Retracted Article: Honeycomb-like Co–B amorphous alloy catalysts assembled by a solution plasma process show enhanced catalytic hydrolysis activity for hydrogen generation†
Dong Ge
Tong
*ab,
Wei
Chu
*c,
Ping
Wu
ab and
Li
Zhang
ab
aMineral Resources Chemistry Key Laboratory of Sichuan Higher Education Institutions, College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China. E-mail: tongdongge@163.com; Fax: +86-28-8407 9074
bInstitute of green catalysis and synthesis, College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China
cCollege of Chemical Engineering, Sichuan University, Chengdu 610065, China. E-mail: chuwei65@yahoo.com.cn; Fax: +86-28-8540 3397
First published on 30th January 2012
Abstract
A novel solution plasma process was developed to assemble honeycomb-like Co–B amorphous alloy catalysts with 253.33 m2 g−1 specific surface area using triethanolamine (TEA) as a soft template. The sample was characterized by X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy and transmission electron microscopy. During catalytic hydrolysis of hydrous hydrazine (H2NNH2) for hydrogen generation, the hydrolysis activity and hydrogen selectivity of the honeycomb-like Co–B is superior to Rh nanoparticles and Co–B nanospheres. Meanwhile, the sample also exhibits excellent catalytic performance in the catalytic hydrolysis of ammonia borane (NH3BH3) and sodium borohydride (NaBH4) for hydrogen generation, respectively. The H2 generation rate of 28.9, 105.8, and 262.8 L h−1 g−1catalyst for the hydrolysis reactions of H2NNH2, NH3BH3 and NaBH4, respectively, can be achieved. Meanwhile, the honeycomb-like Co–B is found to be a highly stable catalyst as it provides 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360, 54
360, 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 93
000, and 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 total turnovers in the hydrolytic dehydrogenations of H2NNH2, NH3BH3 and NaBH4, respectively, after being tested for 30 h. The enhanced catalytic performance for the honeycomb-like Co–B catalyst is attributed to the high specific surface area and the enhanced intrinsic activity resulting from its unique honeycomb-like structure.
727 total turnovers in the hydrolytic dehydrogenations of H2NNH2, NH3BH3 and NaBH4, respectively, after being tested for 30 h. The enhanced catalytic performance for the honeycomb-like Co–B catalyst is attributed to the high specific surface area and the enhanced intrinsic activity resulting from its unique honeycomb-like structure.
Introduction
In the past few years, Co–B amorphous alloy catalysts have been widely studied owing to their low cost and high catalytic activity.1–7 Recently, the assembly of nanoparticles into nano/microstructures, such as spheres, tubes, rods, tapes, and fibers, has evolved as an attractive strategy for fabricating materials with tunable physical and chemical properties via control of their composition, size, and shape.8–13 Therefore, designing novel synthetic routes to fabricate Co–B nanostructures will open up exciting opportunities for developing a new class of catalysts.In 2007, mesoporous Co–B with excellent catalytic performance was described by the groups of Tong and Li.14,15 Soon after, Tong and Ma et al. prepared Co–B nanoflowers and hollow spheres (using a soft template technique), which were able to hydrolyze metal borohydrides to produce H2.16,17 In contrast Chen et al. used lyotropic liquid crystals with a layered structure (formed by mixing nonionic and anionic surfactants) as templates to obtain Co–B nanotubes.18 More recently, yolk-shell and hollow nanospindles have been described by Li and Tong's groups, respectively.19,20 All of these samples showed enhanced performance in comparison with regular Co–B amorphous alloys.14–20 Even though these approaches can provide Co–B amorphous alloys with interesting nanostructures, high surface area Co–B amorphous structures are still scarce.
Recently, solution plasma processing (SPP), in which plasma is introduced in an aqueous environment containing chemical agents, has attracted a great deal of attention.21–23 In 2010, Tong and coworkers prepared Co–B nanospheres with a high active surface area through SPP in the presence of hexadecyltrimethylammonium bromide (CTAB).22 The Co–B nanospheres can readily facilitate hydrous hydrazine decomposition to hydrogen under ambient conditions.22 However, for practical use, the improvement of the active surface area for Co–B is still desired. Kim and coworkers reported that the shape of Co–B nanoparticles can be adjusted by changing the stabilizer during SPP.23 Inspired by these results, we synthesized honeycomb-like Co–B amorphous alloyx with high specific surface area (253.33 m2 g−1) using triethanolamine (TEA) as a soft template via SPP for the first time.
Hydrogen is considered as one of the best alternative energy carriers to satisfy the increasing demand for an efficient and clean energy supply.24–27 The main technical challenge for its widespread application is real-time production and storage.24–27 Thus, various kinds of materials for hydrogen storage have been investigated. Among them, hydrous hydrazine (H2NNH2),28–32ammonia borane (NH3BH3)33–39 and sodium borohydride (NaBH4)40–48 have attracted much research interest as potential hydrogen storage materials. Thus, in this work, the catalytic performance of the prepared honeycomb-like Co–B during hydrolysis of H2NNH2, NH3BH3 and NaBH4 for hydrogen generation was studied.
Experimental
Sample preparation
The self-designed reactor used for the preparation of honeycomb-like Co–B viaSPP was illustrated in our previous study.23 The pulsed direct current voltage was 450 V (duty: 50%, frequency: 12 kHz). The electrodes were tungsten wire (diameter: 2 mm) and the gap between the cathode and anode was 1 mm.In a typical synthesis, 40 mL of 25% NH3·H2O solution was mixed with 10 mL of cobalt acetate (0.0085 mol) to form a Co(NH3)62+ complex. Then 32 mL of 1.0 M KBH4 aqueous solution, and 0.0 M–0.3 M triethanolamine (TEA) was added at 298 K under an argon atmosphere. No significant reaction occurred in the absence of plasma. Then, SPP was carried out. After 7 min, the obtained product was washed with deionized water, followed by three washings with absolute alcohol (EtOH). Finally, the sample was stored in EtOH until use. For comparison, the regular Co–B sample and Co–B nanospheres were prepared via the direct reduction of cobalt acetate by KBH4 and the SPP approach, respectively, as described in our previous work.23 The commercial catalysts were provided by Changzhen High Technology Limited Company.
Characterization
The bulk composition was determined by inductively coupled plasma optical emission spectrometry (ICP; Irris, Advantage). The reproducibility was checked by repeating runs at least three times and was found to be within acceptable limits (± 0.5%). The amorphous structure was verified by X-ray diffraction (XRD; Rigaku D/Max-RB with Cu-Kα radiation). The surface morphology was examined viafield emission scanning electron microscopy (FESEM, JEOL JSM-6500FE) and transmission electron microscopy (TEM; JEOL JEM 2100F, 200 kV). The surface electronic states were investigated by X-ray photoelectron spectroscopy (XPS; Perkin–Elmer PHI 5000C ESCA using AlKα radiation). All the binding energy (BE) values were calibrated by using C1s = 284.6 eV as a reference. The active surface area (SCo) was measured by hydrogen chemisorption on a Quantachrome CHEMBET 3000 system, assuming H/Co(s) = 1 and a surface area of 6.5 × 10−20 m2 per Co atom.49 The hydrogen temperature programmed desorption (H2–TPD) curves were obtained on the same instrument in an argon flow at a ramping rate of 20 K min−1. N2 adsorption–desorption isotherms were measured at 77 K using a Quantachrome Nova 4000 analyzer. The pore size distribution and the average pore size were determined by using the BJH model. The room temperature magnetic characterization of the sample was performed using a vibrating sample magnetometer (MagLab-12, Oxford).Activity test
The hydrolysis of hydrazine was carried out at room temperature. Hydrogen generation tests of the samples were initiated by pumping 200 mL of 0.5 M hydrazine monohydrate aqueous solution into a reactor containing 0.5 g catalyst at 298 K. The gas released during the reaction was passed through a trap containing 1.0 M hydrochloric acid to ensure the absorption of ammonia, of which the volume was monitored using a flowmeter.The hydrolysis of ammonia borane was performed according to a previously reported procedure.20 5 mg catalyst was placed in a three-necked glass container and mixed with 50 mL 0.5 wt% NH3BH3 aqueous solution under constant stirring. The generated amount of H2 was measured via a gas flow meter.
The hydrolysis of NaBH4 aqueous solution over the samples was initiated by pumping 50 ml of 2 wt% NaBH4 + 7 wt% NaOH solution into a reactor containing 0.013 g catalyst at 298 K. The solution temperature was maintained within 1 °C of its set point using an external water jacket. The volume of hydrogen gas generated was measured by a gas flow meter.
The analysis of the gas released during the reaction was performed with an on-line gas chromatograph (HP Series 6890) equipped with a Poropak N column and a thermal conductivity detector. The reproducibility of the results was checked by repeating each result at least three times and was found to be within ±5%. The catalytic lifetime of the honeycomb-like Co–B in the hydrolysis was determined by measuring the total turnover number (TTON). The test time was 30 h. The reactivation of the deactivated Co–B were carried out in the same SPP reactor used for the preparation of the honeycomb-like Co–B but only in the presence of 0.05 M potassium chloride (KCl) solution.
Results and discussion
A typical SEM image of the honeycomb-like Co–B amorphous alloy obtained is shown in Fig. 1a, and a magnified image revealing the morphology of the honeycomb-like Co–B is shown in Fig. 1b. The SEM images reveal that the honeycomb-like structure comprises of numerous nano-scale chambers constructed of nanoflakes a few nanometres thick. The TEM images further confirmed the honeycomb-like structure (Fig. 1c–e). The appearance of halos in the selected area electron diffraction (SAED) pattern (Fig. 1f) indicated that the sample was present in a typical amorphous state. This was also confirmed by the XRD result. | ||
| Fig. 1 SEM images (a, b); TEM images (c–e) and the SAED pattern (f) of the obtained honeycomb-like Co–B. | ||
The XRD pattern of the sample is shown in Fig. 2. From Fig. 2, only one broad peak around 2θ = 45.5° is observed, which indicates that the sample has a typical amorphous alloy structure.14–20 ICP analysis demonstrated that the obtained product displayed the same atomic composition as the regular Co–B (Co63.0B37.0). Furthermore, the small-angle XRD pattern (the inset of Fig. 2) displays a well-resolved diffraction peak around 2θ = 1.52°, implying the presence of a mesoporous structure in the sample.14,50 The formation of the mesoporous structure is also confirmed by the nitrogen adsorption–desorption isotherm and BJH pore size distribution (Fig. 3). The isotherm of the sample shows an H2 type hysteresis loop, which implies the presence of a network of pores of different sizes.14,50 This is consistent with the pore size distribution plot in the inset of Fig. 3, where three well-distinguished maxima can be observed. It is known that apart from the surface morphology, factors like inter-particle spacing and internal voids also contribute to the characteristic pore size distribution of a material.50 Therefore, the honeycomb-like shaped porous network and the random attachment of porous balls are likely reasons for the multimodal pore size distribution of the sample. The specific surface area of the honeycomb-like Co–B (253.33 m2 g−1) was much larger than those previously reported,14–20 and larger than that of the regular Co–B (65.00 m2 g−1). The specific pore volume of the samples was 0.47 cm3 g−1.
 | ||
| Fig. 2 XRD patterns of the honeycomb-like Co–B. The inset is the small angle XRD pattern of the sample. | ||
 | ||
| Fig. 3 N2 adsorption–desorption isotherm of the honeycomb-like Co–B. The inset is the pore size distribution curve of the sample. | ||
The XPS spectra (Fig. S1, ESI†) revealed that the Co species in the Co–B sample are present in the metallic state with a binding energy of 778.1 eV, which agrees with that reported previously.14–20 The B species (Fig. S1, ESI†) displayed one peak around 188.2 eV, which is positively shifted by 1.1 eV in comparison with the pure B (187.1 eV).51 The shift in the binding energy of the B species indicates the existence of a synergistic effect between the Co and B in the prepared Co–B, similar to the effects reported for the regular Co–B amorphous alloy.14–20 As a result, electrons are partially transferred from the alloying B to the vacant d-orbital of the metallic Co, making the Co electron-rich and, in turn, the B electron-deficient. The lack of an observed binding energy shift for the Co species could be attributed to the relatively larger size of the Co atoms in comparison with the B atoms.14–20
To understand the formation process of the honeycomb-like Co–B, we carried out time-dependent experiments during which samples were collected as a function of time and examined using SEM. Fig. 4a–h show the SEM images of the samples obtained with various plasma treatment periods. As shown in Fig. 4a and b, during the first minute, the sample was composed of a large number of small nanoparticles (∼30 nm across). The sample obtained at the 3rd minute changed to a sphere-like structure and small nanoflakes began to form on the outside of the spheres (Fig. 4c and d). As the reaction proceeded, interconnected nanoparticles with honeycomb structures began to appear (Fig. 4e and f). Meanwhile, many microspheres with an incomplete honeycomb-like structure, in which the nanoflakes were much larger than those obtained after the 3rd minute, appeared. After about 7 min of the reaction, perfect 3D honeycomb-like nanostructures with well-arranged nanoflakes were obtained (Fig. 4g and h). The extremely high energy level of localized supersaturation due to evaporation of the solvent in the bubbles created by the formation, growth, and implosive collapse of bubbles in the solution plasma field apparently triggers the reaction between Co(NH3)62+ and BH4− to produce the Co–B amorphous alloy.21–23
 | ||
| Fig. 4 The morphology evolution of the honeycomb-like Co–B products during SPP: (a, b) 1 min; (c, d) 3 min; (e, f) 5 min; (g, h) 7 min. | ||
To highlight the influence of triethanolamine on the formation of the Co–B honeycomb-like microspheres, we carried out a series of comparison experiments where we varied the concentration of triethanolamine while holding the other reaction parameters constant. Fig. 5 shows SEM images of the Co–B samples obtained using various amounts of triethanolamine. From Fig. 5a and b, it can be seen that without triethanolamine, irregular particles with a rough surface were formed. By increasing the concentration of triethanolamine to 0.05 M, microspheres with some nanoflakes on the surface can be observed (Fig. 5c and d). When the concentration of triethanolamine reached 0.1 M, nano-chambers appeared while some irregular aggregates still existed (Fig. 5e and f). As the amount of triethanolamine increased to 0.15 M, perfect 3D honeycomb-like nanostructures with well-arranged nanoflakes were obtained (Fig. 5g and h). Further increases in the amount of triethanolamine (0.3 M) did not result in further changes in the morphology and particle size (not shown here). These observations show that the presence of an appropriate amount of triethanolamine is beneficial to the formation of the honeycomb-like structure, and adjusting the amount of triethanolamine is crucial for controlling the morphology and size of the Co–B honeycomb-like microspheres.
 | ||
| Fig. 5 SEM images of the honeycomb-like Co–B prepared by SPP with different concentrations of triethanolamine (a, b) 0; (c, d) 0.05; (e, f) 0.10; (g, h) 0.15 M. | ||
Furthermore, we tried many other surfactants, such as Pluronic P123, ethylenediamine, sodium dodecyl benzene sulfonate (SDBS) and PVA under the same reaction conditions. Unfortunately, they did not form a honeycomb-like nanostructure. The SEM images of the nanoparticles grown with the other surfactants showed that all of the products were of different shapes, including flakes, rods, etc. (Fig. S2a–d, ESI†). However, if additional triethanolamine was added into the reaction system using Pluronic P123, ethylenediamine, sodium dodecyl benzene sulfonate (SDBS) or PVA as the stabilizer, a similar honeycomb-like structure could still be obtained (Fig. S2e and f, ESI†).
Based on experimental observation and analysis, the formation mechanism of the honeycomb-like Co–B in our case can be described as follows. First, nano-nuclei were formed in the solution during the initial reaction stage. The freshly-formed nano-nuclei were thermodynamically unstable due to their high surface energy, and tended to aggregate together to minimize interfacial energy, and thus forming many agglomerates. Earlier studies have shown that the extrinsic modulation of crystal growth can occur by selective adsorption of solvents, inorganic additives or surfactants to certain crystallographic planes modifying the relative order of surface energies.52–54 In other words, in our case, triethanolamine may act as a surface modifier for the particles, leading to the oriented development of the subunits into flake shapes during SPP. Therefore, in the subsequent stage, the formed particles deposited on the small raised areas of the agglomerates and assembled to form small nanoflakes along a specific direction with the assistance of triethanolamine. When the concentration of triethanolamine was increased, the proportion of nano-chambers increased (Fig. 5e–h). It revealed that more Co–B nanoflakes were connected together by ethylenediamine and gradually developed into honeycomb-like nanostructures. As the reaction went on, each nanoflake continued to grow along its axis until the complete honeycomb-like structures formed. Therefore, triethanolamine molecules in this work were believed to take the role of “connecter”, causing a certain amount of newly produced Co–B nuclei to form small chambers as the rudiments of the honeycomb. However, finding the detailed mechanism for the formation of honeycomb-like structures during SPP will be a great challenge.
Hydrazine can be decomposed in two ways: 1) complete decomposition as shown in equation (1) H2NNH2 → N2↑+ 2H2↑; 2) incomplete decomposition as shown in equation (2) 3H2NNH2 → 4NH3 + N2↑.22,28–33 According to our previous study, the hydrolysis of hydrazine over Co–B amorphous alloy can be expressed as the equation: (3) 3H2NNH2 → (1 + 2x)N2 + 6xH2 + 4(1 − x)NH3, where x is the H2 selectivity.22Table 1 summarizes some structural parameters and the catalytic performance of different catalysts for the hydrolysis of H2NNH2 to H2. The active surface area (SCo) was obtained by hydrogen chemisorption. The specific activity (Rm) refers to the conversion of H2NNH2 per h and per gram of Co. The turnover frequency (TOF) is the number of molecules formed per cobalt active site per second.55 In our case, it can be calculated based on Rm and SCo with the assumption that the surface atoms concentration for cobalt was 1.53 × 10−19m−2,49 and may be considered to be an intrinsic activity. The catalyst lifetime was evaluated by using total turnovers (TTON) for the hydrolysis reactions of H2NNH2 over 30 h before deactivation. In order to facilitate the evaluation, the average TOF values (ATOF) were also calculated. Comparing TOF values and SCo, the honeycomb-like Co–B exhibited more intrinsic activity and contained more Co active sites than the Co–B nanospheres and regular Co–B. This can obviously be attributed to its unique structure. During hydrolysis, the chambers in the honeycomb-like structure may provide contact between catalysts and H2NNH2 at both the inner and outer surfaces. The hydrogen temperature programmed desorption (H2–TPD) curves reveal only one broad peak at 514 K for the honeycomb-like Co–B (Fig. 6). This indicated that the catalytic active sites in this sample are more uniform than those in the Co–B nanospheres and regular Co–B (which exhibited two desorption peaks at around 493, 625 K and four desorption peaks at around 517, 563, 625, and 744 K in our previous study, respectively23).
 | ||
| Fig. 6 H2–TPD curves obtained on the honeycomb-like Co–B. | ||
| Precursors | Catalyst | S Co /m2 g−1 | H2 generation rate/L h−1 g−1catalyst | R m/mol h−1 g−1Co | TOF/s−1 | H2 selectivity/% | TTON | ATOF/s−1 |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions in this work: 0.5 g catalyst, 200 ml 0.5 M H2NNH2 aqueous, T = 298 K. b Reaction conditions in this work: 0.005 g catalyst, 50 ml 0.5 wt% NH3BH3 aqueous, T = 293 K. c Reaction conditions in this work: 0.013 g catalyst, 50 ml of 2 wt% NaBH4 + 7wt% NaOH solution, T = 298 K. d Reported by Xu's group.28 e Reported by Xu's group.35 f Reported by Patels's group.44 | ||||||||
| H2NNH2a | Regular Co–B | 24.3 | 0.59 | 0.17 | 0.076 | 8.7 | — | — |
| Honeycomb-like Co–B | 90.2 | 28.9 | 1.72 | 0.21 | 41.8 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
0.17 | |
| Co–B nanospheres | 81.1 | 5.67 | 0.66 | 0.089 | 21.3 | — | — | |
| Commercial Rh | — | 1.90 | — | — | 42.7 | — | — | |
| Rh | — | 1.96d | — | — | 43.8d | — | — | |
| NH3BH3b | Regular Co–B | 24.3 | 28.4 | 0.47 | 0.21 | — | — | |
| Honeycomb-like Co–B | 90.2 | 105.8 | 1.75 | 0.7 | — | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.5 | |
| Co–B nanospheres | 81.1 | 53.8 | 0.89 | 0.40 | — | — | ||
| Commercial Pt black | — | 60.7 | — | — | — | — | — | |
| Pt black | — | 54.1e | — | — | — | — | — | |
| NaBH4c | Regular Co–B | 24.3 | 57.3 | 0.79 | 0.35 | — | — | — |
| Honeycomb-like Co–B | 90.2 | 262.8 | 3.26 | 1.20 | — | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 727 |
0.87 | |
| Co–B nanospheres | 81.1 | 121.7 | 1.51 | 0.57 | — | — | — | |
| Commercial Ru | — | 35.7 | — | — | — | — | — | |
| Commercial Co–B-film | — | 215 | — | — | — | — | — | |
| Commercial Co–P–B | — | 240 | — | — | — | — | — | |
| Commercial Pt/C | — | 960 | — | — | — | — | — | |
| Ru | — | 28.8f | — | — | — | — | — | |
| Co–B-film | — | 198f | — | — | — | — | — | |
| Co–P–B | — | 249f | — | — | — | — | — | |
| Pt/C | — | 1380f | — | — | — | — | — |
For determining the activation energy, we measured the temperature dependence of the hydrogen generation rate using 0.5 M aqueous H2NNH2 solution with 0.5 g honeycomb-like Co–B in 293–333 K. Under our experimental conditions, the reaction rate constant k is constant for a given temperature. The result showed that the catalytic hydrolysis of H2NNH2 proceeds according to zero order kinetics with respect to H2NNH2 concentration.35 In other words, the hydrogen generation rate is controlled by a surface reaction. Thus, the hydrogen generation rate (R) can be given as: R = k[H2NNH2]0. Through the Arrhenius treatment of the temperature dependent reaction rates, the activation energy (Ea) is determined to be 54.3 ± 2.7 kJ mol−1 (Fig. S3, ESI†).
Meanwhile, the H2 selectivity and catalytic activity for the honeycomb-like Co–B remained unchanged as the hydrazine concentration increased from 0.5 to 5 M (Fig. S4 and 5, ESI†). Based on the hydrolysis activity and hydrogen selectivity, the catalytic performance of the honeycomb-like Co–B is superior to those of commercial and reported Rh nanoparticles (Table 1).28 Considering the price difference between Rh (59.7 CNY per mmole) and Co–B (0.218 CNY per mmole), the honeycomb-like Co–B is more cost-effective.
However, to meet practical applications, the H2 selectivity of hydrazine decomposition over Co–B needs to be enhanced. Generally, the product selectivity shows a critical dependency on the catalyst used.56–61 Because the electronic and structural properties of the catalyst surfaces are closely related to their catalytic activities, the precise modification of the catalyst surfaces by the introduction of a second component or change of the morphology could facilitate tuning of the catalytic performance.56–61 Recently, Xu's group demonstrated that Rh4Ni, Ni0.93Pt0.07, or Ni0.95Ir0.05 nano-catalysts make it possible to achieve 100% conversion of hydrous hydrazine to hydrogen at room temperature at a low H2 generation rate.29–31 They discovered that the synergistic effect of Ni and the second metal gives rise to the drastic enhancement of the hydrogen selectivity. This is attributed to the fact that the alloying of Ni and the second metal leads to a modification of the Ni surface and tunes the interactions of Ni with the N–N and N–H bonds, as well as the stability of the reaction intermediates on the catalyst surface. More recently, Xu's group developed a highly efficient and low-cost catalyst of non-precious nickel and iron nanoparticles in alloy form, which also achieves 100% hydrogen selectivity from hydrous hydrazine decomposition in an alkaline solution at 343 K.32 The present finding demonstrates the importance of combining hydrous hydrazine hydrolysis conditions to develop active nanocatalysts whose distinct surface properties enable them to outperform their parent metals. These results have inspired us to search for more selective Co–B amorphous catalysts with distinct surface properties via alloying other transition metals and combining hydrolysis reaction conditions with this promising system. This work is currently underway.
H2 can be released by decomposition of NH3BH3via the reaction (4) NH3BH3 + 2H2O → NH4+ + BO2− + 3H2↑. In our work, the NH3BH3 concentration is 0.5 wt%, which is lower than 6 wt%. According to Xu and Liu's work,62,63 the quantity of ammonia liberated during the hydrolysis of NH3BH3 can be negligible. Meanwhile, control tests using acid/base indicator also indicated no ammonia evolution in detectable amounts. During the hydrolysis of NH3BH3 to H2, the honeycomb-like Co–B also showed much higher activity (Rm) than the Co–B nanospheres and regular Co–B (Table 1). According to the hydrogen yield, the catalytic performance of honeycomb-like Co–B is superior to those of commercial and reported Pt black.35
Furthermore, during the hydrolysis of NaBH4 to H2, the honeycomb-like Co–B still exhibited much higher activity (Rm) than the Co–B nanospheres and regular Co–B (Table 1). The reaction can be expressed as follows: (5) NaBH4 + 2H2O → 4H2 + NaBO2. Comparing the efficiency of honeycomb-like Co–B to that of catalysts reported previously in the hydrolysis of NaBH4 shows that the hydrogen generation rate of honeycomb-like Co–B is far better than many other commercial and reported catalysts, such as Ru, Co–B film and Co–P–B, etc.44 Only carbon supported Pt (Pt/C) shows a higher H2 generation rate (1380 L h−1 g−1catalyst) than honeycomb-like Co–B.44 However, Pt is very expensive and not preferable for practical use. Besides the higher dispersion degree of Co active sites (Table 1 and Fig. 6), the higher activity of honeycomb-like Co–B could be mostly attributed to the enhanced intrinsic activity (TOF).
The catalyst lifetime of honeycomb-like Co–B was also investigated. From Table 1, it can be seen that the honeycomb-like Co–B is a highly stable catalyst, providing 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360, 54
360, 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 93
000, and 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 total turnovers (TTON) for the hydrolysis reactions of H2NNH2, NH3BH3 and NaBH4 over 30 h before deactivation, respectively. The average TOF values (ATOF) were calculated as 0.17, 0.5, and 0.87 s−1, respectively. The hydrogen generation slows down as the reaction proceeds and ultimately stops. ICP analysis reveals that the leaching of Co species was <1.0 ppm, which can rule out the loss of active phases. Moreover, TEM, SEM and XRD results indicated that there had no occurrence of crystallization and structure change for honeycomb-like Co–B after repetitive use at room temperature. It appears that the oxidation of B on the active surface of Co–B is responsible for the deactivation, as shown by XPS (Fig. S6, ESI†) and XRD results (Fig. S7, ESI,† JCPDS 73-1550). In particular, the boron oxide film resulted in a decrease in SCo due to blocking of the Co active sites and the reduction of Co electron density.7
727 total turnovers (TTON) for the hydrolysis reactions of H2NNH2, NH3BH3 and NaBH4 over 30 h before deactivation, respectively. The average TOF values (ATOF) were calculated as 0.17, 0.5, and 0.87 s−1, respectively. The hydrogen generation slows down as the reaction proceeds and ultimately stops. ICP analysis reveals that the leaching of Co species was <1.0 ppm, which can rule out the loss of active phases. Moreover, TEM, SEM and XRD results indicated that there had no occurrence of crystallization and structure change for honeycomb-like Co–B after repetitive use at room temperature. It appears that the oxidation of B on the active surface of Co–B is responsible for the deactivation, as shown by XPS (Fig. S6, ESI†) and XRD results (Fig. S7, ESI,† JCPDS 73-1550). In particular, the boron oxide film resulted in a decrease in SCo due to blocking of the Co active sites and the reduction of Co electron density.7
During the hydrolysis of NH3BH3 and NaBH4, the blocking of catalyst sites by the accumulation of hydrolysis by-product BO2− on the catalyst surfaces may be the another reason for deactivation of the honeycomb-like Co–B.33,34,64 BO2− is known to be water soluble and can be washed away.65,66 However, the washed honeycomb-like Co–B still exhibited poor activity in comparison with the fresh sample. These results indicated that the oxidation of B on the Co–B active surfaces is also the main reason for Co–B deactivation during NH3BH3 or NaBH4 hydrolysis. Thus, acid washing is an effective approach to reactivate honeycomb-like Co–B. But, acid washing greatly shortened the catalyst lifetime of honeycomb-like Co–B due to the leaching of Co and B species and the destruction of the honeycomb-like structure. Plasma techniques are known to be powerful approaches for catalyst reactivation.67 In this work, we attempted to reactivate Co–B by the solution plasma process in the presence of potassium chloride (KCl) for the first time. Surprisingly, the reactivated honeycomb-like Co–B exhibits the same activity as the fresh sample (Fig. S8, ESI†). As shown by XPS and XRD results, the oxidized B was reduced (Fig. S6 and 7 in the ESI). It is believed that electrons yielded during the SPP are the main factors for the reduction of B2O3 on the Co–B surface.21,23 The refresh process can be expressed as follows: (6) B2O3 + 6e− + 3H2O → 2B + 6OH−. However, the real scheme of Co–B reactivation by SPP requires further study, which is currently underway. Meanwhile, there was no significant effect of the plasma treatment on the morphology and composition of the honeycomb-like Co–B after reactivation. In other words, SPP may be a potential pathway to reactivate the deactivated catalyst due to surface oxidation.
Meanwhile, the easy magnetic separation of honeycomb-like Co–B is very important for its lifetime in the solution system.20,23 The magnetic properties of the sample is shown in Fig. 7. For honeycomb-like Co–B, the typical coercivity (Hc), saturation magnetization (Ms), and remnant magnetization (Mr) at 298 K are 240.1 Oe, 56.16 emu/g, and 15.5 emu/g, respectively. The magnetic properties enable effective separation of the honeycomb-like Co–B by a magnet in a solution system.20,23
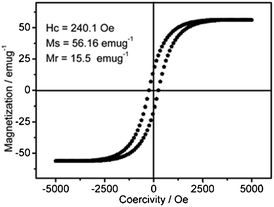 | ||
| Fig. 7 Hysteresis loop and magnetic properties of the honeycomb-like Co–B. | ||
According to the previous research, a hydrogen generation rate of 1.0 L min−1 can give sustainable H2 supply for a 162 W Proton Exchange Membrane Fuel Cell (PEMFC) at 100% H2 utilization.41,44 Therefore, a reactor filled with 1.0 g honeycomb-like Co–B may produce more than 0.54, 1.96, 4.86 L within 1 min for H2NNH2, NH3BH3 and NaBH4, respectively. In other words, it may give a sustainable H2 supply for a 87.5, 317, 886 W PEMFC at a 100% H2 utilization for H2NNH2, NH3BH3 and NaBH4, respectively.
Conclusion
In summary, in this work we developed a new approach to assemble honeycomb-like Co–B amorphous alloy catalysts with high specific surface area (253.33 m2 g−1) by using triethanolamine as a soft template in a solution plasma process. The sample exhibited excellent hydrolysis activity for hydrogen generation owing to the high surface area resulting from the unique structure. The reaction system consisting of a honeycomb-like Co–B catalyst and H2NNH2, NH3BH3 or NaBH4, may potentially be used for small hydrogen fuel cells. Furthermore, the present approach can be extended to assemble other honeycomb-like nano-composites.Acknowledgements
This work was financially supported by the Research Funds of the CDUT (2007-YG2 and HZ0033), the Natural Science Foundation of Sichuan Province (07ZA004), the Cultivating programme of Middle-aged backbone teachers (HG0092) and the Cultivating programme for Excellent Innovation Team of Chengdu University of Technology.References
- H. X. Li, X. F. Chen, Y. P. Wang and Y. P. Xu, Appl. Catal., A, 2002, 225, 117 CrossRef CAS.
- C. Wu, F. Wu, Y. Bai, B. Yi and H. Zhang, Mater. Lett., 2005, 59, 1748 CrossRef CAS.
- H. X. Li, H. Li, J. Zhang, W. L. Dai and M. H. Qiao, J. Catal., 2007, 246, 301 CrossRef CAS.
- S. U. Jeong, E. A. Cho, S. W. Nam, I. H. Oh, U. H. Jung and S. H. Kim, Int. J. Hydrogen Energy, 2007, 32, 1749 CrossRef CAS.
- D. G. Tong, W. Chu, Y. Y. Luo, X. Y. Ji and Y. He, J. Mol. Catal. A: Chem., 2007, 265, 195 CrossRef CAS.
- N. Patel, R. Fernandes, G. Guella, A. Kale, A. Miotello, B. Patton and C. Zanchetta, J. Phys. Chem. C, 2008, 112, 6968 CAS.
- H. Li, J. Liu, S. H. Xie, M. H. Qiao, W. L. Dai and H. X. Li, J. Catal., 2008, 259, 104 CrossRef CAS.
- K. Mori and H. Yamashita, Phys. Chem. Chem. Phys., 2010, 12, 14420 RSC.
- S. Singamaneni, V. N. Bliznyuk, C. Binekc and Y. Evgeny, J. Mater. Chem., 2011, 21, 16819 RSC.
- F. Li, D. P. Josephson and A. Stein, Angew. Chem., Int. Ed., 2011, 50, 360 CrossRef CAS.
- R. H. Eduardo, P. Aranda, M. Dardera and M. Ogawa, Chem. Soc. Rev., 2011, 40, 801 RSC.
- C. J. Jia and F. Scuth, Phys. Chem. Chem. Phys., 2011, 13, 2457 RSC.
- M. R. Jones, K. D. Osberg, R. J. Macfarlane, M. R. Langille and C. A. Mirkin, Chem. Rev., 2011, 111, 3736 CrossRef CAS.
- D. G. Tong, W. Chu, Y. Y. Luo, H. Chen and X. Y. Ji, J. Mol. Catal. A: Chem., 2007, 269, 149 CrossRef CAS.
- H. Li, H. X. Yang and H. X. Li, J. Catal., 2007, 251, 233 CrossRef CAS.
- D. G. Tong, X. Han, W. Chu, H. Chen and X. Y. Ji, Mater. Res. Bull., 2008, 43, 1327 CrossRef CAS.
- H. Ma, W. Q. Ji, J. Z. Zhao and J. Chen, J. Alloys Compd., 2008, 474, 584 CrossRef.
- Y. Zhu, F. P. Liu, W. P. Ding, X. F. Guo and Y. Chen, Angew. Chem., Int. Ed., 2006, 45, 7211 CrossRef CAS.
- H. Li, D. Q. Zhang, G. S. Li, Y. Xu, Y. F. Lu and H. X. Li, Chem. Commun., 2010, 46, 791 RSC.
- D. G. Tong, X. L. Zeng, W. Chu, D. Wang and P. Wu, J. Mater. Sci., 2010, 45, 2862 CrossRef CAS.
- O. Takai, “Solution plasma processing” in The 5th international symposium advanced plasma process and diagnostics & The 1st international symposium on flexible electronics technology, 2007, p23 Search PubMed.
- D. G. Tong, X. L. Zeng, W. Chu, D. Wang and P. Wu, Mater. Res. Bull., 2010, 45, 442 CrossRef CAS.
- S. M. Kim, G. S. Kim and S. Y. Lee, Mater. Lett., 2008, 62, 4354 CrossRef CAS.
- A. Züttel, A. Borgschulte and L. Schlapbach, Hydrogen as a Future Energy Carrier; Wiley-VCH, Weinheim, Germany, 2008 Search PubMed.
- N. Z. Muradov and T. N. Veziroğlu, Int. J. Hydrogen Energy, 2008, 33, 6804 CrossRef CAS.
- B. L. Chen, S. C. Xiang and G. D. Qian, Acc. Chem. Res., 2010, 43, 1115 CrossRef CAS.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS.
- S. K. Singh, X. -B. Zhang and Q. Xu, J. Am. Chem. Soc., 2009, 131, 9894 CrossRef CAS.
- S. K. Singh and Q. Xu, J. Am. Chem. Soc., 2009, 131, 18032 CrossRef CAS.
- S. K. Singh and Q. Xu, Chem. Commun., 2010, 46, 6545 RSC.
- S. K. Singh and Q. Xu, Inorg. Chem., 2010, 49, 6148 CrossRef CAS.
- S. K. Singh, A. K. Singh, K. Aranishi and Q. Xu, J. Am. Chem. Soc., 2011, 133, 19638 CrossRef CAS.
- T. J. Clark, G. R. Whittell and I. Manners, Inorg. Chem., 2007, 46, 7522 CrossRef CAS.
- F. Cheng, H. Ma, Y. Li and J. Chen, Inorg. Chem., 2007, 46, 788 CrossRef CAS.
- M. Chandra and Q. Xu, J. Power Sources, 2007, 168, 135 CrossRef CAS.
- S. B. Kalidindi, M. Indirani and B. R. Jagirdar, Inorg. Chem., 2008, 47, 7424 CrossRef CAS.
- T. Umegaki, J. M. Yan, X. B. Zhang, H. Shioyama, N. Kuriyama and Q. Xu, J. Power Sources, 2010, 195, 8209 CrossRef CAS.
- Y. Yamada, K. Yano, Q. Xu and S. Fukuzum, J. Phys. Chem. C, 2010, 114, 16456 CAS.
- H. L. Jiang and Q. Xu, Catal. Today, 2011, 170, 56 CrossRef CAS.
- B. H. Liu, Z. P. Li and S. Suda, J. Alloys Compd., 2006, 415, 288 CrossRef CAS.
- Y. Bai, C. Wu, F. Wu and B. Yi, Mater. Lett., 2006, 60, 2236 CrossRef CAS.
- G. Guella, C. Zanchetta, B. Patton and A. Miotello, J. Phys. Chem. B, 2006, 110, 17024 CrossRef CAS.
- N. Patel, B. Patton, C. Zanchetta, R. Fernandes, G. Guella, A. Kale and A. Miotello, Int. J. Hydrogen Energy, 2008, 33, 287 CrossRef CAS.
- N. Patel, R. Fernandes and A. Miotello, J. Power Sources, 2009, 188, 411 CrossRef CAS.
- U. B. Demirci and P. Miele, Phys. Chem. Chem. Phys., 2010, 12, 14651 RSC.
- N. Patel, R. Fernandes, N. Bazzanella and A. Miotello, Catal. Today, 2011, 170, 20 CrossRef CAS.
- H. B. Dai, Y. Liang and P. Wang, Catal. Today, 2011, 170, 27 CrossRef CAS.
- T. F. Hung, H. C. Kuo, C. W. Tsai, H. M. Chen, R. S. Liu, B. J. Weng and J. F. Lee, J. Mater. Chem., 2011, 21, 11754 RSC.
- J. J. F. Scholten, A. P. Pijers and A. M. L. Hustings, Catal. Rev. Sci. Eng., 1985, 27, 151 CAS.
- R. E. Xu, W. Q. Pang, J. H. Yu, Q. S. Hu and J. S. Chen, Chemistry of Molecular Sieve and Porous Materials, Science Press, Beijing, 2004, ch 9, pp. 528–557 Search PubMed.
- Reference: Manual for Operator for PHI PC Windows Software Version 1.2b, Physical Electronic Division, Perkin-Elmer..
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576 CrossRef.
- G. M. Whitesides and M. Boncheva, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4769 CrossRef CAS.
- J. F. Banfield, S. A. Welch, H. Zhang, T. T. Ebert and R. L. Penn, Science, 2000, 289, 751 CrossRef CAS.
- M. Boudart, Chem. Rev., 1995, 95, 661 CrossRef CAS.
- J. A. Rodriguez and D. W. Goodman, Science, 1992, 257, 897 CAS.
- J. Greeley and M. Mavrikakis, Nat. Mater., 2004, 3, 810 CrossRef CAS.
- V. R. Stamenkovic, B. S. Mun, J. J. Arenz, M. Mayrhofer, C. A. Lucas, G. Wang, P. N. Ross and N. M. Markovic, Nat. Mater., 2007, 6, 241 CrossRef CAS.
- R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 845 CrossRef CAS.
- J. Greeley, I. E. L. Stephens, A. S. Bondarenko, T. P. Johansson, H. A. Hansen, T. F. Jaramillo, J. Rossmeisl, I. Chorkendorff and J. K. Nørskov, Nat. Chem., 2009, 1, 552 CrossRef CAS.
- B. Lim, M. Jiang, P. H. C. Camargo, E. C. Cho, J. Tao, X. Lu, Y. Zhu and Y. Xia, Science, 2009, 324, 1302 CrossRef CAS.
- M. Chandra and Q. Xu, J. Power Sources, 2006, 156, 190 CrossRef CAS.
- B. H. Liu, Z. P. Li and S. Suda, J. Alloys Compd., 2006, 415, 288 CrossRef CAS.
- C. A. Jaska, T. J. Clark, S. B. Clendenning, D. Groeza, A. Turak, Z. H. Lu and I. Manners, J. Am. Chem. Soc., 2005, 127, 5116 CrossRef CAS.
- X. J. Zhen, Handbook of Boron Composites, Chemical Industry Press, Beijing, 201035 Search PubMed.
- H. Erdǒgan, Ö. Metin and S. Özkar., Catal. Today, 2011, 170, 93 CrossRef.
- X. Z. Zou and D. K. Chen, Plasma Treatment Technique, Mechanical Industry Press, Beijing, 1990, p.48 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra01321e |
| This journal is © The Royal Society of Chemistry 2012 |
