Shape control of Pd-based nanocrystals via quasi-solid-state reactions†
Lei
Zhang
ab,
Gang
Xie
b,
Junfeng
Hui
ab,
Biao
Xu
a,
Guolei
Xiang
a and
Xun
Wang
*a
aDepartment of Chemistry, Tsinghua University, Beijing 100084, P. R. China. E-mail: wangxun@mail.tsinghua.edu.cn; Fax: 8610-62792791; Tel: 8610-62792791
bCollege of Chemistry and Materials Science, Northwest University, Xi'an, 710069, P. R. China
First published on 29th February 2012
Abstract
We demonstrated here a quasi-solid-state method for the shape-controlled growth of Pd-based nanocrystals. Tiny amount of solvent is needed to form clusters which can aggregate and then evolve into nanocrystals with different morphologies. It is proposed that the shape-controlled growth processes proceed primarily by the aggregation of the clusters and the subsequent interparticle growth via solid-state self-focusing process.
Pd-based catalysts are widely used in the fields of reducing automobile pollutants, hydrogenation and dehydrogenation reactions in the petrochemical industry, carbon chain prolonging reactions and electro–chemo–luminescence, etc.1,2 As a useful catalytic material, its performances are affected by many factors, especially its size and shape. To learn the effects of shape on the properties of the nanocrystals, a lot of effort has been focused on the shape-controlled growth of Pd-based nanocrystals. Xia's group have synthesized Pd with different shapes, such as nanobars, nanorods, icosahedra and Pd–Pt bimetallic nanocrystals.3 Recently Xu has prepared concave Pd@Au core-shell nanocrystals.2 Nearly all of the current preparation results are realized via solution-based methods due to the good control over the shape and composition of the nanocrystals.4 In solution-based synthesis methods, the solid precursors can be fully mixed for dissolution in solvents, and nucleation occurs as the concentrations of atoms reach super-saturation, then the nucleus grow into nanoparticles with different shapes.5 By contrast, the solventless strategy might be more advantageous in term of economics and sustainability. Korgel et al. have pioneered a new solvent-less synthesis route for the shape-controlled growth of nanoparticles.6–9 Wu also synthesized monodisperse silver nanodisks in the absence of solvents.10 Korgel et al. proposed that in the solventless environment, the particle growth proceeded primarily by monomer addition to the particle surface—leading to a monodisperse distribution in size and shape with interparticle growth occurring rarely.7 However, the currently developed solventless methods generally rely on the structures of the molecular precursors, which need to be carefully designed and prepared in solution first. Herein, we demonstrate a new quasi-solid-state method to prepare Pd and Pd–Pt nanocrystals. In this quasi-solid state approach, precursor design and preparation are not required and we used simple commercially available inorganic salts as precursors. A small amount of solvent is involved in this process, so that the atoms readily reach the point of super-saturation and the quasi-solid state becomes completely solid during the heat treatment process. The clusters that formed during the reaction aggregated with each other to recrystallize into nanocrystals of different shapes. In this method, the in situ diffusion and solid state self-focusing process play important roles in controlling the morphology of the nanocrystals.11
We prepared Pd and Pd–Pt nanocrystals with different shapes such as cubic, dendrite-like, truncated octahedron and octahedron morphology via a quasi-solid state reaction. Pd nanocubes were synthesized as follows: first, PdCl2 (10 mg) and PVP (75 mg) were mixed by grinding in a mortar, into which KI (20 mg) was added while grinding continued until the formation of a brownish black powder. Then the mixture was transferred to a glass surface plate and 100 μL formamide was added. Obviously, the proportion of solvent to solid precursor is as low as one can reach, nearly 100 μL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 mg. After fully grinding, the mixture looks like a paste, and if put on a glass surface plate, the paste-like mixture will not slide down (see ESI for details†). The schematic pictures are shown in Fig. 1. The Pd nanocubes were formed by annealing the above mixture for 4 h at 150 °C. The morphological characterization by transmission electron microscopy (TEM) is shown in Fig. S1, ESI.† From the TEM image we can see that most of the Pd nanocubes are within the size range of 9 ± 2 nm and possess barely truncated corners. In this system, KI plays an important role in controlling the shape of Pd nanocrystals. After adding KI and continuing to grind the mixture, the color of the solid reactants changed to brownish black, which meant the KI had reacted with PdCl2 and influenced the morphology of the Pd nanocrystals. If no KI was used in this system the morphology of most of the Pd nanocrystals will be octahedral instead of nanocubes (Fig. S2†).
105 mg. After fully grinding, the mixture looks like a paste, and if put on a glass surface plate, the paste-like mixture will not slide down (see ESI for details†). The schematic pictures are shown in Fig. 1. The Pd nanocubes were formed by annealing the above mixture for 4 h at 150 °C. The morphological characterization by transmission electron microscopy (TEM) is shown in Fig. S1, ESI.† From the TEM image we can see that most of the Pd nanocubes are within the size range of 9 ± 2 nm and possess barely truncated corners. In this system, KI plays an important role in controlling the shape of Pd nanocrystals. After adding KI and continuing to grind the mixture, the color of the solid reactants changed to brownish black, which meant the KI had reacted with PdCl2 and influenced the morphology of the Pd nanocrystals. If no KI was used in this system the morphology of most of the Pd nanocrystals will be octahedral instead of nanocubes (Fig. S2†).
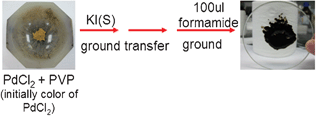 | ||
| Fig. 1 A schematic image of the preparation of a Pd nanocrystal cube. | ||
Keeping the same conditions used for the synthesis of Pd nanocubes, Pd–Pt alloy nanocubes were prepared with 10 mg H2PtCl6·6H2O added to the system. The TEM images are given in Fig. 2d. It is clear that the alloys also possess a cubic morphology but the size is almost two times bigger than the Pd nanocubes, about 20 nm. Interestingly if the annealing time was shortened to 2 h and 1 h, more and more dendrite-like nanoparticles exist (Fig. 2c and Fig. 2b). The elemental mapping shows they are also Pd–Pt alloys and covered by (200) facets (Fig. 2b and Fig. 2e). When the time was shortened to 5 min or less, only small clusters were obtained (Fig. 2a) which could not be precipitated by centrifugation. In all the experiments, by adding tiny amount of solvent and grinding, it was hard to dissolve and mix the solid precursor very well. Based on the experimental results shown above, it was found that at the initial stage of the reaction, the large precursor particles would be reduced and etched into small nanoparticles. As the solvent evaporated, the system became completely solid state and the cluster formation procedure stopped, but the interparticle growth driven by surface diffusion continued, as by prolonging the ripening time, the solid state self-focusing process played a key role in the morphology evolution. The dendrites evolved into cubic-like or other polyhedron nanocrystals with diverse shapes depending on different conditions. The shape controlled growth processes proceeded primarily by the aggregation of small nanoparticles and a secondary interparticle growth process. Since there is no involvement of large quantities of solvents, the temperature of the reactants can be quenched rapidly to room temperature which provided the possibility of studying the time-dependent morphology evolution of nanocrystals without the influence of surface-absorbed solvent molecules. In a series of experiments we found that by prolonging the heating time more clusters for aggregation were made. Fig. 3 demonstrates a possible mechanism for the formation of Pd–Pt nanocrystals via this quasi-solid state procedure, which was further proved by subsequent experimental results.
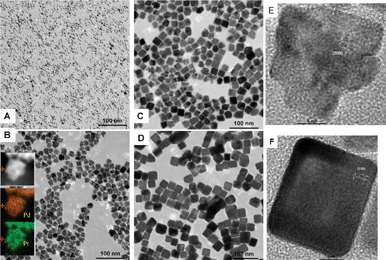 | ||
| Fig. 2 TEM images of Pd–Pt nanocrystals obtained using the same procedure, except for different annealing times: (A) 5 min, (B) 1 h, (C) 2 h, (D) 4 h, (E) 1 h, (F) 4 h. (Insert of B is the STEM image and elemental mapping correspond to the Pd and Pt elements respectively.) | ||
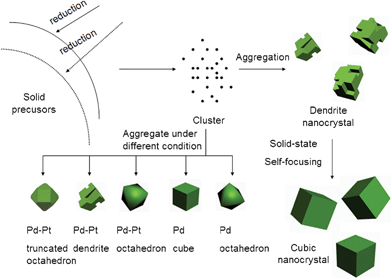 | ||
| Fig. 3 Schematic illustrating of a possible mechanism that lead to Pd-based nanocrystals with different shapes. | ||
For the preparation of bimetallic nanocrystals, if KI was removed from the system, Pd–Pt alloys could also be obtained with the shape changing into truncated octahedron or octahedron morphology instead of nanocubes at different temperatures. Fig. 4 shows the typical TEM image of the as-synthesized Pd–Pt nanocrystals. As shown in Fig. 4a, most of the truncated octahedron prepared at 120 °C have different projections under TEM, which may correspond to the various orientation of the nanoparticles located on the TEM copper grid. Fig. 4b shows two typical projected shapes from different orientations. At a higher temperature of 150 °C, the truncated corners developed so that most of nanoparticles become octahedral (Fig. 4c). At 150 °C, when increasing the amount of H2PtCl6·6H2O with the other conditions unchanged, the morphology changed from octahedral to dendrite-like structures (Fig. 4d and Fig. S4, ESI†). From the elemental mapping, we found that they were also Pd–Pt alloys, but the elements did not disperse homogeneously in the nanoparticles. The elemental mapping images (insert of Fig. 4d) clearly show that the area of the Pt element is larger than the area of the Pd element. The line scanning also confirms that the shells possess more Pt than Pd, especially in the branch of the nanoparticles while the cores possess more Pd.
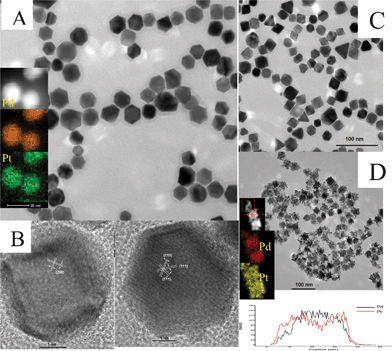 | ||
| Fig. 4 (A) TEM image of Pd–Pt truncated octahedrons synthesized at 120 °C (The insert is the elemental mapping of the Pd–Pt truncated octahedrons). (B) HRTEM image of Pd–Pt truncated octahedrons from different orientations. (C and D) TEM images of Pd–Pt octahedrons synthesized at 150 °C with 10 and 30 mg of H2PtCl6, respectively. (the insert of image D is the elemental mapping and line scanning of Pd–Pt dendrite-like nanocrystals.) | ||
In the quasi-solid-state system, formamide could be replaced by ethylene glycol to prepare Pd octahedrons with citric acid added. Although this system used only a tiny amount of ethylene glycol, the size of the Pd octahedron could be well controlled by altering the concentration of PdCl2, in a similar way to solution-based methods. Fig. 5 shows typical TEM images of the Pd octahedron with a size of 5 nm and 10 nm, respectively. Higher concentrations of PdCl2 favored the formation of nanocrystals with larger sizes.
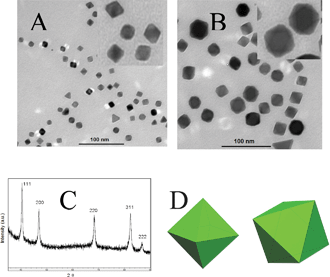 | ||
| Fig. 5 (A and B) TEM images of Pd octahedron produced with 10 mg and 20 mg PdCl2, respectively. (the insert of A and B show enlarged images) (C) XRD pattern of a 10 nm Pd octahedron (D) The corresponding geometric model of octahedron Pd from different directions. | ||
In summary, we have developed a simple quasi-solid state method to produce Pd-based nanocrystals with controlled shapes and sizes. In this quasi-solid-state reaction procedure, only a tiny amount of solvent is needed. After annealing at a high temperature for a short time, all the solvent evaporates and the system becomes completely solid-state. After this, the aggregation of clusters and solid-state self-focusing by diffusion occurs. Although little solvent is involved in the reaction, we can also regulate the shape and size of the nanocrystals by changing the reaction temperature and concentration of the precursors. We believe that the method opens up a new way for the shape control of nanoparticles and gives us a new view to reconsider the nanocrystal growth mechanism.
We gratefully acknowledge support from NSFC (Grant 91127040, 20921001) and the State Key Project of Fundamental Research for Nanoscience and Nanotechnology (Grant 2011CB932402).
References
- (a) Y. Li, X. M. Hong, D. M. Collard and M. A. El-Sayed, Org. Lett., 2000, 2, 2385 CrossRef CAS; (b) S.-W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642 CrossRef CAS; (c) Y. Nishihata, J. Mizuki, T. Akao, H. Tanaka, M. Uenishi, M. Kimura, T. Okamoto and N. Hamada, Nature, 2002, 418, 164 CrossRef CAS.
- L. Zhang, W. X. Niu, Z. Y. Li and G. B. Xu, Chem. Commun., 2011, 47, 10353 RSC.
- (a) Y. J. Xiong, H. G. Cai, B. J. Wiley, J. G. Wang, M. J. Kim and Y. N. Xia, J. Am. Chem. Soc., 2007, 129, 3665 CrossRef CAS; (b) Y. J. Xiong, J. M. McLellan, Y. D. Yin and Y. N. Xia, Angew. Chem., Int. Ed., 2007, 46, 790 CrossRef CAS; (c) H. Zhang, M. S. Jin, J. G. Wang, W. Y. Li, P. H. C. Camargo, M. J. Kim, D. R. Yang, Z. X. Xie and Y. N. Xia, J. Am. Chem. Soc., 2011, 133, 6078 CrossRef CAS.
- (a) Q. Yuan and X. Wang, Nanoscale, 2010, 2, 2328 RSC; (b) Q. Yuan, Z.Y. Zhou, J. Zhuang and X. Wang, Chem. Commun., 2010, 46, 1491 RSC; (c) Q. Yuan, J. Zhuang and X. Wang, Chem. Commun., 2009, 6613 RSC; (d) X. Wang, J. Zhuang, Q. Peng and Y. D. Li, Nature, 2005, 437, 121 CrossRef CAS.
- Y. N. Xia, Y. J. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60 CrossRef CAS.
- M. B. Sigman and B. A. Korgel, Chem. Mater., 2005, 17, 1655 CrossRef CAS.
- T. H. Larsen, M. Sigman, A. Ghezelbash, C. Doty and B. A. Korgel, J. Am. Chem. Soc., 2003, 125, 5638 CrossRef CAS.
- A. Ghezelbash, M. B. Sigman and B. A. Korgel, Nano Lett., 2004, 4, 537 CrossRef CAS.
- M. B. Sigman, A. Ghezelbash, T. Hanrath, A. E. Saunders, F. Lee and B. A. Korgel, J. Am. Chem. Soc., 2003, 125, 16050 CrossRef CAS.
- Y. Wang, J. Chen, L. Chen, Y. B. Chen and L. M. Wu, Crystal Growth & Design, 2010, 10, 1579 Search PubMed.
- Y. F. Chen, E. Johnson and X. G. Peng, J. Am. Chem. Soc., 2007, 129, 10937 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterizations and additional TEM, XRD, EDS results. See DOI: 10.1039/c2ra00888b |
| This journal is © The Royal Society of Chemistry 2012 |
