Paper-based fluoroimmunoassay for rapid and sensitive detection of antigen†
Jing
Liang
,
Yanyan
Wang
and
Bin
Liu
*
Department of Chemical and Biomolecular Engineering, 4 Engineering Drive 4, National University of Singapore, 117576, Singapore. E-mail: cheliub@nus.edu.sg; Fax: +65 67791936; Tel: +65 65168049
First published on 17th February 2012
Abstract
A paper strip assay is developed for fast, simple and inexpensive detection of IgG protein in a quantitative manner with high sensitivity and selectivity. In the first step, polystyrene (PS) microbeads conjugated with primary anti-goat IgG are immobilized on paper strips. Upon capturing target goat IgG and subsequent incubation with Cy3-labeled secondary anti-goat IgG, a sandwich type immunoassay is formed on paper strips. Detailed study reveals that BSA blocking is effective in preventing non-specific protein adsorption on paper strips and the concentration of the signalling anti-goat IgG has to be controlled to minimize background signal. The paper-based fluoroimmunoassay has shown high specificity for goat IgG detection with a detection limit of 20 ng mL−1 using a fluorescence array scanner under optimized conditions and demonstrated generic applications in practical samples. To test the feasibility of the assay for colorimetric detection, gold nanoparticles are conjugated to the secondary anti-goat IgG to replace Cy3. Small Au nanoparticles (NPs) are retained on the paper strip upon target capturing and sandwich assay formation. The detection is signalled by a visible color change of the PS spot from white to blue with Au development. The Au NP stained colorimetric immunoassay requires no equipment, has simple operating steps, and enables detection of 5 μg mL−1 IgG with the naked eye.
Introduction
Immunoassay, which relies on antibody based recognition, is one of the most important biological techniques for protein detection and quantification due to its wide applications in clinical diagnosis and food and environment inspections.1 The conventional immunoassay enzyme linked immunosorbent assay (ELISA) is based on enzymatic catalysis of a substrate for signal amplification of a reporter molecule.2 It has high sensitivity but requires special storage and time consuming manipulations.3,4 Other techniques like radioimmunoassay have limitations in real applications due to possible health hazards.3 Fluoroimmunoassay represents one of the most promising immunoassays due to its extremely high sensitivity and relatively simple technique.5 Many groups have performed fluoroimmunoassay using various fluorescent reporters such as quantum dots,6–8 polymeric materials9,10 and noble nanoparticles for fluorescence enhancement11 or quenching.12 Despite their high sensitivity, most of them rely on the use of bulky and expensive equipment, such as fluorescence imagers and fluorometers.It remains a challenge to develop rapid, simple, inexpensive and sensitive detection methods that are suitable for clinical diagnosis and treatment.13 This is especially desirable in less developed areas that lack sophisticated equipment and fabrication techniques. The development of ‘bioactive’ paper-based bioassays has attracted much attention due to its accessibility, portability and disposability.14 Most paper based assays involves immobilization of target specific biomolecules on paper surfaces that recognize a wide range of targets through antigen-antibody recognition,15–17 aptamer-target interaction18,19 and DNA hybridization.20,21 Previous work has reported several paper-based bioassays in which bioprobes are directly dipped onto paper surface,15,16 which provided limited surface area for subsequent reaction. A few groups have also used microgels19,20 as supporting particles or sol–gel encapsulation22 of biomolecules on paper strips for biosensing. However, these methods require relatively complex procedures for substrate modification and surface functionalization.
Herein, we report a paper-based fluoroimmunoassay employing polystyrene (PS) microbeads as the protein carrier for antigen detection. In our design, the protein molecules are immobilized on PS microbeads via chemical reaction. The use of microbeads not only provides increased surface area for probe immobilization,23 but also enables localization of probe molecules in a defined reaction area, allowing for easy signal readout. As compared to previously reported nanoparticle based fluoroimmunoassays,6–12 the use of paper substrate has enabled fast and simple fluoroimmunoassay detection with distinct advantages. Firstly, it eliminates the use of cumbersome centrifuge in the immunoassay step to remove the unbound molecules. Secondly, regular filter papers are suitable for the assay, and they are abundant, cheap and environmental friendly. In addition, the high porosity of paper renders rapid absorption and entrapment of the probe immobilized PS beads through capillary forces and physical adsorption for easy assay substrate preparation.
This paper is organized as follows. We start with the anti-goat IgG-PS conjugates and paper strips preparation. We then study the effect of bovine serum albumin (BSA) treatment on paper strips for non-specific protein adsorption. This is followed by PS beads stability study and optimization of the signalling antibody concentration. The fluoroimmunoassay sensitivity and selectivity tests are subsequently conducted for goat IgG detection using a dye labeled antibody in a sandwich assay. To eliminate the use of equipment, a colorimetric immunoassay is further developed to detect IgG using gold nanoparticles as the signal reporter. The signal is easily detectable with the naked eye after Au enhancement.
Material and methods
Chemicals
Goat IgG, anti-goat IgG, anti-goat IgG-Cy3, lysozyme, bovine serum albumin (BSA), N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and bromophenol blue (BPB) were purchased from Sigma. Human α-thrombin was ordered form HTI. Carboxylated PS microbeads with a diameter of 500 nm were purchased from Polysciences Inc. Hydrogen tetrachloroaurate(III) (HAuCl4·4H2O, 99.99%), citric acid monohydrate (>98%) and sodium citrate tribasic dehydrate (> 99.0%) were purchased from Sigma. Fetal bovine serum (FBS) was purchased from Invitrogen (Life Technologies). Blocker™ Casein in PBS (Pierce) and 10× phosphate buffer saline (PBS, pH 7.4, 1st BASE) were commercial products and used as received without further purification. Milli-Q water (18.2 MΩ) was used to prepare all stock solutions and PBST buffer (1×PBS, with 0.1% Tween 20, pH 7.4).Characterization
The absorption spectra of protein were measured using UV-vis spectrometer (Shimadzu, UV-1700, Japan). The fluorescence images of paper strips were obtained using GenePix 4100A fluorescence scanner (Molecular Devices, USA) and analyzed using ImageJ software.Preparation of anti-goat IgG-PS conjugates
PS-COOH microbeads (50 μL, 2.5%) were washed according to the manufacturer's guidance. The resultant PS-COOH beads were redispersed in freshly prepared 1% EDC in sodium phosphate buffer (100 μL, pH = 4.5), followed by addition of anti-goat IgG (100 μL, 2 mg mL−1). The PS-COOH suspension was incubated overnight at room temperature. The excess anti-goat IgG was removed by centrifugation, and the anti-goat IgG-PS conjugates were washed with 1×PBS buffer (1 mL, 3×). The antibody-PS conjugates were redispersed in 1% (w/v) casein blocking buffer (1 mL), and the mixture was incubated and shaken at room temperature for 2 h. The NP suspension was then centrifuged and thoroughly washed with PBS buffer (1 mL, 3×), and was finally resuspended in PBS buffer (50 μL) before use.Paper strip preparation and BSA treatment
A series of paper strips with the size of ∼3 × 25 mm were cut from Whatman filter paper #1. Paper strips were soaked in 100 μL 1×PBS buffer containing 1 mg mL−1 BSA for 1 h and dried in the air. Both a natural paper strip and a BSA treated paper strip were soaked in citric buffer solution (250 mM, pH = 1.8) separately for 10 min and air-dried. Finally, they were immersed in BPB solution (10 μL, 9 mM in ethanol) separately for 10 min followed by air drying. A photo of the strips was taken for color comparison.Effect of BSA treatment
An aliquot (2 μL) of the as-prepared anti-goat IgG-PS conjugates was carefully spotted onto both a natural paper strip and BSA blocked paper strip using a pipette. After air-drying for ∼20 min at room temperature, each paper strip was dipped into a 1.5 mL microcentrifuge tube containing 500 μL of 1×PBS buffer. After rocking gently by hand for 30 s, the binding buffer was removed from the tube. Another 500 μL of the binding buffer was added to the tube to wash the strips and then removed. The paper strips were then immersed in IgG solution (1 μg mL−1, in 100 μL PBST buffer) for 30 min. These strips were washed twice in 500 μL of PBST buffer with gentle rocking after 30 min incubation. The resulting paper strips were soaked in anti-goat-IgG-Cy3 (5 μg mL−1 in 100 μL PBST) and incubated for 30 min, and washed twice in 500 μL of PBST buffer. Finally, the paper strips were stuck on a glass slide and scanned for fluorescence.Effect of soaking time on assay fluorescence
The fluorescent PS beads were synthesized by immobilization of Cy3 labeled anti-goat IgG on PS microbeads. 10 μL of the EDC activated PS beads (2.5%) prepared using the steps described above were added into the anti-goat IgG-Cy3 solution (20 μL, 2 mg mL−1) for 30 min. The beads were centrifuged and washed with 1×PBS buffer (200 μL, 3×) and redispersed in 10 μL of PBS buffer. 2 μL of the obtained fluorescent PS beads were dropped onto a BSA treated paper strip. After air drying, the paper strip was fixed on a glass plate for fluorescence scanning using a fluorescence scanner upon excitation of Cy3 at 532 nm. The paper strip was then soaked in PBST buffer for 1 h and again scanned for fluorescence.Optimization of the Cy3-labeled anti-goat IgG
The BSA treated paper strips were spotted with 2 μL of as-prepared anti-goat IgG-PS beads for 30 min. They were air dried and soaked in a series of anti-goat IgG-Cy3 solutions at different concentrations (0, 1, 2.5, 5, 7.5 and 10 μg mL−1, in 100 μL PBST buffer) for 30 min. After washing with PBST buffer and air drying, the strips were stuck onto a glass slide for fluorescence scanning.Fluoroimmunoassay selectivity study
The paper strips immobilized with anti-goat IgG-PS beads prepared previously were incubated with goat IgG (1 μg mL−1, in 100 μL PBST buffer) and three other interference proteins BSA, thrombin and trypsin (1 μg mL−1 each) in 100 μL PBST buffer, respectively for 30 min. After washing with PBST buffer, the paper strips were then incubated in anti-goat IgG-Cy3 solutions (5 μg mL−1, in 100 μL PBST buffer) for 30 min. The paper strips were gently washed with PBST buffer, air dried and fixed for fluorescence imaging.Fluoroimmunoassay sensitivity study
The obtained anti-goat IgG-PS beads adsorbed paper strips were immersed in various concentrations of IgG solutions (0, 50, 100, 250, 500, 750, and 1000 ng mL−1 in 100 μL PBST). After careful washing, the paper strips were further incubated in anti-goat IgG-Cy3 solution (5 μg mL−1, in 100 μL PBST) followed by washing and air drying. A fluorescence image was taken for the paper strips. Another five measurements were conducted using the same procedures for protein quantification. The fluorescence intensity was analyzed by ImageJ software for six independent measurements.Synthesis of Au NPs and anti-goat IgG-Au conjugates
The 14 nm Au NPs was synthesized by the citrate reduction of HAuCl4 as previously reported.24,25 Trisodium citrate (1%, 3.5 mL) was added to a boiling, rapidly stirred solution of HAuCl4 (100 mL, 0.01%). The solution was kept boiling and stirred for 20 min, and then cooled to room temperature. The prepared Au NPs (2.94 nM) were stored at 4 °C.The obtained Au NPs were then modified with anti-goat IgG according to the published protocol.26 Typically, 11 μL of 2 mg mL−1 anti-goat IgG solution (10% more than the minimum amount determined by aggregation test) was added to Au colloids (1 mL, 2.94 nM) adjusted to pH 9.0 using Na2CO3 to reach a final protein concentration of ∼21 μg mL−1. After 30 min of incubation, the excess anti-goat IgG was removed by centrifugation. The anti-goat IgG-Au conjugates were incubated with 1% (w/v) casein blocking buffer (1 mL) under shaking for 1 h to block the free protein binding sites and subsequently washed with 0.3×PBS buffer (1 mL, 2×) and 1×PBS buffer (1 mL, 1×), respectively. The antibody-NP conjugates were redispersed in PBST buffer (1 mL).
Colorimetric immunoassay on paper strips
The procedure of paper strip preparation and anti-goat IgG-PS beads deposition onto paper strip was the same as that described above. A series of concentrations of IgG (0, 5, 10, 50 and 100 μg mL−1) in 100 μL of PBST buffer were prepared, and the paper strips were immersed in the mixture for 30 min. The paper strips were washed with 500 μL of PBST buffer with gentle rocking after incubation. The resulting paper strips were soaked in anti-goat IgG-Au NP solution in PBST (100 μL) and incubated for 20 min. Next, the strips were washed with 500 μL of PBST twice for 1 min followed by gentle rocking. The gold developer solution was then prepared by mixing Tween 20, ascorbic acid and HAuCl4 to give final concentrations of 0.5%, 1.9 mM and 1 mM, respectively. Subsequently, each strip was treated with 100 μL of the freshly prepared gold developer solution for ∼30 s, and washed with DI water 3 times.Results and discussion
Fluoroimmunoassay mechanism
The assay mechanism is illustrated in Scheme 1. Anti-goat IgG molecules were conjugated to carboxyl group functionalized PS microbeads through 1-ethyl-3-(3-dimethylamineopropyl) (EDC) activated coupling reaction. Casein blocking was carried out to minimize the non-specific interaction of antibody-PS conjugates with interference proteins.27 Paper strips cut into a defined size were treated with BSA to enhance their hydrophobicity, which were followed by immobilization of the PS microbeads through physical adsorption. These strips were immersed in goat IgG solution for antigen-antibody recognition and then washed with DI water to remove the unbound IgG molecules. They were further immersed in anti-goat IgG-Cy3 solutions to form a sandwich type immunoassay which consisted of the primary antibody on PS microbeads, target antigen and Cy3-labeled secondary antibody. The excess of Cy3-labeled anti-goat IgG was washed away and the paper strip was examined for fluorescence signal. The control strip which had no analyte could not form the sandwich type structure, thereby generated no fluorescence signal. As the fluorescence intensity is dependent on the number of Cy3-labeled secondary anti-goat IgG molecules captured, quantitative measurement of IgG can be realized. To demonstrate the applicability of assay in real biological samples, a parallel experiment was conducted in IgG spiked 5% fetal bovine serum in PBS buffer solutions under the same experimental conditions.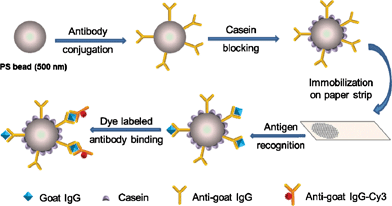 | ||
| Scheme 1 Schematic illustration of the paper-based fluoroimmunoassay. | ||
Preparation of paper strips and anti-goat IgG-PS conjugates
Anti-goat IgG molecules were immobilized onto the PS microbead surface through coupling reaction between the activated carboxyl groups of the microbeads and the amino groups of the proteins in the presence of EDC, while the residual carboxyl groups were blocked with casein (1% in PBS). Whatman No. 1 filter paper was cut into rectangular pieces. After BSA treatment, the BSA-blocked paper strips were examined with bromophenol blue (BPB) based on nonspecific binding of BPB to proteins. As shown in Fig. 1A, the natural paper strip is a yellow color and it turns to blue after BSA treatment. In general, BPB binds to BSA through a combination of electrostatic and hydrophobic interactions.28,29 In addition, the lysine residues of BSA also play an important role in BSA-BPB complexation.30 Under acidic conditions, BPB interacts with BSA to form a stable blue complex due to deprotonation of phenol group with the color change of BPB from yellow to blue.31 This blue color shown in Fig. 1A demonstrates successful BSA blocking on paper strips.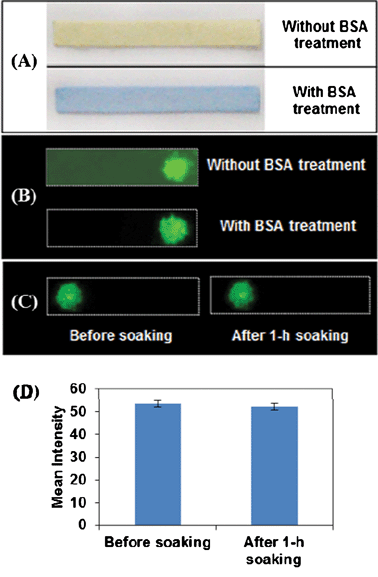 | ||
| Fig. 1 (A) Paper strips with and without BSA treatment after reaction with BPB. (B) Fluorescence images of strips without and with BSA treatment for the detection of IgG. Fluorescence images (C) and their intensity profile (D) of BSA treated paper strips coated with fluorescent PS beads before and after 1 h soaking upon excitation of Cy3 at 532 nm. | ||
Both BSA-blocked and natural paper strips were employed for IgG detection to test the effect of BSA treatment. 2 μL of pre-synthesized antibody-PS conjugates were dropped on paper strips, followed by incubation in 1 μg mL−1 IgG solution and 5 μg mL−1 Cy3-tethered secondary antibody subsequently. The resultant paper strips were washed in PBST buffer and air-dried, and the fluorescence images of paper strips were taken using fluorescent scanner with laser excitation at 532 nm. As shown in Fig. 1B, fluorescent dots with similar intensity exist on both paper strips. In addition, the natural paper strip itself exhibits visible fluorescence while no background fluorescence appears on BSA treated paper. These observations demonstrate that BSA treatment prevents non-specific absorption of proteins on filter paper, which significantly reduces background signal.
The use of filter paper as substrate facilitates the mechanical entrapment of PS beads due to the high roughness and capillary forces which push the PS beads to further penetrate the fibre network during the air-drying process.19 The key issue for successful paper-based detection is that PS beads should be stationary toward chromatographic elution during the incubation and washing steps. As such, fluorescent PS beads spotted paper strips after soaking were examined for fluorescence. Fluorescent PS beads were synthesized by incubation of anti-goat IgG-Cy3 with EDC activated PS beads, deposited onto paper strips. The fluorescence images of paper strips before soaking and after 1 h soaking in buffer were taken, in which the fluorescent spots present the same size and intensity (Fig. 1C and 1D). These data demonstrate no loss or mobility of PS beads in the soaking and washing procedures, which further implies the feasibility of paper strip with physically immobilized PS beads for biosensing.
Assay optimization
For a typical sandwich assay, the concentration of signalling antibody plays a crucial role in assay performance in terms of high sensitivity and low non-specific interaction. We investigated how the assay performance was affected by introducing anti-goat IgG-Cy3 with different concentrations. Anti-goat IgG-PS beads spotted paper strips were air-dried and subsequently soaked in various concentrations of anti-goat IgG-Cy3 solution (0–10 μg mL−1) for 30 min. The obtained paper strips after washing and drying were stuck onto a glass slide, and the fluorescence image was taken using a fluorescent scanner. As shown in Fig. 2A, no fluorescent spots are obtained on the paper strips when less than 5 μg mL−1 of the signalling antibody is used for incubation. However, inevitable fluorescence signals are observed with further increased signalling antibody concentrations. Since there is no sandwich structure formed in the absence of the target antigen, the fluorescence signal indicates the occurrence of non-specific adsorption at high anti-goat IgG-Cy3 concentrations. To avoid non-specific background and to achieve maximum specific signal, 5 μg mL−1 of anti-goat IgG-Cy3 was applied in the assay.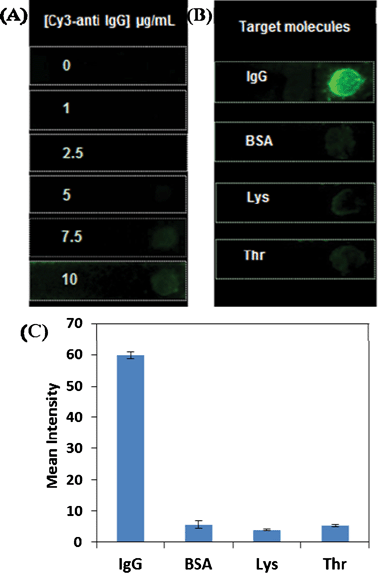 | ||
| Fig. 2 (A) Fluorescence images of anti-goat IgG-PS beads spotted paper strips incubated with signalling anti-goat IgG at different concentrations. Fluorescence images (B) and their intensity profile (C) of anti-goat IgG-PS beads immobilized paper strips upon incubation with IgG and three interference proteins, namely BSA, lysozyme and thrombin with concentration of 1 μg mL−1 each. The signalling antibody concentration was kept as 5 μg mL−1. | ||
Selectivity of paper-based IgG detection
The assay specificity was examined using three typical interference proteins, i.e. BSA, lysozyme and thrombin. The anti-goat IgG-PS conjugates immobilized paper strips were incubated with goat IgG (1 μg mL−1) as well as the interference proteins (1 μg mL−1 each) in PBST buffer, respectively, followed by anti-goat IgG-Cy3 incubation (5 μg mL−1). The resulting paper strips were gently washed, air-dried and fixed on a glass slide for fluorescence scanning. The fluorescence images of the as-prepared paper strips were recorded and shown in Fig. 2B. As expected, intense fluorescent spot is only witnessed in the presence of goat IgG, while negligible Cy3 emission is observed in the presence of interference proteins. These results not only indicate the effectiveness of antibody-antigen interaction on PS beads, but also highlight the good sensing specificity of PS beads spotted paper-based immunoassay.Sensitivity of paper-based IgG detection
To demonstrate protein quantification, different concentrations of goat IgG were prepared and the anti-goat IgG-PS conjugates immobilized paper strips were incubated with different concentrations of goat IgG (ranging from 0 to 1 μg mL−1) in PBST buffer for 30 min. The resulting paper strips were then soaked in anti-goat IgG-Cy3 solution (5 μg mL−1) for another 30 min, followed by washing with PBST. After air-drying, each strip was scanned for fluorescence and the results are given in Fig. 3A. The green fluorescence of the spots on paper strips progressively brightens with increased goat IgG concentrations. This is due to the increased number of sandwich structure formation upon incubation of anti-goat IgG-PS beads with higher goat IgG concentrations, which enables more Cy3-labeled secondary antibody to be retained on paper strips. In addition, the integrated fluorescence intensities of each strip using ImageJ software are plotted as a function of IgG concentration in Fig. 3B. A linear range from 0 to 250 ng mL−1 was observed, which indicates that the paper-based assay can be used to perform quantitative protein detection. The inset shows the linear range of the signal response and the detection limit is estimated to be 20 ng mL−1 based on 3σ from six independent measurements. Taking advantage of the small sample volume (100 μL) needed in this test, the assay can detect 2 ng of IgG using a standard fluorescence scanner, which is more sensitive as compared to that for previously reported solution-based fluoroimmunoassays32,33 and substrate-based immunoassays.34,35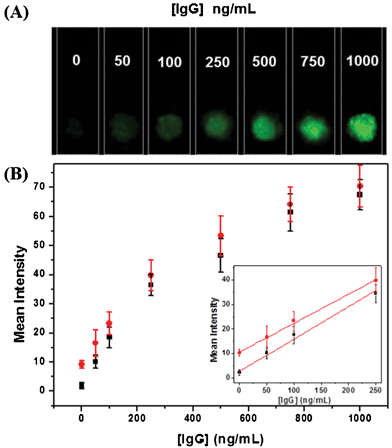 | ||
| Fig. 3 (A) Fluorescence images of paper strips for IgG detection upon excitation at 532 nm. (B) Plot of mean fluorescence intensity as a function of IgG concentrations in assay buffer (■) for six measurements and serum media ( ) for four measurements. The inset shows the linear fluorescence signal response in the range of 0–250 ng mL−1 of IgG in the two media. | ||
To demonstrate the potential application of the fluoroimmunoassay in biological medium, FBS is diluted 20-fold in PBS solutions and spiked with IgG with the same concentrations used in pure form. As shown from Fig. 3B, the signal response of the IgG spiked FBS samples is similar to that of the pure IgG samples. In FBS medium, although the fluorescence background is slightly higher, due to presence of interference proteins, the assay parameters are not affected much, yielding an LOD of ∼30 ng mL−1 from the inset of Fig. 3B. The compatibility of the assay in serum indicates the developed assay is suitable to be used fast screening of proteins in various biological media.
As the above fluoroimmunoassay requires the use of fluorescence scanner for signal readout, a colorimetric immunoassay using Au nanoparticles as the visualizing agent is further developed by taking advantage of the vivid color exhibited by Au NPs for naked-eye sensing. Since the optical properties of Au NPs are highly dependent on their size, shape and dispersion state,36 in this assay, 14 nm Au NPs conjugated with anti-goat IgG was used to form a sandwich structure with the anti-goat IgG-PS conjugates on paper strips upon target capturing. The blue color of Au NPs retained on the paper strips can be visualized by further development which signifies a targeting event.
Colorimetric immunoassay mechanism
The assay mechanism is shown in Scheme 2. Au NPs were functionalized with anti-goat IgG through electrostatic adsorption.37 PS microbeads conjugated with the primary antibody were immobilized on a paper strip using the same steps as described in the previous section. After capturing antigen, the paper strip with antigen-antibody-PS conjugates was immersed in a solution containing anti-goat IgG-Au NP conjugates to form a sandwich type immunoassay. The paper strip was then washed to remove the unbound Au NPs. The Au NPs remained on the paper strip were enlarged by immersing the paper in a developer solution which contains gold precursor, reducing agent and protective agent. The enlarged Au NPs give a visible blue color, indicating the presence of target IgG in solution.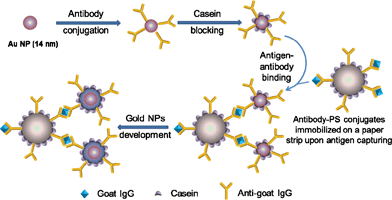 | ||
| Scheme 2 Schematic illustration of the paper-based colorimetric immunoassay. | ||
Preparation of Au NPs and anti-goat IgG-Au conjugates
The Au NPs were prepared by reduction of HAuCl4 using trisodium citrate both as a reducing agent and a protective ligand at the boiling temperature. The synthesized NPs have an average size of 14 nm as confirmed by TEM images and the solution appeared reddish. Au NPs were functionalized with anti-goat IgG through electrostatic interactions.37 The reaction was carried out at pH 9.0, slightly above the isoelectric point of citrate for effective binding between the negatively charged citrate groups on the NPs and the positively charged amino acid side chains of anti-goat IgG.38 Inadequately encapsulated Au NPs will aggregate significantly in salt solution and lead to a redshift of absorption.34,39 The minimum concentration of anti-goat IgG needed to efficiently cap 14 nm Au NPs was determined to be ∼19 μg mL−1 based on the aggregation test (Fig. S1, ESI†).34Colorimetric IgG detection on paper strips
The anti-goat IgG-PS bead immobilized paper strips were air-dried and soaked in a series of concentrations of IgG solutions (0–100 μg mL−1) for 30 min. The paper strips were washed and soaked in anti-goat IgG-Au NP solutions containing 0.36 nM of Au NPs for 20 min. After careful washing with PBST buffer, only the Au NPs bound to the goat IgG captured by anti-goat IgG-PS conjugates on paper would be retained due to the sandwich structure formation. However, the color of the PS spot is almost invisible due to the capturing of limited numbers of Au NPs and low optical cross-sections of small Au NPs.35 The strips were further treated with developing solution containing the precursor HAuCl4, reducing agent ascorbic acid and protective agent Tween 20 for ∼30 s to enlarge Au NPs. The enlarged Au NPs have an increased optical cross-section,40 producing a visible blue color. As shown in Fig. 4, the results show that the PS spot on the control strip is almost colorless. As the concentration of target molecule increases, the color of the spot turns more bluish. The naked-eye detection of IgG is feasible with a detection limit of 5 μg mL−1.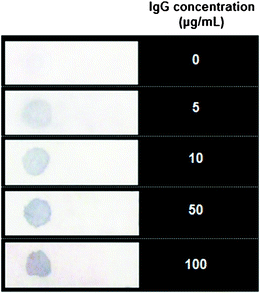 | ||
| Fig. 4 Photograph of paper strips for colormetric detection of IgG at different concentrations. The color of the spot turns blue in the presence of target IgG molecule after Au development. | ||
It is important to note that the Au NP development is sensitive to growth conditions. The ascorbic acid concentration influences the reduction speed of Au3+, which in turn determines the formation of Au NPs and the spot color on paper strip. The optimal concentration of ascorbic acid was found to be 1.9 mM. Higher ascorbic acid concentration will induce fast reduction, rapid formation of Au seeds and slow development of Au NPs on paper strips.40 As a result, a large amount of newly formed seeds tend to adsorb on to the paper strips, resulting in a high background color. On the other hand, lower ascorbic acid concentration will lead to slow reduction for both seed formation and Au enlargement, which requires a longer time for the color development. Some of the newly formed seeds will also deposit onto the paper strips at a longer reaction time to produce a high background color. Therefore, it is important to optimize the reagent concentration and the reaction time to allow for sufficient reaction without producing high background signal.
Conclusions
In summary, we have successfully developed a PS based fluoroimmunoassay for IgG detection. It is found that BSA blocking of the paper strips is crucial to provide surface hydrophobicity for effective immobilization of PS microbeads and biomolecules, as well as high signal to noise ratio. With the optimized secondary anti-goat IgG concentration, the assay shows high specificity against interference proteins, i.e. BSA, lysozyme and thrombin and good sensitivity with a detection limit of 20 ng mL−1. A similar assay performance was achieved in serum medium, indicating good compatibility of the assay in biological fluids. Based on a similar sensing strategy, colorimetric detection is further developed, which uses Au as the signal reporter to eliminate the use of any instruments and can be detected by the naked eye. A semi-quantitative detection can be achieved with a detection limit of 5 μg mL−1 for naked-eye sensing of IgG. It is believed that the principles of these assays can be generalized to other systems for detection of a wide range of biomolecules by changing the probes immobilized on paper. The disposable PS immobilized paper substrate renders it promising and advantageous in many biological applications.Acknowledgements
The authors are grateful to the TDSI (R-279-000-305-592, R-279-000-305-422 and R-279-000-305-232), Singapore Ministry of Defence (R279-000-301-232) and the National University of Singapore (R279-000-301-646), and Singapore Ministry of Education (R-279-000-255-112) for financial support. J. Liang thanks the National University of Singapore for support via a research scholarship.References
- L. Zhao, L. Sun and X. Chu, TrAC, Trends Anal. Chem., 2009, 28, 404–415 CrossRef CAS
.
- H. Buss, T. P. Chan, K. B. Sluis, N. M. Domigan and C. C. Winterbourn, Free Radical Biol. Med., 1997, 23, 361–366 CrossRef CAS
.
- X. Hun and Z. J. Zhang, Talanta, 2007, 73, 366–371 CrossRef
.
- Y. Murakami, T. Endo, S. Yamamura, N. Nagatani, Y. Takamura and E. Tamiya, Anal. Biochem., 2004, 334, 111–116 CrossRef CAS
.
- D. S. Smith, M. H. Al-Hakiem and J. Landon, Ann. Clin. Biochem., 1981, 18, 253–274 CAS
.
- Z. Li, Y. Wang, J. Wang, Z. Tang, J. G. Pounds and Y. Lin, Anal. Chem., 2010, 82, 7008–7014 CrossRef CAS
.
- H. Wang, J. Wang, C. Timchalk and Y. Lin, Anal. Chem., 2008, 80, 8477–8484 CrossRef CAS
.
- Y. Chen, H. L. Ren, N. Liu, N. Sai, X. Liu, Z. Liu, Z. Gao and B. A. Ning, J. Agric. Food Chem., 2010, 58, 8895–8903 CAS
.
- Y. Wang and B. Liu, Biosens. Bioelectron., 2009, 24, 3293–3298 CrossRef CAS
.
- Y. He, Y. Li and X. Hun, Microchim. Acta, 2010, 171, 393–398 CrossRef CAS
.
- J. Zhang, E. Matveeva, I. Gryczynski, Z. Leonenko and J. R. Lakowicz, J. Phys. Chem. B, 2005, 109, 7969–7975 CrossRef CAS
.
- L. Ao, F. Gao, B. Pan, R. He and D. Cui, Anal. Chem., 2006, 78, 1104–1106 CrossRef CAS
.
- D. Mabey, R. W. Peeling, A. Ustianowski and M. D. Perkins, Nat. Rev. Microbiol., 2004, 2, 231–240 CrossRef CAS
.
- A. W. Martinez, S. T. Phillips, E. Carrilho, S. W. Thomas, H. Sindi and G. M. Whitesides, Anal. Chem., 2008, 80, 3699–3707 CrossRef CAS
.
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318–1320 CrossRef CAS
.
- W. Wang, W. Y. Wu, W. Wang and J. J. Zhu, J. Chromatogr., A, 2010, 1217, 3896–3899 CrossRef CAS
.
- L. Wang, W. Chen, D. Xu, B. S. Shim, Y. Zhu, F. Sun, L. Liu, C. Peng, Z. Jin, C. Xu and N. A. Kotov, Nano Lett., 2009, 9, 4147–4152 CrossRef CAS
.
- J. Liu, D. Mazumdar and Y. Lu, Angew. Chem., Int. Ed., 2006, 45, 7955–7959 CrossRef CAS
.
- S. Su, M. M. Ali, C. D. M. Filipe, Y. Li and R. Pelton, Biomacromolecules, 2008, 9, 935–941 CrossRef CAS
.
- M. M. Ali, S. D. Aguirre, Y. Xu, C. D. M. Filipe, R. Pelton and Y. Li, Chem. Commun., 2009, 6640–6642 RSC
.
- K. Glynou, P. C. Ioannou, T. K. Christopoulos and V. Syriopoulou, Anal. Chem., 2003, 75, 4155–4160 CrossRef CAS
.
- R. E. Luckham and J. D. Brennan, Analyst, 2010, 135, 2028–2035 RSC
.
- C. Wang, R. Zhan, K.-Y. Pu and B. Liu, Adv. Funct. Mater., 2010, 20, 2597–2604 CrossRef CAS
.
- J. Turkevitch, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC
.
- H. D. Hill and C. A. Mirkin, Nat. Protoc., 2006, 1, 324–336 CrossRef CAS
.
-
J. Beesley, Colloidal Gold. A New Perspective for Cytochemical Marking, Oxford Science Publications, Oxford University Press, Oxford, England, 1989 Search PubMed
.
- R. F. Vogt, D. L. Phillips, L. O. Henderson, W. Whitfield and F. W. Spierto, J. Immunol. Methods, 1987, 101, 43–50 CrossRef CAS
.
- J. Steinhar, J. Krijn and J. G. Leidy, Biochemistry, 1971, 10, 4005–4015 CrossRef
.
- W. G. Cao, Q. C. Jiao, Y. Fu, L. Chen and Q. Liu, Spectrosc. Lett., 2003, 36, 197–209 CrossRef CAS
.
- V. D. Trivedi, Ital. J. Biochem., 1997, 46, 67–73 CAS
.
- M. J. Pugia, J. A. Lott, J. A. Profitt and T. K. Cast, J. Clin. Lab. Anal., 1999, 13, 180–187 CrossRef CAS
.
- Y. Suzuki and K. Yokoyama, J. Am. Chem. Soc., 2005, 127, 17799–17802 CrossRef CAS
.
- Q.-P. Qin, T. Lövgren and K. Pettersson, Anal. Chem., 2001, 73, 1521–1529 CrossRef CAS
.
- Z. Lin, J. C. Sauceda-Friebe, J. M. Lin, R. Niessner and D. Knopp, Anal. Methods, 2010, 2, 824–830 RSC
.
- N. R. Jana and J. Y. Ying, Adv. Mater., 2008, 20, 430–434 CrossRef CAS
.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS
.
- I. Willner and E. Katz, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef
.
- W. Shenton, S. A. Davis and S. Mann, Adv. Mater., 1999, 11, 449–452 CrossRef CAS
.
- A. Ambrosi, M. T. Castañeda, A. J. Killard, M. R. Smyth, S. Alegret and A. Merkoçi, Anal. Chem., 2007, 79, 5232–5240 CrossRef CAS
.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Mater., 2001, 13, 2313–2322 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c2ra20156a |
| This journal is © The Royal Society of Chemistry 2012 |

