Structural characteristics and flammability of fire retarding EPDM/layered double hydroxide (LDH) nanocomposites
De-Yi
Wang
*ab,
Amit
Das
b,
Andreas
Leuteritz
b,
R.N.
Mahaling
b,
Dieter
Jehnichen
b,
Udo
Wagenknecht
b and
Gert
Heinrich
bc
aCenter for Degradable and Flame-Retardant Polymeric Materials (ERCEPM-MoE), College of Chemistry, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610064, China. E-mail: dywangscu@gmail.com.; Fax: +86-28-85410755
bLeibniz-Institut für Polymerforschung Dresden e.V., Hohe Strasse 6, D-01069 Dresden, Germany
cTechnische Universität Dresden, Institut für Werkstoffwissenschaft, D-01069 Dresden, Germany
First published on 3rd February 2012
Abstract
A high performance elastomeric flame retardant nanocomposite was prepared which was based on maleic anhydride grafted ethylene-propylene-diene terpolymer (mEPDM), a one-step synthesised organo-layered double hydroxide (LDH), and an intumescent flame retardant (FR) comprised of pentaerythritol (PER), ammonium polyphosphate (APP) and methyl cyanoacetate (MCA). The morphology, fire behavior and mechanical properties of the flame-retarded mEPDM/LDH nanocomposite have been studied in detail. Wide angle X-ray scattering (WAXS), small angle X-ray scattering (SAXS) and TEM observation confirmed an exfoliated structure of LDH in a particular composite containing 2 phr (parts per hundred) LDH and 38 phr FR. As an effective flame retardant synergistic agent, MgAl–LDH shows a significant decrease in the heat release rate (HRR), low mass loss (ML) and low fire growth rate (FIGRA) of the nanocomposite. The flame retardant mechanism has been proposed, which is mainly due to the condensed phase flame retardant mechanism to form reinforced char layers during combustion, leading to the low volatiles produced. Moreover, as far as the mechanical properties of the vulcanizates are concerned, in all cases of flame retardant mEPDM and flame retarded mEPDM/LDH nanocomposites, they exhibit superior values compared to the gum compound.
1. Introduction
The application and development of polymeric materials have made a significant contribution to the electrical industry. They have considerable advantages concerning excellent insulation properties, low cost, and ease of fabrication and processing. Ethylene-propylene-diene terpolymer (EPDM) is just one distinguished member in this family. EPDM is known as a rubber with many remarkable properties, like electric properties, heat resistance, chemical resistance, and flexibility at low temperature, etc.1 However, a vital drawback for nearly all polymeric materials including EPDM is its flammability, which restricts the range of their application. Particularly, EPDM being purely hydrocarbon can easily catch fire. For example, in the case of the application of EPDM in the electrical field, overheating in conduits as a result of an imperfect connection could cause a fire. Thus, to be useful in electrical applications and other relevant fields, EPDM has to present good fire retardancy. However, so far there has been little research on the fire retardancy of the neat EPDM,2,3 except for a few publications on the fire retardance of EPDM/PP4 and EPDM/PE.5,6 In the case of the existing study on fire retarding EPDM/PP (or PE), the fire retardants that were mainly used were still halogenated additive retardants, like decabromodiphenyl oxide (FR-10), associated with antimony trioxide,4 and aluminum hydroxide (ATH).7 It was also reported that radiation crosslinking improved the fire retardancy of EPDM/HDPE/silicon rubber compounds.6 In addition, Chang et al.8 used nano-sized hydroxyl aluminum oxalate (HAO) and montmorillonite (MMT) as a flame retarded additive to the EPDM/LDPE system, observing that there was a synergistic flame retardance between HAO and MMT. Traditionally, although the halogen-antimony synergistic system was always thought of as an effective fire retardant method for most polymeric materials,9 its future would face tremendous pressure from the environmental concerns because the evolution of toxic gases and corrosive chemical fumes from combustion or pyrolysis, which can choke people exposed to the fumes and can damage costly equipment.10 So some legislation has been established to restrict the use of some halogen-containing flame retardant materials in some fields, especially as a result of toxicity concerns on various brominated derivatives. With respect to the low cost and toxicity,11,12 ATH could be a suitable fire retardant for EPDM. But the main problem of ATH as a fire retardant for EPDM is its low efficiency on fire retardancy as well as its flammability performance on other thermal plastic materials. For obtaining satisfied fire performance, usually, the loading content of ATH is over 60 wt% in the composite. High loadings of the additive influences the mechanical properties of EPDM, especially its flexibility. Therefore, it should be a very important and urgent task to study the flame retardation of EPDM by the introduction of new methods and technology.Compared with halogen based flame retardants, intumescent fire retardant (IFR) additives are more promising as an effective and “green” fire retardant based on their excellent intumescent carbonization. Many studies focused on IFR have been widely investigated in the fire retardation of polyolefin.13–17 Generally, they act by a condensed phase mechanism when heating or burning.18 On heating, intumescent fire retardant form a foamed cellular charred layer on the polymer surface, which acts as a physical barrier and protects the underlying materials from the action of heat and flame. Usually, such formulations contain three ingredients: an acid source, a blowing agent and a carbonization agent.18 In our earlier work, the intumescent fire retardant systems incorporation with a few amounts of (nano)filler on polylactic acid had been investigated,19 result showed that there was a good synergistic effect on fire retardancy between the intumescent fire retardant and (nano)filler. On the other hand, it is worth noting that, recently, layered double hydroxides (LDH) have drawn enormous attention among researchers as novel nanofillers for polymers due to their various unique properties, which are not common in layered silicates.20 LDH can be represented by the ideal formula [M2+1−xM3+x(OH) 2]x+An−x/n·yH2O, where M2+ and M3+ are divalent and trivalent metal cations, such as Mg2+ and Al3+, respectively, A is an anion, such as CO32−, Cl− and NO3−. Principally, the intercalated anions can be exchanged with wide variety of anionic species both of organic and inorganic origin opening a wide field of potential applications of LDH materials, for example, as catalysts,21 nanofiller22,23 and flame retardants.24–26 In the previous study27 it has been found that LDH could improve the mechanical properties and fire retardancy simultaneously for PE/LDH nanocomposites. However, a limitation in this case is that the efficiency of fire retardancy of LDH is not high enough due to its intrinsic structure based on metal hydroxides. However, LDH incorporation with an effective fire retardant could be a promising way to improve significantly the fire retardancy of the polymeric matrix, like the fire retarded PVA/LDH composites in our earlier work.28
In this paper, intumescent fire retardant technology and nanotechnology has been employed to study the fire retardancy of maleic anhydride grafted EPDM (mEPDM). The intumescent flame retardant (FR) being used in this paper is pentaerythritol (PER), methyl cyanoacetate (MCA) combined with ammonium polyphosphate (APP) at a desirable formulation (weight ratio APP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PER
PER![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MCA = 2
MCA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, according to the previous reports on flame retarded PLA).19 Organomodified MgAl–LDH (shown by LDH) as a nanofiller and synergistic fire retardant are introduced to the fire retarded mEPDM system. The morphology, fire behaviors and mechanical properties of the fire retarded mEPDM/LDH nanocomposite have been investigated. Many interesting results have been reported herein for the first time.
1, according to the previous reports on flame retarded PLA).19 Organomodified MgAl–LDH (shown by LDH) as a nanofiller and synergistic fire retardant are introduced to the fire retarded mEPDM system. The morphology, fire behaviors and mechanical properties of the fire retarded mEPDM/LDH nanocomposite have been investigated. Many interesting results have been reported herein for the first time.
2. Materials and experimental method
2.1 Materials
The metal nitrate salts [Mg(NO3)2 and Al(NO3)3] and sodium dodecyl benzene sulfonate (SDBS) which were used for the synthesis of organomodified MgAl–LDH were obtained from Aldrich Chemical Company. Pentaerythritol (PER) was obtained from Aldrich Chemical Company and used without further purification.Ammonium polyphosphate (APP) was provided by the Budenheim Iberica Company and methyl cyanoacetate (MCA) was supplied by CHEMOS GmbH. The maleic anhydride grafted EPDM (mEPDM), used in this study has an ethylene/propylene ratio of 55/45 and a maleic anhydride content of 1 wt%. Dicumyl peroxide (synthesis grade) was purchased from Merck, Hohenbrunn Germany.
2.2 Organic modification of LDH29
The organic Mg–Al LDHs were prepared by the one-step route as follows. The synthesis was carried out by the slow addition of a mixed metal (Mg2+ and Al3+) salt solution (with Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ equal to 2
Al3+ equal to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and a total metal ion concentration of 0.3 M) to a 0.12 M sodium dodecyl benzene sulfonate (SDBS) solution under continuous stirring maintaining the reaction temperature at 50 °C. During the synthesis the pH value was kept at 10 ± 0.2 by adding suitable amount of 1 M NaOH solution. After the addition of the mixed metal salt solution, the resulting slurry was continuously stirred at the same temperature for 0.5 h and then was allowed to age in heater at 75 °C for 18 h. The final products were filtered and washed several times with distilled water to get rid of any non-reacted surfactant molecules until the pH of the supernatant solution was about 7. The material was then dried in the oven at 80 °C until a constant weight was achieved and is called organomodified LDH.
1 and a total metal ion concentration of 0.3 M) to a 0.12 M sodium dodecyl benzene sulfonate (SDBS) solution under continuous stirring maintaining the reaction temperature at 50 °C. During the synthesis the pH value was kept at 10 ± 0.2 by adding suitable amount of 1 M NaOH solution. After the addition of the mixed metal salt solution, the resulting slurry was continuously stirred at the same temperature for 0.5 h and then was allowed to age in heater at 75 °C for 18 h. The final products were filtered and washed several times with distilled water to get rid of any non-reacted surfactant molecules until the pH of the supernatant solution was about 7. The material was then dried in the oven at 80 °C until a constant weight was achieved and is called organomodified LDH.
The characterization of organomodified LDH was carried out by WAXS and FTIR, respectively. The WAXS patterns of the organomodified LDH revealed that the DBS anion had been efficiently intercalated within the LDH layers by this method, showing the interlayer distance of 2.98 nm for the basal reflection (003). In the unmodified LDH, the first basal reflection (003) at 2θ = 11.8° corresponds to an interlayer distance of 0.77 nm. In FTIR spectrum of organomodified LDH, the characteristic vibration bands were detected for the SO3 group (symmetric stretching at 1037 cm−1 and asymmetric at 1182 cm−1), the benzene group (C–C stretching at 1496 and 1602 cm−1, C–H in plane bending at 1011 and 1131 cm−1) and alkyl group (asymmetric stretching of CH3 and CH2 at 2958 and 2926 cm−1 respectively, symmetric stretching of CH3 and CH2 at 2872 and 2855 cm−1, respectively, and CH2 scissoring at 1466 cm−1). The bands recorded below 800 cm−1, especially the sharp and strong characteristic band around 450 cm−1 arise due to the vibration of the metal–oxygen bond in the brucite-like lattice. These results indicate the presence of DBS molecules in all the modified LDHs and were consistent with the results of WAXS.
2.3 Preparation of the fire retarding mEPDM/LDH nanocomposite
The rubber compounds were prepared by a two-roll mixing mill (Polymix 110L, size: 203 × 102 mm2 Servitech GmbH, Wustermark, Germany) with the friction ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, rotating at 40 °C using a 20 min compounding cycle. All the weights were taken in parts per hundred of rubber and the recipe of the mEPDM compounds is given in Table 1.
1.2, rotating at 40 °C using a 20 min compounding cycle. All the weights were taken in parts per hundred of rubber and the recipe of the mEPDM compounds is given in Table 1.
| Compound | mEPDM | mEPDM–LDH40 | mEPDM–FR40 | mEPDM–FR40–LDH2 |
|---|---|---|---|---|
| a Dicumyl peroxide (DCP) was used as a crosslinking agent. | ||||
| mEPDM | 100 | 100 | 100 | 100 |
| LDH | 0 | 40 | 0 | 2 |
| FR | 0 | 0 | 40 | 38 |
| DCPa | 2 | 2 | 2 | 2 |
Firstly, requisite amounts of LDH and FR were incorporated in the rubber. After addition of those additives the dicumyl peroxide was added for the crosslinking of the rubber matrix. After mixing the rubber according to the above described procedure the compounded sample was obtained, and was subjected to the optimum curing time. In this procedure an uncured mass (∼6 mg) from the compounded rubber was subjected to a moving die rheometer (Scraeabaus SIS-V50) under isothermal condition at 160 °C with a frequency at 1.67 Hz and the generated torque was measure against time. From this torque-time curve the curing time was calculated to that point at which the rheometric torque reached 90% value of its ultimate torque. The rubber samples were then cured until their optimum curing time (t90) by a hot press (FORTUNE Holland, Modell TP 400) at 160 °C, cooled to room temperature and then kept for 24 h before doing any further tests.
2.4 Characterization techniques
Wide angle X-ray scattering (WAXS) was performed using a 2-circle diffractometer XRD 3003 θ/θ (GE Inspection Technologies/Seifert-FPM, Freiberg) with Cu-Kα radiation (λ= 0.1542 nm) in the range of 2θ = 0.5–25° using the step length of 0.05°. The accelerating voltage was 40 kV at a current of 30 mA. The interlayer distance in the LDH materials was calculated using the Bragg's equation.Small angel angle X-ray scattering (SAXS) were executed by means of a Kratky Compact camera (AntonPaar, Graz) equipped with a linear detector (mbraun, Garching) with Cu-Kα radiation (λ = 0.1542 nm) in the range of q = 0.2–6.6 nm−1 (Δq ≈ 0.008 nm−1).
Transmission electron microscopy (TEM) images of the nanocomposite specimens without staining were taken at room temperature. The TEM grids were mounted in a liquid nitrogen cooled sample holder. The ultrathin sectioning (50–70 nm) was performed by ultramicrotomy at low temperature using a Reichert Ultracut E low temperature sectioning system. A transmission electron microscope (JEM-100CX, JEOL) operated at 80 kV was used to obtain images of the nanocomposite specimens.
Scanning electron microscopy (SEM) (microscope model: LEO 435 VP, Carl Zeiss SMT) was used to study morphological features of the powdered samples. The samples were placed on a sample holder using a conducting carbon cement and were then coated with a thin layer of platinum (layer thickness 15 nm) using a sputter coater (BAL-TEC SCD 500 Sputter Coater).
Microscale combustion colorimeter (MCC-1, FTT) was used to investigate the combustion of fire retarded mEPDM according to ASTM D7309-07. In this system, about 5 mg samples was heated to 800 °C at a heating rate of 1 K s−1 in a stream of nitrogen flowing at 80 cm3 min−1. The volatile, anaerobic thermal degradation products in the nitrogen gas stream are mixed with a 20 cm3 min−1 stream of pure oxygen prior to entering a 900 °C combustion furnace. The specific heat release rate HRR (W g−1) and the heat release capacity HRC (J g−1 K) were recorded during the measurement. All samples were measured in triplicate.
Cone calorimeter test (FTT cone calorimeter): Square specimens (100 mm × 100 mm × 3 mm) were irradiated at a heat flux of 35 kW m−2 according to ISO 5660 standard procedures without the use of the “frame and grid” and exhaust flow was set at 24 L s−1.
Mechanical tests: Tensile tests have been done with a Zwick 1456 (model 1456, Z010, Ulm Germany) with cross head speed 200 mm min−1 (ISO 527). Dynamic mechanical analysis (DMA) was done with an Eplexor 2000N (Gabo Qualimeter, Ahlden, Germany) at a frequency of 10 Hz and within the temperature range of −80 to +80 °C. The samples were analyzed in the tension mode with 1% static prestrain and 0.5% oscillating strain. The measurements were performed with a heating rate of 2 K min−1 under liquid nitrogen flow. It was observed that the stress-strain properties were increased sufficiently after addition of FR and LDH what is very important for mEPDM rubber as this rubber itself without any fillers are very poor in strength.
3. Results and discussion
3.1 Structural characterization of fire retarding mEPDM/LDH nanocomposite
The morphology and structure of layered nanofiller containing polymer nanocomposite were generally characterized by X-ray scattering patterns and TEM analysis. X-ray scattering is one of the powerful technologies to characterize the layered structure of nano-materials, providing a way to determine the interlayer spacing of the LDH in the polymer/LDH nanocomposite. In comparison, TEM shows a qualitative understanding of the internal structure in the polymer nanocompostion through direct visualization. Fig. 1 shows the WAXS patterns of organomodified LDH, mEPDM–LDH40 and mEPDM–FR38–LDH2 composite.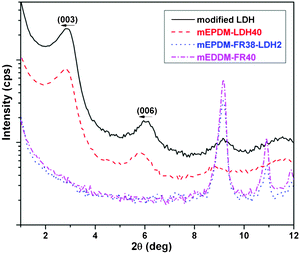 | ||
| Fig. 1 WAXS patterns of the modified LDH and fire retarded mEPDM/LDH composite. | ||
The first order basal diffraction maximum in modified LDH i.e., (003) at 2θ = 2.90° corresponds to an interlayer distance of 3.05 nm. In comparison to (003) basal reflection of modified LDH, for the mEPDM-LDH40 composite, the position of (003) has a minor shift to 2θ = 2.78° (d = 3.16 nm), indicating the inferior dispersion state of most of the LDH in the polymer matrix and higher loading of LDH leads to large agglomerated structures. The slight shift in the distance of the layers of LDH in mEPDM–LDH40 could be caused by somewhat swollen upon the mixing with rubber. In the case of mEPDM–FR38–LDH2 composite, there is almost no peak at the (003) basal reflection, indicating the disordered structure of LDH in the polymer matrix. It may say that a good dispersion of LDH in mEPDM rubber matrix could be obtained in mEPDM–FR38–LDH2, which could be relevant to both the matters of low concentration and some physical-chemistry interactions
In order to confirm the exfoliation of the mEPDM–FR38-–LDH2 nanocomposite, we performed SAXS experiments on the composites of mEPDM–LDH40 and mEPDM–FR38–LDH2. The scattering profiles are shown in Fig. 2. In the SAXS pattern of mEPDM–LDH40, there are two strong peaks at q = 2.0 nm−1 and 4.1 nm−1, meaning that the mean interlayer spacing of the (003) plane for the LDH in the polymer matrix is 3.14 nm (as determined by using the equation d = 2π/q). It means that there are many agglomerations of LDH particles in the mEPDM–LDH40 composite since the interlayer distance of the modified LDH is 3.05 nm. However, there are no peaks in the SAXS pattern of mEPDM–FR38–LDH2 nanocomposite in the wave vector values ranging 0.3–6.0 nm−1(corresponding to characteristic length scale, d, ranging from 21 to 1 nm), indicating that LDH in the nanocompostie has poor long-range order and/or a very small coherence length (due to a minor number of layers only). These results are in accordance with those from the WAXS.
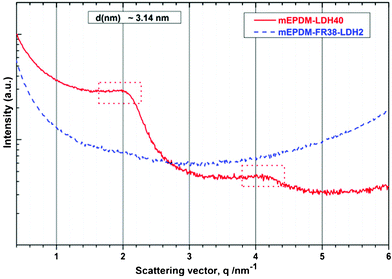 | ||
| Fig. 2 The small angle X-ray scattering (SAXS) patterns of the mEPDM–LDH40 and mEPDM–FR38–LDH2 nanocomposites. | ||
Although the X-ray analysis has shown the dispersion state of LDH in the mEPDM–FR38–LDH2 nanocomposite, more direct information could be observed by transmission electron microscopy (TEM). TEM micrographs of the mEPDM–FR38–LDH2 nanocomposite are presented in Fig. 3. It is clearly visible that the LDH sheets are randomly dispersed in the rubber matrix, providing direct evidence of crystal layer exfoliation. TEM images also show some large ordered parts, which looks like intercalated nanostructures. By measurement, the distances between the sheets in the ordered parts are greater than 18 nm, which match very well with the conclusion derived from the X-ray results. Therefore, based on the X-ray results (WAXS and SAXS) and TEM observation, it can be concluded that an exfoliation structure is formed in the case of the mEPDM–FR38–LDH2 nanocomposite.
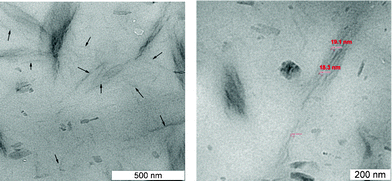 | ||
| Fig. 3 TEM for fire retarded mEPDM/LDH nanocomposite. | ||
3.2 Fire behaviour of the fire retarding mEPDM/LDH nanocomposites
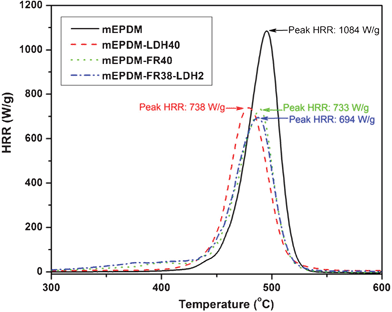 | ||
| Fig. 4 HRR of samples from microscale combustion calorimeter (MCC). | ||
While in mEPDM pHRR is 1084 W g−1, it decreases to 733 and 694 W g−1 in mEPDM–FR40 and mEPDM–FR38–LDH2. This is above 32.4% and 36.0% reduction, respectively. In the case of the pHRR of mEPDM–FR38–LDH2, even after taking into account the dilution effect by the filler it can be considered as an improvement in flame resistance. These results suggest that the degradation pathway of mEPDM is changed when containing FR and LDH. The reason is that the MCC measurement process will not afford an efficient physical barrier, which usually will reduce the heat release rate via limiting the heat and mass transfer. The changed HRR can only be due to modification of the degradation pathway of the polymer, leading to the chemical changes in the evolving phase. This conclusion is accorded to the report of the PLA/f-MWNT nanocomposite.31 The heat release capacity (HRC) is an important parameter used to predict and evaluate the fire hazard. The HRC values are obtained as the sum of all peak HRR values. mEPDM gave the highest HRC of 1212 J g−1 K−1, while the mEPDM–FR40 and mEPDM–FR38–LDH2 nanocomposites showed much lower HRC values, which are 799 and 781 J g−1 K−1, showing a similar trend with pHRR.
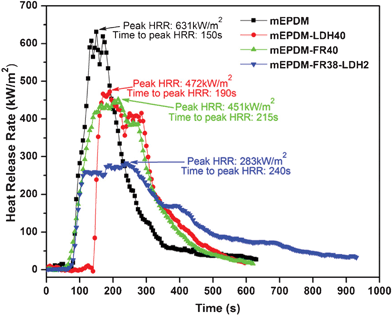 | ||
| Fig. 5 HRR of samples from cone calorimeter test (CCT). | ||
Another notable difference in the experiments using the cone calorimeter comes from the time to peak HRR (t-pHRR). The t-pHRR of mEPDM and mEPDM–FR40 are 190 s and 215 s, respectively. However, the t-pHRR of mEPDM–FR38–LDH2 is 240 s, which is much longer than that of mEPDM. We believe that this results from improvements in the performance of the protective char layer formed after ignition, indicating that a high quality protective char layer was formed after the addition of small quantities of LDH. This is consistent with the observation of the burning process and the residues remaining after the cone calorimeter test. Fig. 6 shows weight loss as a function of combustion time for the materials. It can be seen that there is a significant difference between materials with and without LDH.
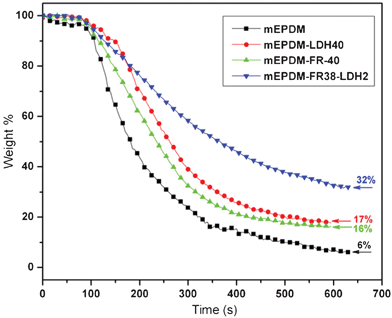 | ||
| Fig. 6 Mass loss of samples from cone calorimeter test. | ||
In the burning test, mEPDM burns rapidly after ignition with little residue left (6 wt%), and mEPDM–FR40 has 17 wt% char residues left after burning. By comparison, 32 wt% char residue remains after burning mEPDM–FR38–LDH2. Furthermore, the residue on the mEPDM–FR40 surface is loose and friable by comparison with the residues from the mEPDM–FR38–LDH2, while the char a residue from mEPDM–FR38–LDH2 shows a more compact char layer. This continuous compact char layer would prevent the effective transfer of gas and volatiles through the surface, and so preventing burning.
FIGRA (fire growth rate), computed as FIGRA = pHRR/time to pHRR, provides an estimation of both the spread rate and the size of a fire.32 So, the FIGRA index is a good indicator of the contribution to fire growth of materials. FIGRA of pHRR for all studied samples has been shown in Fig. 7. A lower value indicates a better cone calorimeter performance and Fig. 7 clearly shows that the mEPDM–FR38–LDH2 performs much better than the others according to this measure. This probably arises from the LDH in the system, but the detailed reason for them will require some further elucidation.
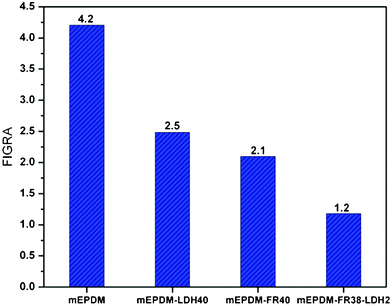 | ||
| Fig. 7 FIGRA (fire growth rate) of all studied samples. | ||
According to the results from both the MCC and CCT, introduction of FR and LDH can improveme the flame retardancy of mEPDM. To understand the flame retardant mechanism, the morphological structures for the chars after combustion have been analyzed as shown in Fig. 8.
 | ||
| Fig. 8 SEM for the residue of the fire retarded EPDM/LDH nanocomposite after combustion. | ||
In the case of neat mEPDM, the material was almost completely burned and little char was left. In comparison, for the flame retardant mEPDM/LDH nanocomposite, there was a large amount of char residues remaining (32 wt% char residues in Fig. 6). From Fig. 8 it can be observed that the morphology of char is the typical intumescent char with mostly unbroken char-bubbles.19 It can be seen that the surface of the char residue is continuous, compact and rough. Besides carbonaceous materials in the char, the rough char layers also comprise some particles to compose composited char layers. The particles enclosed in the char layers may correspond to the decomposition residue of LDH. The reinforced char layer will provide a good barrier to prevent the transfer of heat and volatiles, indicating that it could significantly improve the flame retardance of the flame retardant mEPDM/LDH nanocomposite. The reason for this is that, as it is well known, the fire would be extinguished if the content of heat and fuel (volatiles) was under limitation. Therefore, controlling of the transfer of heat and the amount of volatiles would be very important in the improvement of materials' flame retardance.
3.3 Mechanical properties of fire retarding mEPDM/LDH nanocomposites
The maleic anhydride grafted EPDM and its composites were peroxide cured with dicumyl peroxide and the curing characteristics are presented in Table 2. It is observed from this table that the addition of LDH makes the rubber compounds more scorchy (shorter t2 values) but mEPDM–FR40 has no influence on this property. However, the curing times are reduced in all cases after the addition of LDH and FR. As far as the ultimate rheometric torque is concerned, an improvement of the torque has been observed for 40 phr LDH filled mEPDM but the addition of 40 phr FR and 38 phr FR conjugated with 2 phr LDH do not show any noticeable differences as compared with the rubber without any filler.The physical properties of mEPDM without any filler (gum vulcanizate) are very poor and reinforcement by filler addition is a very important step for the practical application of this rubber. It is found that after the addition of 40 phr FR, the 100% modulus (strength at 100% modulus) increases only a little, but the replacement of 2 phr FR by 2 phr LDH causes the 100% modulus values to be increased further, which makes a noticeable difference as compared with the pure gum. As discussed with the morphology of the sample mEPDM–FR38–LDH2, the existence of intercalated and exfoliated layers in the rubber matrix (TEM figure) clearly reinforces the mEPDM rubber matrix and reflects into the modulus values. Dynamic mechanical analysis is thought to be a useful tool to understand the reinforcing activity of LDH in the mEPDM rubber. It can be observed in Fig. 9, that there is no significant change in the dynamic properties between the mEPDM compounds with the temperature sweep measurements. However, at the rubbery region, the storage modulus of all the filled vulcanizates show that a slight increment has been found. A slight reduction of the tan δ peak height also observed after incorporation of the fillers inside. So in this particular mEPDM composite the effect of reinforcement of LDH has been observed in stress–strain properties and also has been reflected on the dynamic mechanical properties. For all the cases a sharp glass transition temperature has been detected around −34 °C temperature.
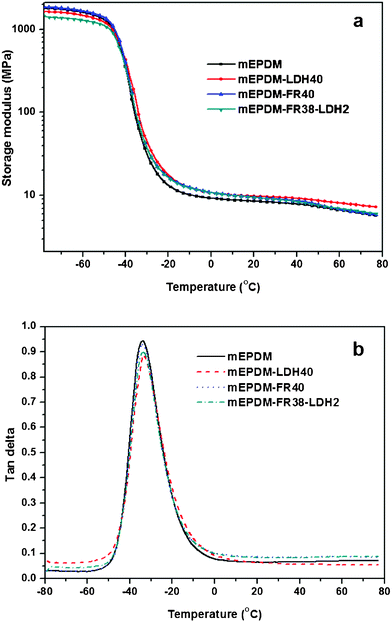 | ||
| Fig. 9 Dynamic mechanical analysis (DMA) of the samples; (a) temperature dependence of storage modulus (b) temperature dependence of tanδ. | ||
4. Conclusion
In this work, the flame retardant mEPDM and their nanocomposites have been prepared, based on the intumecesent flame retardant and a one-step synthesised organo LDH. The morphology, fire behavior, flame retardant mechanism and mechanical properties of the flame retarded mEPDM/LDH nanocomposites have been studied. The results showed that the introduction of a small amount of LDH in the flame retardant mEPDM led to the significant decrease in HRR, low ML and FIGRA. A flame retardant mechanism had been proposed, which is mainly due to the condensed phase flame retardant mechanism to form reinforced char layers during combustion, leading to low volatiles being produced.Acknowledgements
Prof. Dr De-Yi Wang thanks the Alexander von Humboldt (AvH) Foundation, National Natural Science Foundation of China (21074076), and Organisation for the Prohibition of Chemical Weapons (OPCW).References
- A. K. Bhowmick, C. Stein and H. L. Stephens, Polynorbornene rubber, in Handbook of elastomers, Marcel Dekker, New York, 1981, p. 729 Search PubMed.
- D. Kang, D. Kim, S. H. Yoon, D. Kim and C. Barry, Macromol. Mater. Eng., 2007, 292(3), 329 CrossRef CAS.
- Y. L. Lu, F. Y. Ye, L. X. Mao, Y. Li and L. Q. Zhang, eXPRESS Polym. Lett., 2011, 5(9), 777 CrossRef CAS.
- L. Yu, W. J. Wang and W. D. Xiao, Polym. Degrad. Stab., 2004, 86, 69 CrossRef CAS.
- Z. H. Chang, F. Guo, J. F. Chen, J. H. Yu and G. Q. Wang, Polym. Degrad. Stab., 2007, 92, 1204 CrossRef CAS.
- S. J. Jia, Z. C. Zhang, Z. W. Du, R. R. Teng and Z. Z. Wang, Radiat. Phys. Chem., 2003, 66, 349 CrossRef CAS.
- C. Canaud, L. L. Y. Visconte, M. A. Sens and R. C. R. Nunes, Polym. Degrad. Stab., 2000, 70, 259 CrossRef CAS.
- Z. H. Chang, F. Guo, J. F. Chen, L. Zuo, J. H. Yu and G. Q. Wang., Polymer, 2007, 48, 2892 CrossRef CAS.
- S. H. Chiu and W. K. Wang, Polymer, 1998, 39, 1951 CrossRef CAS.
- A. K. Sen, B. Mukheriee, A. S. Bhattacharya, L. K. Sanghi, P. P. De and K. Bhowmich, J. Appl. Polym. Sci., 1991, 43, 1673 CrossRef CAS.
- F. K. Jones, J. L. Laird and B. W. Smith, Rubber World., 1996, 215, 42 CAS.
- R. D. Allen, Improving the high temperature performance of EPDM, in Handbook of polymer science and technology, ed. N. P. Cheremisino, Marcel Dekker, New York, 1989, p. 127 Search PubMed.
- M. L. Bras, M. Bugajny, J. M. Lefebvre and S. Bourbigot, Polym. Int., 2000, 49, 1115 CrossRef.
- D. Y. Wang, X. X. Cai, Y. Liu, J. S. Wang and Y. Z. Wang, Polym. Degrad. Stab., 2008, 93(12), 2186 CrossRef CAS.
- R. C. Xie and B. J. Qu, J. Appl. Polym. Sci., 2001, 80, 1181 CrossRef CAS.
- F. Xie, Y. Z. Wang, B. Yang and Y. Liu Macro, Mater. Eng., 2006, 291, 247 CAS.
- D. Y. Wang, Y. Liu, Y. Z. Wang, C. P. Artiles, T. R. Hull and D. Price, Polym. Degrad. Stab., 2007, 92, 1592 CrossRef CAS.
- G. Camino, Polym. Degrad. Stab., 1989, 23, 359 CrossRef CAS.
- D. Y. Wang, A. Leuteritz, Y. Z. Wang, U. Wagenknecht and G. Heinrich, Polym. Degrad. Stab., 2010, 95(12), 2474 CrossRef CAS.
- F. R. Costa, M. Grenzer, U. Wagenknecht and G. Heinrich, Adv. Polym. Sci., 2008, 210, 101 CrossRef CAS.
- F. Li and X. Duan, Struct. Bonding, 2006, 119, 193 CrossRef CAS.
- A. Das, D. Y. Wang, A. Leuteritz, K. Subramaniam, H. C. Greenwell, U. Wagenknecht and G. Heinrich, J. Mater. Chem., 2011, 21, 7194 RSC.
- P. J. Purohit, J. H. Sanché, D. Y. Wang, F. Emmerling, A. Thünemann, G. Heinrich and A. Schönhals, Macromolecules, 2011, 44, 4342 CrossRef CAS.
- D. Y. Wang, A. Das, F. R. Costa, A. Leuteritz, Y. Z. Wang, U. Wagenknecht and G. Heinrich, Langmuir, 2010, 26(17), 14162 CrossRef CAS.
- D. Y. Wang, A. Das, A. Leuteritz, R. Boldt, L. Häuβler, U. Wagenknecht and Ge. Heinrich, Polym. Degrad. Stab., 2011, 96, 285 CrossRef CAS.
- D. Y. Wang, A. Leuteritz, M. A. Landwehr, U. Wagenknecht and G. Heinrich, J. Alloys Compd., 2011, 509, 3497 CrossRef CAS.
- F. R. Costa, U. Wagenknecht and G. Heinrich, Polym. Degrad. Stab., 2007, 92, 1813 CrossRef CAS.
- C. X. Zhao, Y. Liu, D. Y. Wang, D. L. Wang and Y. Z. Wang, Polym. Degrad. Stab., 2008, 93, 1323 CrossRef CAS.
- D. Y. Wang, F. R. Costa, A. Vyalikh, A. Leuteritz, U. Scheler, D. Jehnichen, U. Wagenknecht, L. Häußler and G. Heinrich, Chem. Mater., 2009, 21(19), 4490 CrossRef CAS.
- P. M. Hergenrother, C. M. Thompson, J. G. Smith, J. W. Connell, J. A. Hinkley, R. E. Lyon and R. Moulton, Polymer, 2005, 46, 5012 CrossRef CAS.
- S. Bourbigot and G. Fontaine, Polym. Chem., 2010, 1, 1413 RSC.
- CENT/TC127N 1424, Reaction to fire tests on building products (“SBI”), Draft, 26 February 1999.
| This journal is © The Royal Society of Chemistry 2012 |
