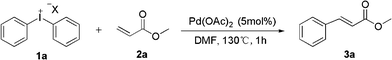
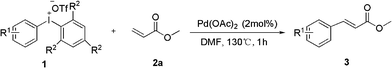
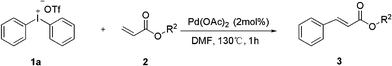
Palladium-catalyzed heck-type arylation of acrylate with diaryliodonium salts†
Jian
Li
*a,
Li
Liu
b,
Yun-yao
Zhou
b and
Sai-nan
Xu
b
aSchool of Pharmaceutical Engineering & Life Sciences, Chang Zhou University, Changzhou, 213164, P.R.China. E-mail: lijianchem@gmail.com
bAnalytical center, Chang Zhou University, Changzhou, 213164, P.R. China
First published on 31st January 2012
Abstract
Highly effective palladium-catalyzed Heck-type arylation of acrylate with diaryliodonium salts has been developed, giving cinnamate in high yields without ligand and base.
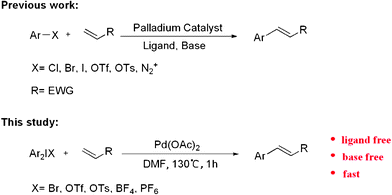
The formation of a carbon–carbon bond by palladium-catalyzed coupling of aryl or vinyl halides with olefins, known as the Heck reaction, has become a powerful tool in organic chemistry1 and occupies a special place for its resourceful versatility.2 Under its usual form, the Heck reaction involves the coupling of aryl, vinyl, benzyl, or allyl halides with olefins in the presence of a homogeneous or heterogeneous source of palladium as catalyst under basic conditions (Scheme 1). However, Pd(II)-precatalysts, such as Pd(OAc)2, PdCl2(PPh3)2 or PdCl2CH3CN are usually preferred in association with stabilizing ligands, such as phosphines or carbenes.3
 | ||
| Scheme 1 Arylation of acrylate. | ||
The use of diaryliodonium salts has recently gained considerable attention in organic synthesis.4 Remarkable progress in both metal-catalyzed and metal-free direct arylations of indoles,5 pyrroles,6 phenols,7 carboxylic acids,8 aniline9 and carbonyls10 has been accomplished through the use of diaryliodonium salts as arylating reagents. In particular, the groups of Gaunt and Olofsson have conducted lots of work with the synthesis and applications of diaryliodonium salts.11,12 Recently, Pd–Ag catalyzed and Pd catalyzed arylation of alkenes with PhI(OAc)2 were reported by the groups of Magedov and Mao.13
As part of our ongoing interest in developing applications of diaryliodonium salts via transition metal catalysis, we herein wish to report a palladium-catalyzed Heck-type arylation of acrylate with diaryliodonium salts without ligand and base (Scheme 1). At the outset of our studies, a set of experiments was carried out using diphenyliodonium salt 1a and methacrylate 2a as model substrates. We tested various reaction conditions for the arylation of methacrylate (Table 1).
| Entry | Catalyst | X | Solvent b | Yield (%)c |
|---|---|---|---|---|
| a Diphenyliodonium salt (0.4 mmol) and methacrylate (2 equiv.) were mixed in solvent (1 mL) at r. t. before addition of Pd(OAc)2 and heating to 130 °C. b Solvent not dried. c Isolated yield. | ||||
| 1 | — | OTs | toluene | — |
| 2 | Pd(OAc)2 | OTs | toluene | 23 |
| 3 | Pd(OAc)2 | OTs | DMF | 83 |
| 3 | Pd(OAc)2 | OTs | DMSO | 65 |
| 4 | Pd(OAc)2 | OTs | DMAC | 74 |
| 5 | Pd(OAc)2 | OTs | NMP | 21 |
| 6 | Pd(OAc)2 | OTs | DCE | 61 |
| 7 | PdCl2 | OTs | DMF | 77 |
| 8 | Pd2(dba)3 | OTs | DMF | 73 |
| 9 | Pd(OAc)2 | BF4 | DMF | 85 |
| 10 | Pd(OAc)2 | PF6 | DMF | 81 |
| 11 | Pd(OAc)2 | Br | DMF | 64 |
| 12 | Pd(OAc)2 | OTf | DMF | 99 |
| 13 | Pd(OAc)2(2% mol) | OTf | DMF | 99 |
| 14 | Pd(OAc)2(0.5% mol) | OTf | DMF | 91 |
First, these two coupling partners were reacted in DMF at 130 °C without the presence of Pd(OAc)2, and no cinnamate product 3a was obtained (entry 1, Table 1). When 5% mol of Pd(OAc)2 was employed, we are pleased to find that the reaction occurred to afford 3a in 23% yield (entry 2, Table 1), from GC-MS we find the major product was diphenyl from the coupling of diphenyl iodine 1a and toluene. So we change toluene into DMF which, gratifyingly, resulted in significant increase of the yield. Screening of solvents with Pd(OAc)2 revealed that the yield of 3a was much higher in DMF than in DMSO, DMAC, NMP and DCE. After screening of various palladium catalysts, we found that Pd(OAc)2 was the most effective catalyst than the other two palladium catalysts, such as PdCl2 and Pd2(dba)3 (entries 7, 8, Table 1). Further studies were thus carried out about the influence of different diphenyliodonium anions, which showed that satisfactory results could be obtained with diaryliodonium tetrafluoroborates, hexafluorophosphates and bromide (entries 9–11, Table 1). Gratifyingly, significant improvement was achieved by the use of diphenyliodonium triflate resulting in clean formation of the product in 99% yield (entry 13). Moreover, decreasing the amount of Pd(OAc)2 to 2% mol led to the same yield and 0.5% mol of catalyst led to the reduction of yields (entry 14, Table 1).
With optimized reaction conditions in hand, we probed the scope of methacrylate with different diaryliodonium triflate employing 2% mol of Pd(OAc)2 in DMF at 130 °C. The results are summarized in Table 2.
| Entry | Ar | Product 3 | Yield (%)b |
|---|---|---|---|
| a Diaryliodonium triflate salt (0.4 mmol) and Methacrylate (2 equiv.) were mixed in solvent (1 mL) at r.t. before addition of Pd(OAc)2 (2% equiv.) and heating to 130 °C. b Isolated yield. | |||
| 1 | C6H51a | 3a | 99 |
| 2 | 4-ClC6H41b | 3b | 93 |
| 3 | 4-FC6H41c | 3c | 92 |
| 4 | 4-NO2C6H41d | 3d | 96 |
| 5 | 4-EtOOCCOC6H41e | 3e | 91 |
| 6 | 4-PhC6H41f | 3f | 86 |
| 7 | 2-MeC6H41g | 3g | 93 |
| 8 | 3-BrC6H41h | 3h | 92 |
| 9 | 2-thiophen 1i | 3i | 76 |
| 10 | 2-pyridine 1j | 3j | 53 |
The reaction scope was subsequently explored by using various asymmetrical diaryl iodonium salts 1 which were prepared with appropriate iodoarene and mesitylene.14 Excellent isolated yields of methyl cinnamate 3 were obtained with 2% mol of Pd(OAc)2 in one hour. Substituted diphenyl iodoniumtriflate with electron-withdrawing substituents worked equally well as those with electron-donating substituents, giving cinnamates 1a–e in high yields (entries 1–6, Table 2). In particular, diaryl iodonium salts of p-nitrobenzene 1d resulted in higher yield of product 3d (entry 4). Likewise, ortho-substituted salts posed no problem in this reaction, as exemplified by o-methyl product 3g (entry 7). Steric bulk in the meta-position was very well tolerated, as demonstrated by arylation with salt 1h to yield high congested product 3h (entry 8). Furthermore, heterocyclics such as pyridine and thiophene, which are privileged scaffold, smoothly proceed to give 3i and 3j in good yield (entries 9, 10).15
The results encouraged us to extend our protocol to investigate this new Heck-type reaction. In this regard substrate acrylate 2 was surveyed under the optimized reaction conditions. To our delight, the results exceeded our expectations, and were somewhat better than those for the methacrylate. We found that this methodology was broadly applicable to a variety of acrylates, affording arylated products in synthetically valuable yields (Table 3). All of the substrates bearing either an ester group or an acid group formed the desired products. Generally, the reactions of substrates with more than two carbons of the ester bond afforded much better results than the methyl one (entries 3–5, Table 3). Excitingly, an extremely efficient reactions were observed in the case of 2c and 2e, products 3ac and 3ae being obtained in high yield after 1 h (97%, entries 3, 5). The substrates of acrylic acid also worked well and afforded products 3ab, 3ag in 81% and 92% yield, respectively (entries 2, 7).
| Entry | 2 | Product 3 | Yield (%)b |
|---|---|---|---|
| a Diaryliodonium triflate salt (0.4 mmol) and methacrylate (2 equiv.) were mixed in solvent (1 mL) at r.t. before addition of Pd(OAc)2 (2% equiv.) and heating to 130 °C. b Isolated yield. | |||
| 1 | Methyl acrylate 2a | 3a | 99 |
| 2 | Acrylic acid 2b | 3ab | 81 |
| 3 | Ethyl acrylate 2c | 3ac | 97 |
| 4 | Butyl acrylate 2d | 3ad | 96 |
| 5 | 2-Ethylhexyl acrylate 2e | 3ae | 97 |
| 6 | 2-Hydroxyethyl acrylate 2f | 3af | 85 |
| 7 | Methacrylic acid 2g | 3ag | 92 |
| 8 | Methyl methacrylate 2h | 3ah | 51 |
It is interesting that the substrate of methyl methacrylate 2h exhibited a poor effect to yield product (entry 8). From GC-MS we found the major product was our expectation, but only 56% yield was afforded; the other side product was methyl-2-phenylacrylate which yields 23%, and the third one was cyclopropane product which yields 9% only. We hypothesize that the cyclopropane product was derived from the major product (Scheme 2). In order to prove our speculation, a reaction was carried out with allyl chloride which was easily transferred into cyclopropane. Rewardingly, the reaction furnished the desired product in 53% yield (Scheme 3).16
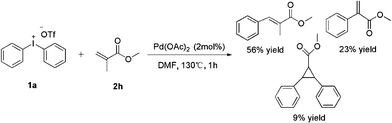 | ||
| Scheme 2 Phenylation of methacrylate. | ||
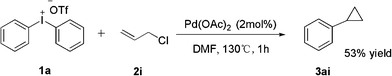 | ||
| Scheme 3 Phenylation of allyl chloride. | ||
Finally, in order to expand the use of the method, an additional attempt on styrene was developed without problem; diphenylethene was obtained in high yield (Scheme 4).
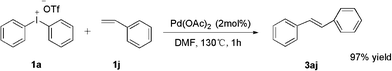 | ||
| Scheme 4 Phenylation of styrene. | ||
The plausible mechanism of this heck-type reaction is depicted in Scheme 5. At first, oxidative addition occurred of Pd(OAc)2 with diaryliodonium salts in the reaction system to start the first catalytic cycle, which affording Pd-complex 4. The next step (4→5) is carbo-palladation followed by β-hydride elimination affording complex 6. Elimination of palladium hydride from intermediate 6 provides the product 3 and the Pd(OAc)2, which is used for the next catalytic cycle.
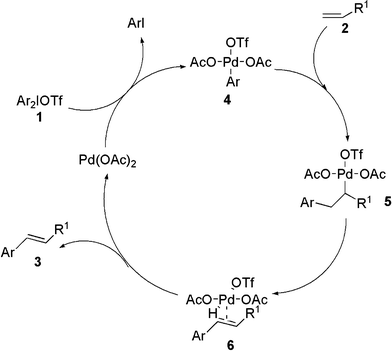 | ||
| Scheme 5 Possible mechanism of the arylation of acrylate reaction. | ||
In conclusion, a fast and high regioselective Heck-type reaction has been developed with acrylate and diaryliodonium salts. Good to excellent yields are obtained without the use of ligand and base in this Palladium-Catalyzed system. Current studies are focused on further exploration of the substrate scope and synthetic utility of this catalyzed system.
Acknowledgements
Financial support from the Foundation of Chang Zhou University (No. ZMF1002130 & ZMF1002100), Changzhou Municipal Bureau of Science and Technology (No. KYZ1102100C) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- (a) R. F. Heck, Acc. Chem. Res., 1979, 12, 146–151 CrossRef CAS; (b) R. F. Heck, Org. React., 1982, 27, 345–390 CAS; (c) G. D. Davis and A. Hallberg, Chem. Rev., 1989, 89, 1433–1445 CrossRef.
- (a) I. P. Beletskaya and A.V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS; (b) N. J. Whitcombe, K. K. Hii and S. E. Gibson, Tetrahedron, 2001, 57, 7449–7476 CrossRef CAS; (c) A. De Meijere and F. E. Meyer, Angew. Chem., 1994, 106, 2473–2506 CrossRef CAS.
- (a) For reviews, see: A. De Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379–2411 CrossRef; (b) S. E. Gibson and R. J. Middleton, Contemp. Org. Synth., 1996, 3, 447–471 RSC; (c) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2963 CrossRef CAS; (d) V. Farina, Adv. Synth. Catal., 2004, 346, 1553–1582 CrossRef CAS.
- (a) For reviews on diaryliodonium salts, see: E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052–9070 CrossRef CAS; (b) A. Varvoglis, The Organic Chemistry of Poly coordinated Iodine; VCH Publishers: New York, 1992 Search PubMed; (c) V. V. Grushin, Chem. Soc. Rev., 2000, 29, 315–324 RSC; (d) J. M. Becht and C. L. Drian, Org. Lett., 2008, 10, 3161–3164 CrossRef CAS; (e) X. Qu, P. Sun, T. Li and J. Mao, Adv. Synth. Catal., 2011, 353, 1061–1066 CrossRef CAS; (f) J. Aydin, J. M. Larsson, N. Selander and K. J. Szabó, Org. Lett., 2009, 11, 2852–2854 CrossRef CAS.
- (a) L. Ackermann, M. Dell'Acqua, S. Fenner, R. Vicente and R. Sandmann, Org. Lett., 2011, 13, 2358–2360 CrossRef CAS; (b) R. J. Phipps, N. P. Grimster and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 8172–8174 CrossRef CAS; (c) N. R. Deprez, D. Kalyani, A. Krause and M. S. Sanford, J. Am. Chem. Soc., 2006, 128, 4972–4973 CrossRef CAS.
- A. M. Wagner and M. S. Sanford, Org. Lett., 2011, 13, 288–291 CrossRef CAS.
- N. Jalalian, E. E. Ishikawa, L. F. Silva Jr and B. Olofsson, Org. Lett., 2011, 13, 1552–1555 CrossRef CAS.
- T. B. Petersen, R. Khan and B. Olofsson, Org. Lett., 2011, 13, 3462–3465 CrossRef CAS.
- (a) H. A. Duong, R. E. Gilligan, M. L. Cooke, R. J. Phipps and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 463–466 CrossRef CAS; (b) C.-L. Ciana, R. J. Phipps, J. R. Brandt, F.-M. Meyer and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 458–462 CrossRef CAS; (c) B. Xiao, Y. Fu, J. J. Xu, T. Gong, J. J. Dai, J. Yi and L. Liu, J. Am. Chem. Soc., 2010, 132, 468–469 CrossRef CAS; (d) O. Daugulis and V. G. Zaitsev, Angew. Chem., Int. Ed., 2005, 44, 4046–4048 CrossRef CAS; (e) D. Kalyani, N. R. Deprez, L. V. Desai and M. S. Sanford, J. Am. Chem. Soc., 2005, 127, 7330–7331 CrossRef CAS.
- (a) J. S. Harvey, S. P. Simonovich, C. R. Jamison and D. W. C. MacMillan, J. Am. Chem. Soc., 2011, 133, 13782–13785 CrossRef CAS; (b) A. Bigot, A. E. Williamson and M. J. Gaunt, J. Am. Chem. Soc., 2011, 133, 13778–13781 CrossRef CAS.
- (a) R. J. Phipps and M. J. Gaunt, Science, 2009, 323, 1593–1597 CrossRef CAS; (b) E. M. Beck and M. J. Gaunt, Top. Curr. Chem., 2010, 292, 85–121 CrossRef CAS.
- E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052–9070 CrossRef CAS.
- (a) X. Qu, P. Sun, T. Li and J. Mao, Adv. Synth. Catal., 2011, 353, 1061–1066 CrossRef CAS; (b) N. M. Evdokimov, A. Kornienko and I. V. Magedov, Tetrahedron Lett., 2011, 52, 4327–4329 CrossRef CAS.
- (a) J.-H. Chun, S. Lu and V. W. Pike, Eur. J. Org. Chem., 2011, 23, 4439–4447 CrossRef; (b) M. Bielawski, M. Zhu and B. Olofsson, Adv. Synth. Catal., 2007, 349, 2610–2618 CrossRef CAS.
- 2, 4, 6-Trimethyliodobenzene and (E)-methyl 3-mesitylacrylate were found with GC-MS in arylation of methacrylate.
- 35% yield of 1, 2-Diphenylcyclopropane was obtained if the reaction temperature was 150 °C.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and the characterization data for all compounds. See DOI: 10.1039/c2ra20165h |
| This journal is © The Royal Society of Chemistry 2012 |