Partial enzymatic hydrolysis of microcrystalline cellulose in ionic liquids by Trichoderma reesei endoglucanases
Ronny
Wahlström
*,
Stella
Rovio
and
Anna
Suurnäkki
VTT Technical Research Center of Finland, P.O. Box 1000, FI-02044 VTT, Espoo, Finland. E-mail: ronny.wahlstrom@vtt.fi; Fax: +358 20 722 7071; Tel: +358 40 02 54 073
First published on 10th April 2012
Abstract
The enzymatic hydrolysis of cellulose in ionic liquid (IL) containing systems has recently received a lot of interest as a pretreatment in biomass conversion to liquid biofuels and chemicals. In this paper we present a study in which the activity and action of two Trichoderma reesei endoglucanases, Cel7B and Cel5A, were evaluated in aqueous solutions containing 0–90% (v/v) of the ionic liquids 1,3-dimethylimidazolium dimethylphosphate or 1-ethyl-3-methylimidazolium acetate, using microcrystalline cellulose (Avicell) as a model substrate. The degree of hydrolysis was analysed by capillary electrophoresis of the hydrolysates and gel permeation chromatography of the remaining cellulose residues. Both of the employed ionic liquids severely inactivated the T. reesei endoglucanases. Only traces of soluble oligosaccharides were present in hydrolysis mixtures containing 40% (v/v) or more of ionic liquids. The employed ILs were found to have a basic impact on the hydrolysis environment, but it could be concluded that the basicity of the ILs was not the only reason for the cellulase inactivation. The effect of an IL on the cellulose binding module in Cel5A was evaluated by comparing the hydrolysis yields of the intact Cel5A and the Cel5A core lacking the cellulose binding module. In this study the cellulose binding module was found to be the most ionic liquid sensitive part of the enzymes used. Comparative data from the partial hydrolysis of an ionic liquid regenerated cellulose is also reported.
Introduction
Cellulose is the world's most common renewable biopolymer, with an annual growth rate estimated at 90 × 109 tons per year.1 The high biosphere production of cellulose makes it an interesting candidate as a raw material for any application, where it could be used to replace fossil resources. Being composed purely of anhydroglucose units, cellulose may be broken down into glucose by chemical (mineral acid) or enzymatic hydrolysis.2 Glucose is a platform chemical, both for the production of fuel ethanol through fermentation, and for many other chemical products via either chemical or biochemical transformations.3 Partial hydrolysis of cellulosic material, yielding water soluble cellooligomers, is another interesting research topic. Cellooligomers could find use as biologically active dietary additives4 and as complex chemical building blocks and model compounds.5Natural cellulose is a semicrystalline polymer consisting of crystalline and amorphous regions.1 It is very recalcitrant towards enzymatic hydrolysis due to its high degree of crystallinity, low surface area for enzyme binding,6 and its general insolubility.2 In lignocellulosics, the presence of other biomass components further shelter the cellulose from hydrolysis.6 A great variety of chemical, physical and biological pretreatment methods have been proposed for lignocellulosic biomass for use prior to enzymatic total or partial hydrolysis.6,7 Typical to all of them is a high consumption of energy and often undesirable by-product formation,8 facts that render them economically and/or environmentally unfeasible for total hydrolysis.
During the last decade a growing interest in the dissolution of cellulose in ionic liquids (ILs), followed by subsequent homogeneous modification, or regeneration by the addition of a counter-solvent, has arisen. ILs are defined as salts with melting points below 100 °C.9 These compounds possess some very interesting solvent properties due to their dual ionic and organic nature. ILs have generally been considered as green solvents, mainly due to their thermal stability and negligible vapour pressure, which eliminates any VOC (volatile organic compound) emissions.10 As early as 1934 Graenacher received a patent on dissolving and processing cellulose in benzylpyridinium chloride.11 However, modifying cellulose in ionic liquids started receiving interest only after Swatloski et al. reported the dissolution of cellulose in 1-n-butyl-3-methylimidazolium chloride [BMIM]Cl in 2002.12 It has also been shown that even wood, in the form of saw dust or wood chips, can dissolve in some ionic liquids.13
Cellulases are the main enzymes for the enzymatic hydrolysis of cellulose. These enzymes have been studied in cellulose hydrolysis both as complex mixtures and in the monocomponent form. Cellulases may be divided into three types of functionalities, which work together synergistically: endoglucanases, which randomly hydrolyse the cellulose chain in its amorphous regions producing cellooligomers, exoglucanases, which hydrolyse the cellulose chains from either the reducing or the non-reducing end producing mainly cellobiose and β-glucosidases, which cleave the resulting cellobiose units produced by the glucanases.14
One of the earliest studies on the enzymatic hydrolysis of cellulose in aqueous ILs was published by Turner et al. in 2003.15 This study clearly demonstrated that ILs greatly inactivate cellulase enzymes. Dadi et al. showed that the enzymatic hydrolysis of cellulose might be greatly enhanced by IL pretreatments of the substrates.16 In this process concept, the enzymatic hydrolysis takes place in a separate process step after the regeneration of cellulose from the IL solution. The increase in reaction rates was attributed to the lower degree of crystallinity in the regenerated cellulose (RC). Kamiya et al. introduced the term “in situ saccharification”, where the regeneration of cellulose and the subsequent enzymatic hydrolysis are carried out in a one-pot procedure.17 The presence of 1,3-dimethylimidazolium diethylphosphate was reported to inactivate the cellulases, with very little enzymatic activity in IL contents over 40% (v/v). Several studies elucidating cellulase inactivation in ILs have been published since, employing different hydrolysis conditions, enzyme cocktails, ILs and substrates.8,18–27
Various factors affecting cellulase inactivation in ionic liquids have been proposed. Basic anions such as Cl−, Br−, NO3− and CF3SO3− in ILs seem to be strongly inactivating as they interfere with the hydrogen bond network keeping the enzyme together.28 Fluorinated anions, such as the BF4− and PF6−, have, on the other hand, been shown to be more compatible with enzymes in some cases. The “enzyme-friendliness” of ILs has been defined in terms of their chao- and chosmotropicity, as well as by using the Hofmeister series for predicting the effects of anions on enzymatic stability.29 The high viscosity of IL solutions also play a significant role in slowing down enzymatic reactions8,28 due to mass transfer constraints. It also remains unclear whether an enzyme is irreversibly denatured or simply inhibited by the presence of ILs.
In this paper we report how the presence of two hydrophilic, cellulose dissolving room-temperature ionic liquids, 1,3-dimethylimidazolium dimethylphosphate [DMIM]DMP and 1-ethyl-3-methylimidazolium acetate [EMIM]AcO affects the partial hydrolysis of microcrystalline cellulose (MCC) by two purified Trichoderma reesei endoglucanases, Cel7B (EGI) and Cel5A (EGII). These two ILs were chosen for this work because they are both hydrophilic, dissolve cellulose, and have been pointed out to be enzyme compatible to a certain degree.24,30 [DMIM]DMP is one of the most studied ILs in enzymatic hydrolysis. It has been found to be a strong candidate for use in biorefinery applications, as it combines good biomass dissolving capability with a certain degree of “enzyme-friendliness”.24 A major advantage is the fact that [DMIM]DMP may be produced on an industrial scale in a one-pot procedure without considerable by-product formation.31 [EMIM]AcO is known as a powerful cellulose solvent, but the enzyme compatibility of this IL has not been clarified conclusively. The enzymatic hydrolysis of MCC was carried out in the presence of 0–90% (v/v) of IL. After hydrolysis, the solid cellulose residue was analysed by gel-permeation chromatography (GPC) for changes in the molecular weight, and the soluble cellooligosaccharides were analysed using capillary electrophoresis (CE) with pre-column derivatization. Based on the results, the two ILs are compared both in terms of their interactions with the substrate and their effect on enzymatic activity.
Experimental
Materials
[DMIM]DMP was prepared as described in the literature.31 [EMIM]AcO (purity > 98%) was purchased from Ionic Liquid Technologies (Heilbronn) and used without further purification. The halide content of the [EMIM]AcO determined by ion chromatography was: chloride < 100 ppm and bromide < 50 ppm. The dry weight of the cellulose substrate (MCC, Serva, research grade, particle size 0.020 mm) was determined as the average mass loss for three parallel samples by keeping the cellulose at 105 °C for 14 h. The cellulase preparations of Trichoderma reesei Cel7B, Cel5A and the core domain of Cel5A were produced, isolated and purified at VTT according to Suurnäkki et al.32 The Cel7B activity was 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 nkat mL−1 (specific activity 3050 nkat mg−1 protein) as determined by the HEC (hydroxyethylcellulose) assay33 where the activity measurement was done in a 1% (w/w) carboxymethylcellulose (CMC) substrate solution in 50 mM sodium citrate buffer (pH 5.0) at 50 °C. The activity measurement time was 10 min and the measurement was terminated by adding a DNS reagent solution to the samples and boiling for 5 min. The endoglucanase activity was determined by measuring the absorption of the boiled samples at 540 nm and comparing the absorption to that of glucose standards treated in the same way as the enzyme samples. The Cel5A activity was 17
700 nkat mL−1 (specific activity 3050 nkat mg−1 protein) as determined by the HEC (hydroxyethylcellulose) assay33 where the activity measurement was done in a 1% (w/w) carboxymethylcellulose (CMC) substrate solution in 50 mM sodium citrate buffer (pH 5.0) at 50 °C. The activity measurement time was 10 min and the measurement was terminated by adding a DNS reagent solution to the samples and boiling for 5 min. The endoglucanase activity was determined by measuring the absorption of the boiled samples at 540 nm and comparing the absorption to that of glucose standards treated in the same way as the enzyme samples. The Cel5A activity was 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 nkat mL−1 (specific activity 2030 nkat mg−1 protein), whereas the Cel5A core domain activity was 13
900 nkat mL−1 (specific activity 2030 nkat mg−1 protein), whereas the Cel5A core domain activity was 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 nkat mL−1 (specific activity 3730 nkat mg−1 protein). The unit katal (kat) is defined by the International Union for Pure and Applied Chemistry (IUPAC) as the number of catalysed reactions per time with the unit of mol s−1.34
800 nkat mL−1 (specific activity 3730 nkat mg−1 protein). The unit katal (kat) is defined by the International Union for Pure and Applied Chemistry (IUPAC) as the number of catalysed reactions per time with the unit of mol s−1.34
Hydrolysis
Hydrolysis mixtures were prepared with 20, 40, 60, 80 and 90% (v/v) IL dosage in sodium citrate buffer (50 mM, pH = 5.0). IL-free hydrolysis mixtures were prepared in a sodium citrate buffer as well as in a sodium phosphate buffer (50 mM, pH = 7.0). 30 mg (dry weight) of microcrystalline cellulose was measured into a test tube, the defined amount of buffer was added and the mixture was stirred to homogeneity. The defined volume of IL was mixed into the mixture, before adding the enzyme preparation corresponding to a total activity of 2000 nkat g−1 cellulose. The total hydrolysis sample volume was 3 mL. The hydrolysis was carried out at 45 °C in closed test tubes in a water bath with continuous magnetic stirring. The hydrolysis time was 2, 24, 48 or 72 h. The hydrolysis was stopped by boiling the sample for 600 s to denature the enzyme. After cooling to room temperature, the reaction tube was centrifuged at 3000 rpm for 10 min and the clear supernatant was separated from the solid cellulose residue. In the 90% (v/v) IL hydrolysis samples the cellulose was partly dissolved and thus needed to be regenerated. This was achieved by adding 3 mL of distilled water after enzyme denaturation by boiling, followed by vigorous mixing before centrifugation. When using regenerated cellulose (RC) as the substrate, the above described procedure was followed with the exception that the substrate was stirred and swollen in buffer overnight before the addition of enzyme. All experiments were carried out in triplicates. Reference samples were treated in the corresponding conditions without the addition of enzyme. Protein containing reference samples were prepared with the inactivated cellulase or the corresponding amount of bovine serum albumin (BSA, model protein). pH-values were followed during hydrolysis with a Knick pH meter 766 Calimatic equipped with a Mettler-Toledo Inlab Semi-Micro electrode (pH range 0–12).Determination of residual enzymatic activity
The enzyme preparations were first incubated in 20, 40, 60, 80, or 90% (v/v) solutions of [DMIM]DMP or [EMIM]AcO in the sodium citrate buffer (50 mM, pH = 5.0) for a specific time at 45 °C. Reference samples were incubated accordingly in pure sodium citrate buffer. 100 μL of the pre-incubated enzyme sample was transferred to the test tubes where the enzyme residual activity measurement was done. The enzyme residual activity was measured as HEC activity as described above. The final IL content was fixed at 9% (v/v) in all cases during the enzyme residual activity measurement and the CMC concentration was kept constant to ensure similar incubation conditions for all samples. The measurement was done in triplicates.Regeneration of microcrystalline cellulose
MCC (4.09 g) was dissolved in [EMIM]AcO (45 mL), by stirring and heating to 80 °C overnight, yielding a 9% (w/v) solution. The cellulose was precipitated by adding de-ionized water (100 mL), stirring and vacuum filtering the cellulose. The cellulose was washed three times by this procedure and finally dried under reduced pressure (13 mbar) overnight. The final product was a brownish brittle solid. The dry weight of the regenerated cellulose was 90.6 w%, determined as the average mass loss for three parallel samples by keeping the cellulose at 105 °C for 14 h.Analyses
In most of the reported hydrolysis studies, the yield of the reducing sugars has been determined by a dinitrosalicylic acid (DNS) photometric assay.35 It has previously been pointed out that ILs may interfere with both photometric assays and HPLC techniques.36 We found the DNS reagent to produce colour together with high contents of ILs (both [DMIM]DMP and [EMIM]AcO). For low concentrations of ILs, the DNS method has been shown to be reliable.21 In our work, CE was found to be a practical technique to separate the derivatized cellooligomers as sugar–borate complexes under alkaline conditions in the presence of ILs. Although the ILs used proved to be very challenging matrices, the CE-method could be optimized allowing the quantification of the cellooligosaccharides in moderate IL concentrations (up to 40% (v/v)).Determination of cellulose dissolution in aqueous ionic liquid solutions
Cellulose dissolution in the studied ILs was evaluated by measuring the light transmission through dispersions/solutions of MCC in aqueous IL solutions. Samples were prepared simulating the employed hydrolysis conditions. A 1% (w/v) MCC solution/dispersion was prepared in an aqueous IL solution and stirred for 1 day at 45 °C in sealed test tubes. The samples were shaken immediately before measuring and analysed at 45 °C with a Turbiscan Lab (Formulaction, France) device. Light transmission was monitored as a measure of the cellulose dissolution. Cellulose dissolution was also followed by visual evaluation and light microscopy. Light microscopy was performed with an Olympus BX61 microscope and digital image recording was performed with the Soft Imaging Systems analySIS® 3.2 software.Capillary electrophoresis
The saccharides in the enzymatic hydrolysates were derivatized with 4-aminobenzonitrile (ABN, samples in aqueous solution or containing [DMIM]DMP) or 4-aminobenzoic acid ethyl ester (ABEE, samples containing [EMIM]AcO), according to a procedure previously described by Dahlman et al.37 Capillary electrophoresis was carried out with a P/ACE MDQ capillary electrophoresis instrument (Beckman Coulter, Fullerton, CA, USA) equipped with a photodiode array (PDA) UV/vis detector. Running conditions were adapted from Sartori et al.,38 but keeping the cartridge temperature at 30 °C and without any addition of organic modifier to the running electrolyte. Standard curves were acquired for glucose, cellobiose and cellotriose against galactose as the internal standard.Gel-permeation chromatography
The solid cellulose residues were washed with distilled water and dissolved and treated as described in the literature.39 The GPC analyses were carried out on a Waters HPLC system using 0.8% (w/v) LiCl/DMAc as eluent with a flow rate of 0.36 mL min−1. The molecular size separation was achieved at 80 °C on two Agilent PL-gel 20 μm Minimix A columns in series preceded by an Agilent PL-gel 20 μm Minimix A guard column. A refractive index (RI) detector was used for monitoring the cellulose elution. The chromatograms were numerically evaluated using Waters Inc. Empower 2 software. The cellulose molecular weight distributions were calculated by comparison with a pullulan standard series with a linear range of 5900 g mol−1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (R2 = 0.996).
000 g mol−1 (R2 = 0.996).
IR spectroscopy of cellulose residues
The cellulose samples were initially air dried and finally dried in a desiccator to remove any remaining free moisture. FTIR spectra of the MCC, RC and solid cellulose hydrolysis residues were measured using a Bio-Rad 6000 spectrometer equipped with a MTEC 300 photoacoustic (PA) detector. The PA cell was kept under an atmosphere of helium during the measurements. The cross-section of the beam inside the cell was ∼1 mm2 and the optical velocity of the interferometer was 0.16 cm s−1. The spectra were collected in the wavenumber region 400–4000 cm−1, with a spectral resolution of 8 cm−1. From the IR spectra the lateral order index (LOI) was calculated as the peak ratio between the absorption at 1437 cm−1 and 899 cm−1 (α1437/α899) as described in the literature.40 The total crystallinity index (TCI) was likewise calculated as the peak ratio between the absorption at 1378 cm−1 and 2900 cm−1 (α1378/α2900) as described in the literature.41Results and discussion
Ionic liquid interactions with microcrystalline cellulose
The interaction of ILs with microcrystalline cellulose (MCC) mixed in IL contents of 20, 40, 60, 80, and 90% (v/v) in buffer was studied. Low contents of ILs did not cause any detectable cellulose dissolution. A high IL content of 90% (v/v) in the mixture lead to visible interactions with the cellulose substrate and the hydrolysis mixture became less opaque, i. e. dissolution occurred. The effect was more pronounced with [EMIM]AcO whereas the changes were barely noticeable for [DMIM]DMP. In the case of [EMIM]AcO, similar interactions were visibly observed already at an IL content of 80% (v/v), suggesting that [EMIM]AcO is a more powerful cellulose solvent than [DMIM]DMP. This is in line with the previously reported comparisons between cellulose dissolution in these two ILs at 50 °C.42 By light microscopy the aqueous cellulose mixtures of [EMIM]AcO were found to contain some cellulose crystals, indicating the dissolution to be partial (Fig. 1). The light transmission measurements showed an increase in the transmission for cellulose suspensions for both ILs already at 80% (v/v) concentration of IL (Fig. 2). The light transmission was stronger for the solutions of [EMIM]AcO than the corresponding solutions of [DMIM]DMP confirming the superior solvation capacity of [EMIM]AcO. The transmission values clearly indicate a change at a concentration of 85–90% (v/v) of [EMIM]AcO, whereas [DMIM]DMP seems to tolerate hardly any water in order to dissolve cellulose efficiently. At a 90% (v/v) [DMIM]DMP solution most of the cellulose was still not dissolved.![Light microscopy pictures of microcrystalline cellulose suspended/dissolved in pure buffer (upper left), or 60 (upper right), 85 (lower left) or 90% (v/v) (lower right) [EMIM]AcO solutions in buffer. The magnification is 100.](/image/article/2012/RA/c2ra01299e/c2ra01299e-f1.gif) | ||
| Fig. 1 Light microscopy pictures of microcrystalline cellulose suspended/dissolved in pure buffer (upper left), or 60 (upper right), 85 (lower left) or 90% (v/v) (lower right) [EMIM]AcO solutions in buffer. The magnification is 100. | ||
![Light transmission (T) for aqueous cellulose suspensions/solutions in [DMIM]DMP and [EMIM]AcO at 45 °C.](/image/article/2012/RA/c2ra01299e/c2ra01299e-f2.gif) | ||
| Fig. 2 Light transmission (T) for aqueous cellulose suspensions/solutions in [DMIM]DMP and [EMIM]AcO at 45 °C. | ||
IR spectroscopy was used to determine the crystallinity of MCC after interactions with the different amounts of ILs in MCC–IL–buffer samples. The lateral order index (LOI), also known as the crystallinity index CrI,43 was calculated as the peak ratio α1437/α899.40 The LOI of commercial MCC was measured to be 1.1. As expected, the LOI of the regenerated cellulose (RC) was significantly lower with a value of 0.41–0.45. Surprisingly, the crystallinity of MCC that had been treated in 90% (v/v) [EMIM]AcO was even lower with values in the range 0.1–0.25. This difference in LOI values might be due to differences in the dissolution and regeneration conditions in preparing RC in pure [EMIM]AcO vs. the treatment of MCC during the hydrolyses. The LOI values of samples that had been treated in 90% (v/v) [DMIM]DMP were in the range 0.7–0.9, confirming that [DMIM]DMP is not capable of dissolving the MCC to a high extent nor in any other way to significantly alter the MCC structure under these conditions. Also the total crystallinity index (TCI) was calculated as the peak ratio α1378/α2900,41 but the TCI values did not correlate with the LOI values or the expected changes in crystallinity. Recently, similar conclusions about the LOI and TCI values for regenerated cellulosic materials were published by Zhao et al.26
Effects of ILs on endoglucanase activity
The hydrolytic activity of cellulases has been reported to be negatively affected by ILs.15,17 IL derived factors such as salt concentration,15 ionic strength8 and chao/chosmotropicity29 have been proposed to be responsible for enzyme inactivation. We studied the effect of the pH, IL type and IL amount on the activity and action of two T. reesei main endoglucanases.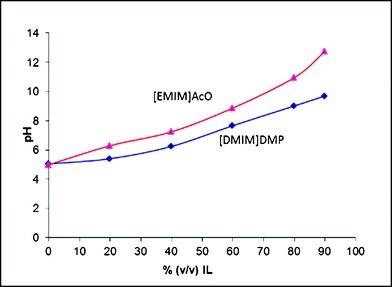 | ||
| Fig. 3 Apparent pH values for ionic liquid in buffer solutions (pH 5.0). | ||
To find out whether the pH drift is one of the major reasons for the previously reported enzyme inactivation,8,15,17 a hydrolysis experiment at pH 7 in buffer was carried out and the saccharide yield and composition was analysed by CE. The hydrolysis yield of soluble oligosaccharides at pH 7 was 50% and 20% for Cel5A and Cel7B, respectively, as compared to the respective yields in buffer at pH 5 (Table 1 and 2). The oligosaccharide distribution was to some extent altered by the change in pH, as measured after 72 h of hydrolysis. The oligosaccharides formed were glucose, cellobiose and cellotriose; no larger oligosaccharides were found with CE. At pH 7 the Cel7B produced traces of cellotetraose and cellopentaose (results not shown), as well as cellotriose, which is not a normal product pattern of Cel7B under optimum conditions45 (Table 1). This would indicate a slowdown of the hydrolysis, yielding intermediary products, due to the deviation from the optimum pH. The fairly well retained hydrolytic activity of Cel5A at pH 7 (Table 2) is expected as this enzyme is known to be active (on β-glucan) over a wide pH window from at least pH 3 to pH 9.45 Cel7B was clearly more sensitive to the deviations from its optimum pH.
| Cel7B | Formed soluble oligosaccharides/yield | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 72 h | |||||||
| Sample | Glc (mg L−1) | CB (mg L−1) | CTr (mg L−1) | Yield (%) | Glc (mg L−1) | CB (mg L−1) | CTr (mg L−1) | Yield (%) |
| MCC, buffer (pH 5) | 29.7 | 112.3 | < LOD | 1.3 | 160.5 | 429.5 | 11.7 | 5.6 |
| MCC, buffer (pH 7) | 7.9 | 41.7 | 13.1 | 0.6 | 14.9 | 79.0 | 24.2 | 1.1 |
| MCC, 20% IL1 | 2.2 | 20.1 | < LOD | 0.2 | 19.0 | 90.8 | 3.6 | 1.0 |
| MCC, 40% IL1 | < LOD | < LOD | < LOD | 0.0 | < LOD | 6.5 | < LOD | < 0.1 |
| MCC, 20% IL2 | < LOD | 4.6 | 5.5 | < 0.1 | Traces | 4.4 | 6.5 | < 0.1 |
| MCC, 40% IL2 | < LOD | < LOD | < LOD | 0.0 | < LOD | < LOD | < LOD | 0.0 |
| RC, buffer (pH 5) | ND | ND | ND | ND | 425.8 | 799.8 | < LOD | 11.4 |
| Cel5A | Formed soluble oligosaccharides/yield | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 72 h | |||||||
| Sample | Glc (mg L−1) | CB (mg L−1) | CTr (mg L−1) | Yield (%) | Glc (mg L−1) | CB (mg L−1) | CTr (mg L−1) | Yield (%) |
| Buffer (pH 5) | 25.8 | 86.6 | 87.1 | 1.9 | 256.2 | 458.9 | 219.4 | 8.8 |
| Buffer (pH 7) | 10.3 | 47.7 | 52.9 | 1.1 | 41.7 | 192.4 | 199.9 | 4.2 |
| 20% IL1 | 2.0 | 17.2 | < LOD | 0.3 | 25.4 | 94.1 | 89.4 | 2.0 |
| 40% IL1 | Traces | Traces | < LOD | 0.0 | < LOD | 3.3 | 4.8 | 0.1 |
| 90% IL1 | < LOD | < LOD | < LOD | 0.0 | < LOD | < LOD | < LOD | 0.0 |
| 20% IL2 | < LOD | 5.9 | 9.4 | 0.1 | Traces | 19.5 | 21.9 | 0.4 |
| 40% IL2 | < LOD | < LOD | < LOD | 0.0 | < LOD | < LOD | < LOD | 0.0 |
| Cel5A Core, buffer pH 5 | ND | ND | ND | ND | 13.5 | 45.6 | 40.5 | 0.9 |
| Cel5A Core, 20% IL1 | ND | ND | ND | ND | 8.3 | 48.1 | 47.9 | 1.0 |
| Cel5A Core, 90% IL1 | ND | ND | ND | ND | < LOD | < LOD | < LOD | 0.0 |
| RC, buffer (pH 5) | ND | ND | ND | ND | 572.2 | 865.6 | 392.3 | 17.1 |
According to the pH curves displayed in Fig. 3, a pH-value of 7.0 corresponds to roughly 60% (v/v) [DMIM]DMP or 40% (v/v) [EMIM]AcO in sodium citrate buffer. For neither Cel7B nor Cel5A no soluble oligosaccharides could be detected after 72 h of hydrolysis in the hydrolysis mixtures containing these amounts of [DMIM]DMP (results not shown) or [EMIM]AcO (Table 1 and 2). This clearly indicates, in the case of Cel5A, that the basic pH drift caused by the ILs is not the only reason for the enzyme's inactivation. Enzymatic activity decreases much faster with increasing IL content than the increase of pH gives reason to. This is also in line with the results of Engels et al.,8 where the pH was adjusted in the IL solutions, yet enzyme inactivation was observed in the presence of ILs. In the case of Cel7B, a major decrease is recorded both at pH 7 as well as when ILs are present allowing no conclusion to be made as to whether the reason for the drop is the changed pH or other effects caused by the presence of ILs.
Enzyme inactivation for Cel5A was studied with prolonged incubation times of 2 h and 72 h in 90% (v/v) [DMIM]DMP in hydrolysis conditions. Interestingly, the measured residual endoglucanase activity was at approximately the same level as that measured after 15 min incubation. This would indicate a very fast decrease in enzymatic activity when the enzyme is mixed with the IL, with an equilibrium settling after which the enzyme activity stays rather stable. It was further noticed that 10 min boiling inactivated the enzyme in 90% (v/v) [DMIM]DMP solution hardly at all. These results were verified to be due to the enzymatic activity by varying the amount of enzyme in the measurements, as well as the incubation time in the residual activity measurement.
To explore the function of the CBM in the presence of ILs, hydrolysis of MCC was carried out in a buffer at pH 5 (optimum conditions) and in 20 and 90% (v/v) of [DMIM]DMP with both intact Cel5A containing CBM and Cel5A without CBM (Cel5A Core) (Table 2). The cellulose hydrolysis yield at optimum conditions with the Cel5A Core was considerably lower than that with the intact Cel5A, resulting in about 10% of the yield of the intact enzyme. Interestingly, the hydrolysis yield of the Cel5A Core was the same in 20% (v/v) of [DMIM]DMP as in the optimum conditions. The product distribution was, however, somewhat different with lower amounts of glucose and higher amounts of cellobiose and cellotriose after the IL containing hydrolysis as compared to that after the hydrolysis in pure buffer. The hydrolysis activity of the intact enzyme decreased, however, drastically in the presence of 20% (v/v) of [DMIM]DMP as compared with its activity in the buffer. According to these results, the action of CBM of Cel5A seems to be highly affected by the presence of [DMIM]DMP. The effect of this IL on the structure of the CBM and its substrate recognition ability, both potential factors affecting the CBM action on the cellulose, needs to be elucidated in further studies. In the high [DMIM]DMP concentration of 90% (v/v) no oligosaccharides could be detected in the hydrolysate for neither Cel5A nor Cel5A Core.
Enzymatic hydrolysis of MCC in the presence of ILs
Both [DMIM]DMP and [EMIM]AcO are very inactivating for the studied endoglucanases, [EMIM]AcO even more so than [DMIM]DMP. More cellobiose and cellotriose and less glucose was produced in the presence of both [DMIM]DMP and [EMIM]AcO as compared to the product distribution in the citrate buffer reference at pH 5 (Table 1 and 2). This suggests an overall slowing down of the hydrolysis. The total yield of solubilized saccharides was significantly decreased as the IL concentration increased. With only 20% (v/v) of [DMIM]DMP present in the hydrolysis mixture, the overall yield decreased to approximately 20% of the reference in 72 h of hydrolysis. 40% (v/v) of this IL only allowed very low hydrolysis rates for both the enzymes. In 20% (v/v) [EMIM]AcO extremely low concentrations of oligosaccharides were detected as compared to the amounts found in 20% (v/v) [DMIM]DMP. In 40% (v/v) of [EMIM]AcO neither of the studied enzymes were able to catalyse the formation of any oligosaccharides. Cel5A, being more active for MCC in the reference buffer system, also seemed to be slightly more tolerant towards the ILs, and especially against [EMIM]AcO, than Cel7B. In hydrolysis samples containing an IL in the range 60–90% (v/v) of IL no soluble oligosaccharides could be detected after enzymatic treatment.
The kinetics of oligosaccharide formation in MCC hydrolysis was different for the two enzymes when [EMIM]AcO was present. Cel7B produced a low amount of cellobiose and cellotriose during the first two hours and was then totally inactivated with no further saccharide formation (Table 1). Cel5A was still able to increase the amount of solubilized saccharides in these circumstances after a two hour hydrolysis (Table 2). In [DMIM]DMP this same difference between the enzymes was not observed, but both enzymes were able to produce saccharides during the course of the 72 h MCC hydrolysis also after the 2 h time point. It could be concluded that the inactivating effect of the ILs was different for the two T. reesei endoglucanases studied and that the inactivation is dependent on both the enzyme and the IL it is exposed to.
Molecular weight of the solid residue after enzymatic hydrolysis
The insoluble cellulose residues, separated from the hydrolysate by centrifugation after enzymatic hydrolysis, were subjected to GPC analysis. When the hydrolysis was carried out in a buffer solution at pH 5 or 7 with either Cel7B, Cel5A or Cel5A Core, no changes in the molecular weight distributions were observed even after 72 h of treatment (Table 3). Endoglucanases are known to hydrolyse primarily the amorphous regions of cellulose.14 A decrease in molecular weight was therefore expected in the aqueous conditions as yields of up to 8% of soluble oligosaccharides were observed (Tables 1 and 2). This conflict between the fairly high yield of saccharides and no decrease in the molecular weight may best be explained with a mechanism where the cellulases are able to hydrolyse only the outermost layer of the cellulose crystals, whereas the internal parts of the crystals remain intact. The same mechanism has previously been suggested based on observations that the crystallinity of MCC was not increased in partial hydrolysis.47| Sample | Time (h) | Mn (g mol−1) | Mw (g mol−1) | StDev(Mw) | Polydispersity |
|---|---|---|---|---|---|
| MCC | |||||
| Reference (MCC, untreated) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5500 | 5.16 | |
| Cel7B, buffer pH 5 | 72 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1600 | 4.02 |
| Cel5A, buffer pH5 | 72 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5000 | 4.33 |
| Cel5A Core, buffer pH5 | 72 | 9000 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3000 | 5.29 |
| Cel7B, buffer pH 7 | 72 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
400 | 4.36 |
| Cel5A, buffer pH 7 | 72 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4300 | 4.89 |
| Reference, buffer | 72 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3800 | 4.62 |
| Cel7B, 90% [DMIM]DMP | 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4900 | 4.50 |
| Cel7B, 90% [DMIM]DMP | 24 | 7000 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
400 | 4.84 |
| Cel7B, 90% [DMIM]DMP | 72 | 8000 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3200 | 4.66 |
| Cel5A, 90% [DMIM]DMP | 2 | 9000 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1000 | 4.57 |
| Cel5A, 90% [DMIM]DMP | 24 | 7000 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6000 | 4.95 |
| Cel5A, 90% [DMIM]DMP | 72 | 9000 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1600 | 4.10 |
| Reference, 90% [DMIM]DMP | 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4400 | 4.68 |
| Reference, 90% [DMIM]DMP | 24 | 8000 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3600 | 5.68 |
| Reference, 90% [DMIM]DMP | 72 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3500 | 4.56 |
| Cel5A Core 90% [DMIM]DMP | 2 | 9000 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
600 | 4.57 |
| Cel5A Core 90% [DMIM]DMP | 72 | 9000 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1800 | 4.33 |
| Cel7B double enzyme dosage, 90% [DMIM]DMP | 72 | 8000 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3700 | 4.75 |
| Cel5A double enzyme dosage, 90% [DMIM]DMP | 72 | 8000 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2100 | 4.50 |
| RC | |||||
| RC (untreated) | 9000 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4200 | 5.15 | |
| RC, Cel7B, buffer pH 5 | 72 | 7000 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
800 | 4.78 |
| RC, Cel5A, buffer pH 5 | 72 | 5000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
500 | 2.45 |
| RC reference, buffer | 72 | 9000 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
600 | 4.91 |
A decrease of 20–30% in the weight average molecular weight (Mw), as well as decreases in the number average molecular weight (Mn) for samples treated enzymatically in 90% (v/v) [DMIM]DMP, could be observed (Table 3). The changes in the molecular weight distribution can clearly be seen when comparing the distributions of the samples (Fig. 4). The decrease in Mw was observed for both Cel7B and Cel5A. Cel5A Core was also able to reduce the Mw, even if not to the same extent as the two intact endoglucanases. This effect was noticed to increase with increasing hydrolysis time. The Mw was not affected either by the presence of BSA or by addition of endoglucanase, which had been inactivated by boiling. It is possible that the 90% (v/v) [DMIM]DMP solution has sufficient dissolving power to alter the MCC structure during hydrolysis to a more accessible substrate for the enzyme, the conditions in this solution at the same time being such that the enzyme still retains some of its cellulose chain scission activity. The decrease in the molecular weight of cellulose was not observed in any other conditions than in the 90% (v/v) [DMIM]DMP solutions. It was shown by the residual activity measurements in 90% (v/v) [DMIM]DMP, that both Cel7B and Cel5A retain about 50% of their activity at least for 15 min in this media.
![Molecular weight distributions for MCC samples after 72 h enzymatic treatments in 90% (v/v) [DMIM]DMP.](/image/article/2012/RA/c2ra01299e/c2ra01299e-f4.gif) | ||
| Fig. 4 Molecular weight distributions for MCC samples after 72 h enzymatic treatments in 90% (v/v) [DMIM]DMP. | ||
Interestingly, the decrease in Mw for MCC noticed in 90% (v/v) [DMIM]DMP was not accompanied by any detectable formation of soluble cellooligomers. This may imply that the enzymes' activity is altered by the conditions applied or that the enzymes are able to carry out random chain scission on the substrate which is in a new, easier accessible form due to the interactions with the IL. Hydrolysis experiments were also carried out with a doubled enzyme dosage (Table 3). Increasing the enzyme dosage did not lead to an increased rate of cellulose chain scission. According to this result, the limiting factor is not the IL induced enzyme inactivation, but must rather be substrate dependent. The possibility that the cellulose could be enzymatically hydrolysed during its regeneration after the hydrolysis cannot be ruled out, especially as we have shown that 10 min boiling does not completely inactivate the enzymes. Further studies are needed to fully clarify the mode of action of T. reesei endoglucanases in the MCC in the presence of high concentrations of [DMIM]DMP.
In [EMIM]AcO, no decrease in Mw was noticed for any concentration of this IL in the hydrolysis mixture (results not shown). Although [EMIM]AcO is a much more powerful cellulose solvent than [DMIM]DMP, it is probable that this IL totally inactivates the studied endoglucanases, in contrast to [DMIM]DMP.
The RC was very efficiently hydrolysed by both Cel7B and Cel5A under optimum conditions at pH 5, both in terms of the produced cellooligomers (Table 1 and 2) and in terms of the decreased Mw (Table 3). The difference between the two endoglucanases was large: Cel7B was able to cause a decrease in Mw of 27% against the reference, whereas Cel5A caused a decrease in Mw of 73%, which is also clearly seen by comparing the molecular weight distributions (Fig. 5). This change in Mw was accompanied by a roughly doubled production of soluble oligosaccharides in the hydrolysate (Table 1 and 2).
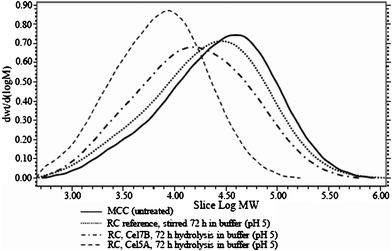 | ||
| Fig. 5 Molecular weight distributions for cellulose regenerated in ionic liquid (RC) samples after 72 h enzymatic treatment in buffer at pH 5. | ||
Conclusions
The literature has little data regarding the effect of ILs on purified monocomponent cellulases. This paper provides results on how the activity of two endoglucanases from the Trichoderma reesei on microcrystalline cellulose is affected by the presence of ILs. Furthermore, the effect of the presence of IL in hydrolysis on the resulting cellooligomer product distribution and the molecular weight of the insoluble cellulose residue is elucidated. Both the employed ILs, [DMIM]DMP and [EMIM]AcO, were found to be severely inactivating for the enzymes used. [EMIM]AcO, being a more powerful cellulose solvent, was also more enzyme inactivating than [DMIM]DMP. The studied endoglucanases, T. reesei Cel7B and Cel5A, displayed some differences in IL tolerance. The product distribution of cellulose hydrolysis was similar under both optimum conditions and in the presence of ILs, though shifting the hydrolysis conditions away from the optimum usually caused the amount of cellobiose and cellotriose to grow at the expense of the glucose concentration. Based on the comparison of the effect of [DMIM]DMP on the cellulose hydrolysis yield by the native Cel5A and Cel5A Core it seems that the action of the cellulose binding module, CBM, present in the native T. reesei Cel5A is highly affected by the presence of this IL.Soluble oligosaccharides were not produced in the enzymatic hydrolysis at IL concentrations of more than 40% (v/v). Surprisingly, both the studied endoglucanases appeared, however, to reduce the molecular weight of the solid cellulose residue in hydrolysis mixtures containing 90% (v/v) [DMIM]DMP. Similar phenomena were not observed in systems containing [EMIM]AcO nor in any other attempted conditions including the optimum conditions in buffer at pH 5. Further studies are needed to fully clarify the mode of action of T. reesei endoglucanases in the MCC in the presence of high concentrations of [DMIM]DMP and during the regeneration of the cellulose sample. IL pretreated, regenerated cellulose was shown to yield by far the best hydrolysis results both in terms of solubilized oligosaccharides and the decreased molecular weight of the cellulose residue.
Both [DMIM]DMP and [EMIM]AcO were found to cause a strong basic drift to the hydrolysis mixtures' pH values, but it could be established that the pH drift was not the main cause for the low observed enzymatic activities in the presence of these ILs. The IL containing matrices were also found to be challenging for the analytics. In particular, the DNS assay and HPLC techniques were disturbed when attempting to analyse samples with high IL concentrations. The cellooligomers could be quantified in the presence of ILs employing CE techniques with pre-column derivatization.
There is a great need to further develop IL compatible analytical methods, if accurate research results are to be obtained in this research field. There is also a clear need for new, more enzyme-friendly ILs, which combine properties such as low price, recyclability, non-toxicity and of course the ability to dissolve biomass. Stabilizing the cellulase enzymes in these systems as well as finding cost effective methods to separate the hydrolysis products from the IL containing hydrolysates are future challenges.
Acknowledgements
The authors wish to thank Finnish Forestcluster Ltd for their financial support through the Future Biorefinery (FuBio) programme. Financial support from the VTT Graduate School is acknowledged (Ronny Wahlström). Prof. Antje Potthast, Dr Anna Bogolitsyna and Dr Ute Henniges at the University of Natural Resources and Life Sciences (BOKU) in Vienna, Austria, are gratefully acknowledged for fruitful analytical consultations. COST action FP0901 “Analytical Techniques for Biorefineries” and the Academy of the Finland Graduate School for Biorefining (BIOREGS) are thanked for their mobility support. Dr Alistair King, Dr Lasse Kyllönen and Prof. Ilkka Kilpeläinen at the University of Helsinki are thanked for their cooperation concerning ionic liquid issues, as well as Dr Riitta Partanen at VTT who is thanked for her help with the light transmission measurements. Mia Löija is thanked for her help with the IR experiments and Dr Tiina Liitiä and Eila Turunen are thanked for their help with the GPC measurements. Prof. Kristiina Kruus, Lic. Hannu Mikkonen and Dr Jarmo Ropponen and Matti Siika-aho at VTT, as well as Prof. Reija Jokela at Aalto University are greatly acknowledged for their support and fruitful discussions during the course of this work.References
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728, DOI:10.1021/cr9001947.
- A. P. Dadi, S. Varanasi and C. A. Schall, Biotechnol. Bioeng., 2006, 95, 904–910 CAS.
- T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass: Volume 1—Results of Screening for Potential Candidates from Sugars and Synthesis Gas, 2004 Search PubMed , DOE/GO-102004-1992.
- O. Akpinar, R. J. McGorrin and M. H. Penner, J. Agric. Food Chem., 2004, 52, 4144–4148, DOI:10.1021/jf035305m.
- H. Kamitakahara, F. Nakatsubo and D. Klemm, Cellulose, 2006, 13, 375–392, DOI:10.1007/s10570-005-9003-6.
- R. P. Chandra, R. Bura, W. E. Mabee, A. Berlin, X. Pan and J. N. Saddler, Adv. Biochem. Eng. Biotechnol., 2007, 108, 67–93 CAS.
- C. E. Wyman, B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch and Y. Y. Lee, Bioresour. Technol., 2005, 96, 1959–1966 CAS.
- P. Engel, R. Mladenov, H. Wulfhorst, G. Jager and A. C. Spiess, Green Chem., 2010, 12, 1959–1966 CAS.
- U. Kragl, M. Eckstein and N. Kaftzik, Curr. Opin. Biotechnol., 2002, 13, 565–571, DOI:10.1016/S0958-1669(02)00353-1.
- R. A. Sheldon, R. M. Lau, M. J. Sorgedrager, F. van Rantwijk and K. R. Seddon, Green Chem., 2002, 4, 147–151, 10.1039/b110008b.
- C. Graenacher, Cellulose solution, US 1943176, 1934 Search PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975, DOI:10.1021/ja025790m.
- I. Kilpeläinen, H. Xie, A. King, M. Granström, S. Heikkinen and D. S. Argyropoulos, J. Agric. Food Chem., 2007, 55, 9142–9148, DOI:10.1021/jf071692e.
- M. K. Bhat and S. Bhat, Biotechnol. Adv., 1997, 15, 583–620, DOI:10.1016/S0734-9750(97)00006-2.
- M. B. Turner, S. K. Spear, J. G. Huddleston, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5, 443, 10.1039/b302570e.
- A. P. Dadi, C. A. Schall and S. Varanasi, Appl. Biochem. Biotechnol., 2007, 137–140, 407–421 CAS.
- N. Kamiya, Y. Matsushita, M. Hanaki, K. Nakashima, M. Narita, M. Goto and H. Takahashi, Biotechnol. Lett., 2008, 30, 1037–1040, DOI:10.1007/s10529-008-9638-0.
- R. Bodirlau, C. Teaca and I. Spiridon, Int. J. Polym. Anal. Charact., 2010, 15, 460–469 CAS.
- R. Bodirlau, C. Teaca and I. Spiridon, Monatsh. Chem., 2010, 141, 1043–1048 CAS.
- M. Paljevac, M. Habulin and Z. Knez, Chem. Ind. Chem. Eng. Q., 2006, 12, 181–186 CAS.
- Y. Wang, M. Radosevich, D. Hayes and N. Labbé, Biotechnol. Bioeng., 2011, 108, 1042–1048, DOI:10.1002/bit.23045.
- R. Bharadwaj, A. Wong, B. Knierim, S. Singh, B. M. Holmes, M. Auer, B. A. Simmons, P. D. Adams and A. K. Singh, Bioresour. Technol., 2011, 102, 1329–1337, DOI:10.1016/j.biortech.2010.08.108.
- S. Datta, B. Holmes, J. I. Park, Z. Chen, D. C. Dibble, M. Hadi, H. W. Blanch, B. A. Simmons and R. Sapra, Green Chem., 2010, 12, 338–345 CAS.
- Q. Li, X. Jiang, Y. He, L. Li, M. Xian and J. Yang, Appl. Microbiol. Biotechnol., 2010, 87, 117–126, DOI:10.1007/s00253-010-2484-8.
- F. Yang, L. Li, Q. Li, W. Tan, W. Liu and M. Xian, Carbohydr. Polym., 2010, 81, 311–316, DOI:10.1016/j.carbpol.2010.02.031.
- H. Zhao, C. L. Jones, G. A. Baker, S. Xia, O. Olubajo and V. N. Person, J. Biotechnol., 2009, 139, 47–54, DOI:10.1016/j.jbiotec.2008.08.009.
- M. F. Thomas, L. Li, J. M. Handley-Pendleton, D. van der Lelie, J. J. Dunn and J. F. Wishart, Bioresour. Technol., 2011, 102, 11200–11203, DOI:10.1016/j.biortech.2011.09.069.
- S. Bose, D. W. Armstrong and J. W. Petrich, J. Phys. Chem. B, 2010, 114, 8221–8227, DOI:10.1021/jp9120518.
- H. Zhao, O. Olubajo, Z. Song, A. L. Sims, T. E. Person, R. A. Lawal and L. A. Holley, Bioorg. Chem., 2006, 34, 15–25, DOI:10.1016/j.bioorg.2005.10.004.
- H. Zhao, L. Jackson, Z. Song and O. Olubajo, Tetrahedron: Asymmetry, 2006, 17, 2491–2498, DOI:10.1016/j.tetasy.2006.09.009.
- C. J. Bradaric, A. Downard, C. Kennedy, A. J. Robertson and Y. Zhou, Green Chem., 2002, 5, 143–152, 10.1039/b209734f.
- A. Suurnäkki, M. Tenkanen, M. Siika-aho, M. -L. Niku-Paavola, L. Viikari and J. Buchert, Cellulose, 2000, 7, 189–209 Search PubMed.
- T. K. Ghose, Pure Appl. Chem., 1987, 59, 257–268 CAS.
- R. Dykbaer, Pure Appl. Chem., 2001, 73, 927–931 Search PubMed.
- G. L. Miller, Anal. Chem., 1959, 31, 426–428, DOI:10.1021/ac60147a030.
- S. Klembt, S. Dreyer, M. Eckstein and U. Kragl, in Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH Verlag GmbH & Co, Weinheim, 2nd edn, 2008, vol. 1, ch. 8, pp. 641–644 Search PubMed.
- O. Dahlman, A. Jacobs, A. Liljenberg and A. I. Olsson, J. Chromatogr., A, 2000, 891, 157–174, DOI:10.1016/S0021-9673(00)00619-1.
- J. Sartori, A. Potthast, A. Ecker, H. Sixta, T. Rosenau and P. Kosma, Carbohydr. Res., 2003, 338, 1209–1216, DOI:10.1016/S0008-6215(03)00115-0.
- U. Westermark and K. Gustafsson, Holzforschung, 1994, 48, 146–150, DOI:10.1515/hfsg.1994.48.s1.146.
- F. G. Hurtubise and H. Krässig, Anal. Chem., 1960, 32, 177–181, DOI:10.1021/ac60158a010.
- M. L. Nelson and R. T. O'Connor, J. Appl. Polym. Sci., 1964, 8, 1325–1341, DOI:10.1002/app.1964.070080323.
- M. Zavrel, D. Bross, M. Funke, J. Büchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587, DOI:10.1016/j.biortech.2008.11.052.
- R. T. O'Connor, E. F. DuPré and D. Mitcham, Text. Res. J., 1958, 28, 382–392 CAS.
- D. Becker, C. Braet, H. Brumer, M. Claeyssens, C. Divne, B. R. Fagerström, M. Harris, T. A. Jones, G. J. Kleywegt, A. Koivula, S. Mahdi, K. Piens, M. L. Sinnott, J. Ståhlberg, T. T. Teeri, M. Underwood and G. Wohlfahrt, Biochem. J., 2001, 356, 19–30 CAS.
- J. Karlsson, M. Siika-aho, M. Tenkanen and F. Tjerneld, J. Biotechnol., 2002, 99, 63–78, DOI:10.1016/S0168-1656(02)00156-6.
- J. Pottkämper, P. Barthen, N. Ilmberger, U. Schwaneberg, A. Schenk, M. Schulte, N. Ignatiev and W. R. Streit, Green Chem., 2009, 11, 957–965, 10.1039/b820157a.
- P. A. Penttilä, A. Varnai, K. Leppänen, M. Peura, A. Kallonen, P. Jääskeläinen, J. Lucenius, J. Ruokolainen, M. Siika-aho, L. Viikari and R. Serimaa, Biomacromolecules, 2010, 11, 1111–1117, DOI:10.1021/bm1001119.
| This journal is © The Royal Society of Chemistry 2012 |
