3)4 to more than 15 mol % showed no substantial improvement in the yield, whereas the yield was decreased by decreasing the amount of catalyst to 5 mol %. Moreover, it was observed that the reaction did not proceed efficiently in the absence of Zr(NO3)4 after a long time (8 h). Moreover, other nitrate salts such as Bi(NO3)3, Ni(NO3)2 and Co(NO3)2 were applied in which the product was obtained in low yields and in long reaction times (Table 1, entries 5–7). The model reaction was also examined in the presence of ZrOCl2 and ZrCl4 under the optimized conditions (Table 1, entries 12, 13). As can be seen from Table 1, ZrOCl2 shows poor activity for this reaction. Though ZrCl4 catalyzed the reaction in a good time and admissible yield, it was not reusable under applied conditions (Table 1, entry 13). It is well known that zirconium tetrachloride immediately hydrolyzes when it comes into contact with humidity. Hence, zirconium polyoxo species and hydrochloric acid are formed. Therefore, the catalytic activities of zirconium oxide, hydrochloric acid and nitric acid for the model reaction were studied (Table 1, entries 14–16).
1 at 80 °C, which furnished the corresponding 3-(1H-pyrrol-1-yl)indolin-2-one 3a in 91% yield within 45 min. Increasing the amount of Zr(NO3)4 to more than 15 mol % showed no substantial improvement in the yield, whereas the yield was decreased by decreasing the amount of catalyst to 5 mol %. Moreover, it was observed that the reaction did not proceed efficiently in the absence of Zr(NO3)4 after a long time (8 h). Moreover, other nitrate salts such as Bi(NO3)3, Ni(NO3)2 and Co(NO3)2 were applied in which the product was obtained in low yields and in long reaction times (Table 1, entries 5–7). The model reaction was also examined in the presence of ZrOCl2 and ZrCl4 under the optimized conditions (Table 1, entries 12, 13). As can be seen from Table 1, ZrOCl2 shows poor activity for this reaction. Though ZrCl4 catalyzed the reaction in a good time and admissible yield, it was not reusable under applied conditions (Table 1, entry 13). It is well known that zirconium tetrachloride immediately hydrolyzes when it comes into contact with humidity. Hence, zirconium polyoxo species and hydrochloric acid are formed. Therefore, the catalytic activities of zirconium oxide, hydrochloric acid and nitric acid for the model reaction were studied (Table 1, entries 14–16).
In the next step, the scope and efficiency of the catalyst were explored under the optimized reaction conditions for the condensation of various isatin and 11H-indeno[1,2-b]quinoxalin-11-one derivatives with 4-hydroxyproline in the presence of Zr(NO3)4, to furnish the corresponding products (Scheme 1). The results are displayed in Table 2. As can be seen, the 11-(1H-pyrrol-1-yl)-11H-indeno[1,2-b]quinoxaline and 3-(1H-pyrrol-1-yl)indolin-2-one derivatives were obtained in high yields and short reaction times.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 80 °C
1 at 80 °C
| Entry | Substrate | Product | Time (min) | Yield (%)a | M.P. (Rep.) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | 1 | 3a | 45 | 91 | 141–142 (142–144)21 |
| 2 | 2 | 3b | 60 | 89 | 173–176 |
| 3 | 3 | 3c | 45 | 90 | 175–177 (173–174)21 |
| 4 | 4 | 3d | 65 | 87 | 163–164 (165–166)21 |
| 5 | 5 | 3e | 50 | 89 | 136–138 (134–136)21 |
| 6 | 6 | 3f | 55 | 83 | 124–126 (125–127)21 |
| 7 | 7 | 3g | 100 | 89 | 180–182 (181–182)21 |
| 8 | 8 | 3h | 130 | 83 | 233–235 (230–232)21 |
The formation of the desired products may be explained by the formation of an azomethine ylide via decarboxylation and subsequent 1,5-proton shift to give the more stable zwitterion, which can easily transform to the more stable product to gain aromatic character (Scheme 2).
![Proposed mechanism for the condensation reaction between isatin and/or 11H-indeno[1,2-b]quinoxalin-11-one derivatives with 4-hydroxyproline in the presence of Zr(NO3)4.](/image/article/2012/RA/c2ra20294h/c2ra20294h-s2.gif) | ||
| Scheme 2 Proposed mechanism for the condensation reaction between isatin and/or 11H-indeno[1,2-b]quinoxalin-11-one derivatives with 4-hydroxyproline in the presence of Zr(NO3)4. | ||
In another study to establish the reusability of the catalyst, the model reaction was examined in the presence of recovered Zr(NO3)4 (Fig. 1). As indicated in Fig. 1, no loss of catalytic activity was observed even after six cycles of the reaction.
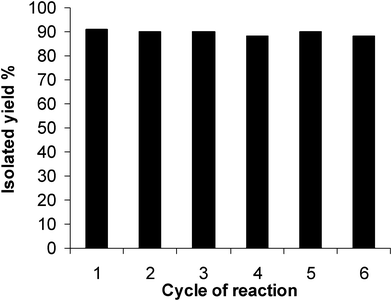 | ||
| Fig. 1 The catalytic activity of Zr(NO3)4 in six cycles for the reaction of isatin with 4-hydroxyproline. | ||
4. Conclusion
In conclusion, an extremely efficient method has been developed for the synthesis of 11-(1H-pyrrol-1-yl)-11H-indeno[1,2-b]quinoxaline and 3-(1H-pyrrol-1-yl)indolin-2-one derivatives using Zr(NO3)4 as a water tolerant Lewis acid catalyst. This method is bestowed with several unique merits, such as high conversions, simplicity of operation and cost efficiency. Use of a reusable solid acid catalyst and application of aqueous media significantly contribute to the practice of green chemistry.Acknowledgements
The authors thank the Research Council of the Persian Gulf University of Bushehr for financial support of this work.References
- R. Ragno, G. R. Marshall, R. Di Santo, R. Costi, S. Massa, R. Rompei and M. Artico, Bioorg. Med. Chem., 2000, 8, 1423 CrossRef CAS.
- R. Di Santo, R. Costi, M. Artico, S. Massa, G. Lampis, D. Deidda and R. Rompei, Bioorg. Med. Chem. Lett., 1998, 8, 2931 CrossRef CAS.
- C. Y. De Leon and B. Ganem, Tetrahedron, 1997, 53, 7731 CrossRef CAS.
- R. B. Thompson, FASEB J., 2001, 15, 1671 CrossRef CAS.
- P. Cozzi and N. Mongelli, Curr. Pharm. Des., 1998, 4, 181 CAS.
- A. Furstner, H. Szillat, B. Gabor and R. Mynott, J. Am. Chem. Soc., 1998, 120, 8305 CrossRef.
- J. M. Muchowski, Adv. Med. Chem., 1992, 1, 109 CAS.
- J. A. H. Lainton, J. W. Hoffman, B. R. Martin and D. R. Compton, Tetrahedron Lett., 1995, 36, 1401 CrossRef CAS.
- R. A. Jones and G. P. Bean, The Chemistry of Pyrroles, Academic Press, London, 1977, pp. 1–5 Search PubMed.
- T. L. Gilchrist, J. Chem. Soc., Perkin Trans 1, 1998, 615 Search PubMed.
- R. K. Dieter and H. Yu, Org. Lett., 2000, 2, 2283 CrossRef CAS.
- N. Iwasawa, K. Maeyama and M. Saitou, J. Am. Chem. Soc., 1997, 119, 1486 CrossRef CAS.
- A. Furstner, H. Weintritt and A. Hupperts, J. Org. Chem., 1995, 60, 6637 CrossRef.
- A. Katritzky, J. Jiang and P. J. Steel, J. Org. Chem., 1994, 59, 4551 CrossRef CAS.
- (a) A. Hantzsch, Ber. Dtsch. Chem. Ges., 1890, 23, 1474 CrossRef; (b) H. S. Broadbent, W. S. Burnharm, R. K. Olsen and R. M. J. Sheely, J. Heterocycl. Chem., 1968, 5, 757 CrossRef CAS; (c) H. O. Bayer, H. Gotthardt and R. Huisgen, Chem. Ber., 1970, 103, 2356 CrossRef CAS; (d) R. Huisgen, H. Gotthardt, H. O. Bayer and F. C. Schaefter, Chem. Ber., 1970, 103, 2611 CrossRef CAS; (e) J. V. Cooney and W. E. McEwen, J. Org. Chem., 1981, 46, 2570 CrossRef CAS; (f) F. Berree, E. Marchand and G. Morel, Tetrahedron Lett., 1992, 33, 6155 CrossRef CAS; (g) A. Arcadi and E. Rossi, Tetrahedron, 1998, 54, 15253 CrossRef CAS; (h) M. Periasamy, G. Srinivas and P. Bharati, J. Org. Chem., 1999, 64, 4204 CrossRef CAS.
- (a) E. D. Brock, D.M. Lewis, T. I. Yousaf and H. H. Harper, WO 9951688, 1999 Search PubMed; (b) A. Gazit, H. App, G. McMahon, J. Chen, A. Levitzki and F. D. Bohmer, J. Med. Chem., 1996, 39, 2170 CrossRef CAS; (c) U. Sehlstedt, P. Aich, J. Bergman, H. Vallberg, B. Norden and A. Graslund, J. Mol. Biol., 1998, 278, 31 CrossRef CAS.
- K. C. Joshi, R. Jain and P. Chand, Heterocycles, 1985, 23, 957 CrossRef CAS.
- J. Azizian, A. R. Karimi, Z. Kazemizadeh, A. A. Mohammadi and M. R. Mohammadizadeh, Tetrahedron Lett., 2005, 46, 6155 CrossRef CAS.
- J. Azizian, A. R. Karimi, Z. Kazemizadeh, A. A. Mohammadi and M. R. Mohammadizadeh, Synthesis, 2005, 7, 1095 CrossRef.
- J. S. Yadav, B. V. S. Reddy, R. Jain and Ch. S. Reddy, Tetrahedron Lett., 2007, 48, 3295 CrossRef CAS.
- A. R. Karimi, F. Behzadi and M. Mohammadpour Amini, Tetrahedron Lett., 2008, 49, 5393 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, R. Jain and U. V. S. Reddy, J. Mol. Catal. A: Chem., 2007, 278, 38 CrossRef CAS.
- S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209 CrossRef CAS.
- H. Firouzabadi and M. Jafarpour, J. Iran. Chem. Soc., 2008, 5, 159 CrossRef CAS.
- M. A. Zolfigol, P. Salehi, A. Ghaderi and M. Shiri, Catal. Commun., 2007, 8, 1214 CrossRef CAS.
- (a) A. Hasaninejad, A. Zare, M. Shekouhy and J. Ameri, Green Chem., 2011, 13, 958 RSC; (b) A. Hasaninejad, M. Shekouhy, A. Zare, N. Golzar and M. M. Doroodmand, Appl. Catal., A, 2011, 402, 11 CrossRef CAS; (c) A. Hasaninejad, A. Zare and M. Shekouhy, Tetrahedron, 2011, 67, 390 CrossRef CAS; (d) A. Hasaninejad, A. Zare, M. Shekouhy and J. Ameri, J. Comb. Chem., 2010, 12, 844 CrossRef CAS.
- (a) V. E. Plyushchev, L. I. Yuranova and L. N. Komissarova, Zh. Neorg. Khim., 1965, 10, 643–646 CAS; (b) F. G. R. Gimblett, A. Hussain and K. S. W. Sing, J. Chem. Tech. Biotechnol., 1988, 41, 277–286 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
