Template-free synthesis of a hierarchical flower-like platinum counter electrode and its application in dye-sensitized solar cells†
Ziying
Tang
,
Qunwei
Tang
,
Jihuai
Wu
*,
Yan
Li
,
Qin
Liu
,
Min
Zheng
,
Yaoming
Xiao
,
Gentian
Yue
,
Miaoliang
Huang
and
Jianming
Lin
Institute of Materials Physical Chemistry, Huaqiao University, Quanzhou, 362021, P. R. China. E-mail: jhwu@hqu.edu.cn; Fax: (+86) 595-22692229; Tel: (+86) 595-22692229
First published on 27th March 2012
Abstract
A large-scale hierarchical flower-like platinum counter electrode was successfully prepared by a template-free route. The platinum flower is composed of numerous of petal-like platinum nanosheets with a thickness of 25–30 nm, with a significantly enhanced active surface-area and electrochemical performance in DSSCs. The DSSC based on the flower-like Pt counter electrode shows an efficiency of 7.94%, which is enhanced by 19.2% compared to that of a conventional Pt nanoparticle electrode.
Dye-sensitized solar cells (DSSC) have aroused widespread, intensive and increasing research interests since they were first reported by O'Regan and Gratzel1 in 1991. It is considered to be a strong candidate for next-generation solar cells and a credible alternative to conventional silicon solar cells due to its low cost, simple preparation procedure and high photoelectric conversion efficiency.2,3 Usually, a DSSC is composed of a dye-sensitized TiO2 film anode electrode, a I−/I3− redox electrolyte, and a catalytic material coated counter electrode.4 The electron is generated at the TiO2 anode electrode, and is subsequently transported to the counter electrode through a I−/I3− redox reaction and collected at the counter electrode. The electrolyte is one of the most important components in DSSCs based on liquid electrolytes, a high photoelectric conversion efficiency of 12% for DSSC has been achieved.2,3 However, the potential problems caused by the liquid electrolytes, such as the leakage and volatilization of liquid, are considered as some of the critical factors limiting the long-term performance and practical use of DSSCs4,5. It has been pointed out by Gratzel2 that long-term stability is a key requirement for all types of solar cells, and a vast amount of tests have therefore been carried out over the last 20 years to scrutinize the stability of DSSCs. Recently, many efforts have been made to replace liquid electrolytes with all solid-state electrolytes in order to enhance the stability of DSSCs,6–9 such as the use of ionic liquid electrolytes10,11 and quasi solid-state gel electrolytes.5,12 Another important component in DSSCs is the counter electrode, which should have low resistance and high electrocatalytic activity for the I−/I3− redox reaction in order to keep a low overvoltage and decelerated charge recombination.13 Great efforts have been made to fabricate counter electrodes using conductive nanomaterials such as platinum (Pt), graphene, carbon black, carbon nanotubes, polyaniline, PbS, poly(3,4-ethylenedioxythiophene), metal titanium and aluminium.14–20 However, Pt has established itself as the preferred material in DSSCs because of its high conductivity, excellent electrocatalytic activity for the I−/I3− redox reaction and good corrosion stability against iodine in the electrolyte.21 Generally, the Pt counter electrode is prepared by coating a layer of Pt nanoparticles onto the surface of a conductive substrate, and the structure of the Pt nanoparticles greatly influences the electrochemical properties of the Pt counter electrode.
More recently, there has been increasing interest in hierarchically nanostructured materials for their large numbers of active sites and unique multidimensional structure.22,23 Even though numerous research on hierarchical flower-like nanomaterials, such as TiO2,22 tungsten oxide,24 SnO2,23 and Au,25 has been carried out, as far as we know, there are few reports on manufacturing flower-like Pt nanostructures and their use as an effective counter electrode for DSSC applications. In the current work, a template-free method was applied to synthesize a large-scale hierarchical flower-like Pt counter electrode by a modified electrodeposition method in highly concentrated H2PtCl6 solution. The surface of the Pt flower is composed of numerous of petal-like Pt nanosheets with a thickness of 25–30 nm, which greatly improves the active surface-area and roughness of the counter electrode. Compared with a traditional Pt nanoparticle electrode, the resultant Pt flower-like electrode shows an enhanced active surface-area and electrochemical catalytic activity for the I−/I3− redox reaction. A DSSC based on the flower-like Pt counter electrode was fabricated, and a photoelectric conversion efficiency of 7.94% was achieved, indicating an enhancement of 19.2% in comparison with that of a conventional Pt nanoparticle assembled DSSC.
The electrodeposition of a hierarchical flower-like Pt counter electrode was performed in a three-electrode system on an electrochemical workstation (CHI660C, CHI Instruments, Shanghai) modified by the references.26,27 Fluorine-doped tin oxide (FTO) glass was used as the working electrode and dipped in a 0.02 M H2PtCl6 solution containing of 1.0 M HCl; Pt wire and Ag/AgCl were used as the counter electrode and reference electrode, respectively. The electrodeposition process was carried out at a potential of −0.35 V for 600 s at room temperature. The conventional ball-like Pt particle electrode was also prepared for comparison and the experimental parameters were similar to the preparation of the flower-like Pt counter electrode. Generally, the conventional Pt nanoparticle electrode was performed in a lower concentration H2PtCl6 solution of 0.002 M. The preparation and assembly processes of the DSSCs as well as the characterization of the Pt counter electrodes were performed in previous reports.5,8,28
Fig. 1 and Fig. S1† represent the morphologies of conventional and flower-like Pt electrodes. From Fig. 1a, one can see that the conventional Pt electrode is composed of Pt nanoparticles with a diameter of 150–200 nm, giving a smooth but compact surface. Such a structure is disadvantageous for the liquid electrolyte permeating into the inside of the Pt counter electrode, thus, the I3− has poor contact with the Pt catalysts, which ultimately leads to a low catalytic efficiency for the I3− reduction in DSSCs. Fig. 1b–d show the tilted-section and top-view SEM images of the flower-like Pt counter electrode, and show that the electrode is composed of flower-like Pt nanostructures. Furthermore, each Pt flower consists of numerous of petal-like Pt nanosheets with a thickness of around 25–30 nm, which is expected to significantly enhance the active surface-area and roughness of Pt counter electrode. Additionally, more liquid electrolyte can be absorbed by the hierarchical flower-like Pt because of its unique structure, demonstrating an effective contact between Pt nanoflowers and the electrolyte containing the I−/I3− redox couple. Compared with conventional Pt particles, the unique structure of hierarchical Pt flowers provide more electrocatalytic active sites for the reduction of I3− (reaction: I3− + 2e− = 3I−) in the counter electrode, this largely benefits the electrocatalytic activity of the counter electrode and logically leads to an improved DSSC performance.
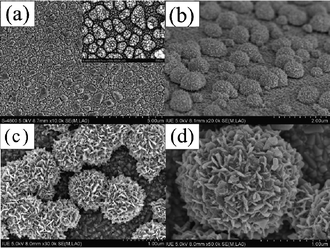 | ||
| Fig. 1 SEM images of the Pt counter electrodes: (a) top-view SEM image of a conventional Pt nanoparticle electrode; (b) tilted-section, and (c) & (d) top-view SEM images of the flower-like Pt electrode. | ||
Fig. 2A shows the cyclic voltammogram (CV) of the conventional and flower-like Pt electrodes at a scan rate of 50 mV s−1, in which two pairs of redox peaks can be observed for both of the electrodes. The left redox peaks correspond to eqn (1) and the right ones correspond to eqn (2)29,30
| 3I− − 2e = I3− | (1) |
| I3− + 2e = 3I− | (2) |
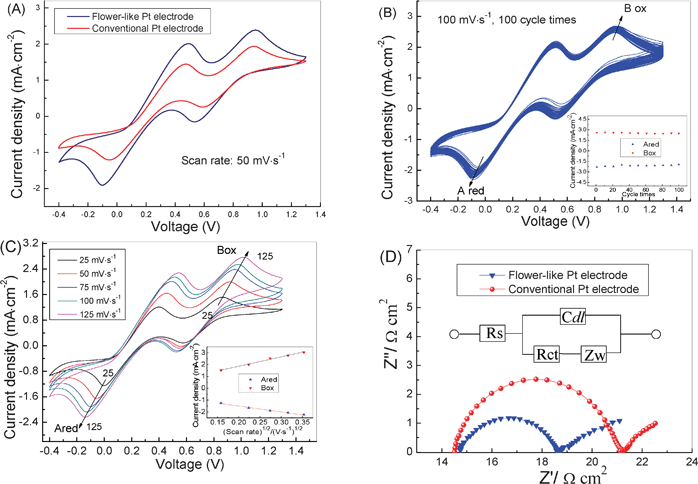 | ||
| Fig. 2 Electrochemical characterization for conventional and flower-like Pt counter electrodes. (a) The CV curves of the conventional and flower-like Pt electrodes in acetonitrile solution containing 0.1 M LiClO4, 0.01 M LiI, 0.001 M I2 as supporting electrolyte at a scan rate of 50 mV s−1. (b) 100 cycles of the CVs on the flower-like electrode at a scan rate of 100 mV s−1; the inset shows the relationship between cycle number and the maximum redox peak current densities. (c) CVs of the flower-like Pt electrode at different scan rates (from inner to outer: 25, 50, 75, 100, 125 mV s−1); insets: the redox peak current versus square root of scan rates. (d) EIS spectra analysis of conventional and flower-like Pt counter electrodes. | ||
It can be clearly seen from Fig. 2A that the flower-like Pt electrode shows much larger redox peak densities compared to that of a conventional Pt electrode, indicating an improved electrocatalytic activity of the flower-like Pt electrode compared to a conventional Pt nanoparticle electrode. Fig. 2B shows 100 times continuous cycle scans of the flower-like Pt electrode at a scan rate of 100 mV s−1, and the relationship between cycle times and maximum redox peak current densities is also inserted in the figure. After 100 cycles, the maximum redox peak current densities scarcely decline, showing an excellent electrochemical stability of the flower-like Pt counter electrode. Fig. 2C presents the CVs of the flower-like Pt electrode at various scan rates, and the inserted figure illustrates the relationship between the redox peaks current versus square root of scan rates. With increasing scan rates, the oxidation and reduction peaks gradually and regularly shift to the more positive and negative positions, respectively. From the inserted figure, it can be seen that the current density versus (scan rate)1/2 plots have a good linear relationship, indicating there is no specific interaction between the I−/I3− redox couple and the Pt flowers.30,31Fig. 2D is the electrochemical impedance spectroscopy (EIS) analysis of the conventional and flower-like Pt electrodes, in which the flower-like Pt counter electrode includes a lower charge-transfer resistance (Rct) and ohmic serial resistance (Rs). A small Rct indicates a facile electron transfer, whereas a large Rct implies a sluggish electron transfer.32 The lower Rct for the flower-like Pt electrode may be responsible for its higher active surface area, better electrolyte absorption property and much higher electrocatalytic activity.
Tafel polarization measurement is an efficient way to characterize the electrochemical catalytic activities of counter electrodes. To further investigate the interfacial charge-transfer properties of the I−/I3− redox couple on the electrode surface, Tafel polarization characterizations were carried out in a dummy cell similar to the one used in the EIS measurements.33Fig. 3 shows the Tafel curves of the dummy cells based on flower-like Pt and conventional Pt electrodes. It shows the logarithmic current density (log J) as a function of the voltage (V) for the reduction of I3− to I−. In the Tafel zone, the anodic and cathodic branches of the flower-like Pt electrode show a larger slope than that of a conventional Pt electrode, which indicates the presence of a larger exchange current density (J0) on the flower-like Pt electrode surface.33 This means the flower-like Pt electrode has a more efficient catalytic activity for the reduction of I3− to I−. According to Ref. 33J0 is inversely proportional to Rct based on eqn (3). Combined with the EIS results, the flower-like Pt electrode shows a lower Rct, which is in agreement with Tafel polarization measurements on the whole.
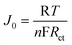 | (3) |
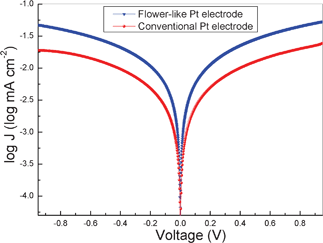 | ||
| Fig. 3 Tafel curves of different dummy cells that are similar to the ones used for the EIS measurements. | ||
where R is the gas constant, T is the temperature, F is the Faraday constant, and n is the number of electrons exchanged in the reaction at the electrolyte and counter electrode interface. In Fig. 3, we also found that the flower-like Pt electrode not only shows a larger J0 but also gives a larger limiting current density (Jlim). The Jlim depends on the diffusion coefficient of the I−/I3− redox couple in the DSSC system.10According to eqn (4),33 we can infer that there is a larger diffusion coefficient in the symmetrical dummy cell.
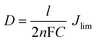 | (4) |
Where D is the diffusion coefficient of the I3−, l is the spacer thickness, n is the number of electrons involved in the reduction of I3− at the electrode, F is the Faraday constant, and C is the I3− concentration.
Briefly, the CV and EIS measurement and Tafel polarization results indicate that the flower-like Pt electrode has an enhanced electrocatalytic activity compared to that of a conventional Pt electrode, and it can be logically expected that a DSSC based on a flower-like Pt counter electrode can achieve a much improved photovoltaic performance.
The photocurrent–voltage curves and photovoltaic parameters of the DSSCs based on conventional and flower-like Pt counter electrodes are shown in Fig. 4 and Table. 1. The DSSCs based on flower-like Pt achieve a short circuit current density (JSC) of 14.9 mA cm−2 and a light-to-electric conversion efficiency (η) of 7.69%. It is clearly demonstrated that all the photovoltaic parameters for the flower-like Pt electrodes are better than those of conventional Pt counter electrodes because of the special features in the hierarchical flower-like Pt counter electrode.
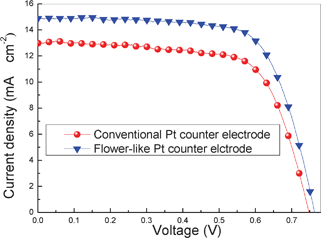 | ||
| Fig. 4 The photocurrent–voltage curves of the DSSCs based on conventional and flower-like Pt counter electrodes. | ||
| Counter electrode | J SC (mA cm−2) | V oc | FF | η (%) |
|---|---|---|---|---|
| Conventional Pt | 13.0 | 0.747 | 0.687 | 6.66 |
| Flower-like Pt | 14.9 | 0.764 | 0.697 | 7.94 |
According to Fig. 1, the hierarchical flower-like Pt electrode shows a rough surface for the numerous petal-like Pt nanosheets on the surface of the Pt flower. The larger surface area of the flower-like Pt electrode has a positive effect, enhancing the electrolyte|electrode contact area,35 giving more electrocatalytic sites for the reduction of I3− at the counter electrode. From Fig. 2 and Fig. 3, it's illustrated that the flower-like Pt counter electrode shows a higher electrocatalytic activity, a lower charge-transfer resistance, a larger J0 and Jlim compared to that of a conventional Pt electrode, resulting in fast reaction kinetics in the reduction of I3− at the counter electrode and a lower charge recombination and a suppressed dark current. Finally, the energy transfer efficiency can be greatly improved and better cell performances can be achieved for DSSCs based on the flower-like Pt counter electrode.
Summarily, a hierarchical flower-like Pt counter electrode was successfully prepared by a template-free method. The flower-like Pt electrode is composed of numerous of petal-like nanosheets with a thickness of 25–30 nm, showing a lower charge transfer resistance and a higher electrocatalytic activity for the I−/I3− redox reaction. A DSSC based on the flower-like Pt counter electrode achieves a relatively high light-to-current transfer efficiency of 7.94%, which is enhanced by 19.2% compared to that of a convention ball-like Pt nanoparticle coated counter electrode. The improved photoelectric conversion efficiency and the facile preparation process of the flower-like Pt counter electrode have significant importance for the practical usage of DSSCs.
Financial support from the National High Technology Research and Development Program of China (No. 2009AA03Z217), and the National Natural Science Foundation of China (Nos. 90922028, 50842027) is greatly appreciated. Technician Bin Xu at the Institute of Urban Environment, Chinese Academy of Sciences is also acknowledged for his assistance with the SEM measurements.
References
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Gratzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef CAS.
- M. Gratzel, Nature, 2001, 414, 338 CrossRef CAS.
- J. H. Wu, S. C. Hao, Z. Lan, J. M. Lin, M. L. Huang, Y. F. Huang, L. Q. Fang, S. Yin and T. Sato, Adv. Funct. Mater., 2007, 17, 2645 CrossRef CAS.
- J. H. Wu, Z. Lan, J. M. Lin, M. L. Huang, S. C. Hao, T. Sato and S. Yin, Adv. Mater., 2007, 19, 4006 CrossRef CAS.
- P. Prene, E. Lancelle-Beltran, C. Boscher, P. Belleville, P. Buvat and C. Sanchez, Adv. Mater., 2006, 18, 2579 CrossRef.
- S. X. Tan, J. Zhai, M. X. Wan, Q. B. Meng, Y. L. Li, L. Jiang and D. B. Zhu, J. Phys. Chem. B, 2004, 108, 18693 CrossRef CAS.
- J. H. Wu, S. C. Hao, Z. Lan, J. M. Lin, M. L. Huang, Y. F. Huang, P. J. Li, S. Yin and T. Satot, J. Am. Chem. Soc., 2008, 130, 11568 CrossRef CAS.
- M. Biancardo, R. Argazzi and C. A. Bignozzi, Inorg. Chem., 2005, 44, 9619 CrossRef CAS.
- S. M. Zakeeruddin and M. Grätzel, Adv. Funct. Mater., 2009, 19, 2187 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, J. E. Moser and M. Gratzel, J. Phys. Chem. B, 2003, 107, 13280 CrossRef CAS.
- R. Komiya, L. Y. Han, R. Yamanaka, A. Islam and T. Mitate, J. Photochem. Photobiol., A, 2004, 164, 123 CrossRef CAS.
- A. Nattestad, A. J. Mozer, M. K. R. Fischer, Y. B. Cheng, A. Mishra, P. Bauerle and U. Bach, Nat. Mater., 2010, 9, 31 CrossRef CAS.
- G. Veerappan, K. Bojan and S.-W. Rhee, ACS Appl. Mater. Interfaces, 2011, 3, 857 CAS.
- Z. Tachan, M. Shalom, I. Hod, S. Ruühle, S. Tirosh and A. Zaban, J. Phys. Chem. C, 2011, 115, 6162 CAS.
- S.-Q. Fan, B. Fang, J. H. Kim, B. Jeong, C. Kim, J.-S. Yu and J. Ko, Langmuir, 2010, 26, 13644 CrossRef CAS.
- B. Fang, S.-Q. Fan, J. H. Kim, M.-S. Kim, M. Kim, N. K. Chaudhari, J. Ko and J.-S. Yu, Langmuir, 2010, 26, 11238 CrossRef CAS.
- S. Zhang, C. Ji, Z. Bian, R. Liu, X. Xia, D. Yun, L. Zhang, C. Huang and A. Cao, Nano Lett., 2011, 11, 3383 CrossRef CAS.
- C. L. Lin, K. C. Huang, J. H. Huang, C. H. Wu, C. Y. Liu, H. W. Chen, C. W. Chu, J. T. Lin and K. C. Ho, J. Mater. Chem., 2011, 21, 10384 RSC.
- J. S. Jang, D. J. Ham, E. Ramasamy, J. Lee and J. S. Lee, Chem. Commun., 2010, 46, 8600 RSC.
- T. N. Murakami and M. Grätzel, Inorg. Chim. Acta, 2008, 361, 572 CrossRef CAS.
- F. Shao, J. Sun, L. Gao, S. Yang and J. Luo, ACS Appl. Mater. Interfaces, 2011, 3, 2148 CAS.
- H. B. Wu, J. S. Chen, X. W. Lou and H. H. Hng, J. Phys. Chem. C, 2011, 115, 24605 CAS.
- C. Ng, C. Ye, Y. H. Ng and R. Amal, Cryst. Growth Des., 2010, 10, 3794 CAS.
- B. K. Jena and C. R. Raj, Langmuir, 2007, 23, 4064 CrossRef CAS.
- M. H. Yang, F. L. Qu, Y. S. Lu, Y. He, G. L. Shen and R. Q. Yu, Biomaterials, 2006, 27, 5944 CrossRef CAS.
- J. I. Lee, S. H. Cho, S. M. Park, J. K. Kim, J. K. Kim, J. W. Yu, Y. C. Kim and T. P. Russell, Nano Lett., 2008, 8, 2315 CrossRef CAS.
- Y. M. Xiao, J. H. Wu, G. T. Yue, J. M. Lin, M. L. Huang and Z. Lan, Electrochim. Acta, 2011, 56, 8545 CrossRef CAS.
- D. H. G. Alexander I. Popov, J. Am. Chem. Soc., 1958, 80, 5346 CrossRef.
- Q. B. Meng, H. C. Sun, Y. H. Luo, Y. D. Zhang, D. M. Li, Z. X. Yu and K. X. Li, J. Phys. Chem. C, 2010, 114, 11673 Search PubMed.
- Y. Saito, W. Kubo, T. Kitamura, Y. Wada and S. Yanagida, J. Photochem. Photobiol., A, 2004, 164, 153 CrossRef CAS.
- C. Yoon, R. Vittal, J. Lee, W. Chae and K. Kim, Electrochim. Acta, 2008, 53, 2890 CrossRef CAS.
- L. Liu, S. H. Yoo and S. Park, Chem. Mater., 2010, 22, 2681 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20194a/ |
| This journal is © The Royal Society of Chemistry 2012 |
