Fabrication, magnetic properties and self-assembly of hierarchical crystalline hexapod magnetites†
Xuzhen
Wang
a,
Jieshan
Qiu
*a,
Jiangying
Qu
a,
Zhiyu
Wang
a and
Dangsheng
Su
b
aCarbon Research Laboratory, State Key Laboratory of Fine Chemicals, and Liaoning Key Laboratory for Energy Materials & Chemical Engineering, Faculty of Chemical, Environmental & Biological Science and Technology, Dalian University of Technology, Dalian, 116024, Liaoning, China. E-mail: jqiu@dlut.edu.cn; Fax: + 86-411-84986080
bShenyang National Laboratory of Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang, 110016, Liaoning, China
First published on 28th March 2012
Abstract
We report a facile and surfactant-free solvothermal approach for the shape controlled synthesis of magnetite (Fe3O4). Hierarchical branched hexapod magnetites with various secondary structures can be prepared from ferrocene by using different solvents and tailoring experimental parameters. The as-prepared hexapods were characterized by X-ray diffraction, room temperature 57Fe Mössbauer spectroscopy, and scanning and transmission electron microscopy. Compared with bulk magnetite, the obtained Fe3O4 hexapods are single crystals and exhibit different ferrimagnetic behavior with relatively high specific saturation magnetization (90–128 emu g−1) and large coercivity (223–238 Oe) values depending on their size and morphology. Self-organization of the shape-anisotropic hexapod crystals has been achieved for the first time by evaporation-mediated assembly. The well-defined hexapod Fe3O4 crystals will be attractive as promising building blocks for the bottom-up design of advanced materials and devices which may have applications in many fields.
Introduction
Controllable syntheses of nano- and micrometre scale semiconductors, metals and metal oxides have gained tremendous attention in recent years due to their size-, shape- and material-dependent properties, as well as their various potential applications.1–5 Apart from typical quantum dots, spheres and one-dimensional (1D) nanorods/wires/tubes, hierarchical structures with three-dimensional (3D) complex morphologies, such as branches,4 tetrapods,3,6 star-shapes,6,7 hexapods8 and dendrites9etc., have also been addressed and achieved through solution or gas phase-based approaches.1–4,10 Among them, research into the design of novel shaped and nano-/micro-size magnetic iron oxides (magnetite, maghemite) has been greatly intensified because of their unique nanomagnetism and important technological applications in catalysts, magnetic information storage, biological assays, chemical sensors and superparamagnets.11,12In addition to the octahedra and truncated octahedra resulting from inherent crystal habit,13,14 the final shape of magnetite nanocrystals can be effectively tailored through the delicate control of external factors, including the nucleation, growth mode and attachment mode.3–5,15 Accordingly, Hyeon's group16 prepared magnetite nanocubes ranging in size from 20 to 160 nm through the thermal reaction of Fe(acac)3. Chen et al.17 synthesized relatively uniform 50 nm-sized magnetite nanocubes from a solvothermal reaction of ferrocene with H2O2. By means of soft-template-assisted methods or magnetic-field-induced growth, anisotropic 1D magnetite nanorods,18a and nanowires18b have also been fabricated. Besides, single-crystalline Fe3O4 nanorings18c were delicately obtained from the reduction of hematite (α-Fe2O3) nanorings. As for the preparation of micrometre-scale magnetite with complex 3D structure, dodecahedral Fe3O4 microcrystals possessing active basal facets,19a microsized Fe3O4 particles with peanut-like morphology,19b Fe3O4 sub-micrometre spheres comprising of single-crystalline Fe3O4 nanorods,19c 3D dendritic magnetite crystals,20a and Fe3O4 novel fractal crystals20b have been successively obtained.
In most of the above cases, a surfactant-assisted process is usually employed to give the shape-controlled product. Both a classic Ostwald ripening mechanism and an oriented attachment (OA) growth mechanism have been proposed to be involved in the growth process. Compared with the classical crystal growth mechanism of nanosized particles, the formation of complicated morphologies has been attributed to the highly oriented self-assembly and subsequently oriented attachment growth of small Fe3O4 nanoparticles.15 However, detailed reports of the formation of iron oxides with hierarchical structures in surfactant-free systems are rare, and facile synthetic approaches for building 3D hierarchical architectures are still in strong demand.
Herein, we introduce a flexible strategy for the synthesis of magnetite micrometre crystals through a one-pot solvothermal approach without using a template or capping agent with ferrocene as the iron source. Depending on the organic solvent adopted, both hierarchical star-like hexapod Fe3O4 with branched stems and pyramidal stems can be selectively fabricated in the temperature range of 350–450 °C. The hexapod magnetite exhibits strongly size- and shape-dependent magnetic properties, and self-organization of the shape-anisotropic microcrystals in the mode of hand-in-hand has been achieved for the first time by evaporation-mediated assembly.
Experimental
Synthesis
In a typical procedure, 30 mg of ferrocene [Fe(C5H5)2] was dissolved in 2 mL isopropanol (abbreviated as IPA). After sonication for 10 min, the solution was transferred into a 10 mL stainless-steel autoclave and sealed in air. The autoclave was put into a muffle furnace preheated to 350 °C and a time hold maintained from 30 min to 24 h. Afterward, the autoclave was taken out of the furnace, and cooled down naturally to room temperature. Black precipitates were obtained and collected by the assistance of a magnet, and washed three times with absolute ethanol. Finally, the black products were dried at 40 °C in a vacuum oven. For comparison, methanol (abbreviated as Meth), acetone (abbreviated as Ace), benzene and cyclohexane have also been employed and investigated as solvents.Characterization
Powder X-ray diffraction (XRD, D/MAX 2400), scanning electron microscopy (SEM, JSM-5600LV), field-emission scanning electron microscopy (FESEM, JSM 6700F) and transmission electron microscopy (TEM, Philips Tecnai G2 20) were applied to characterize the size, shape, composition, and structure of the samples. Magnetic measurements were carried out at room temperature using 57Fe Mössbauer spectroscopy (Topologic 500A) and a vibrating-sample magnetometer (VSM, JDM-13).Results and discussion
Phases and structures
Fig. 1(a) shows XRD patterns of the typical products with three-dimensional (3D) structure synthesized in different solvents (including isopropanol, methanol and acetone). It can clearly be seen that all of the peaks in every profile are consistent with Bragg reflections of the standard face-centered cubic (fcc) phase of Fe3O4 with cell constants a = 8.391 Å (space group: Fd3m(227), JCPDS No. 85-1436). Further confirmed by its 57Fe Mössbauer spectrum (Fig. 1(b)), the product contains only inverse spinel magnetite and no maghemite.13 Moreover, from the XRD patterns of the samples obtained at different reaction intervals in isopropanol (Fig. S1, ESI†), the fact that diffraction peaks are stronger and sharper on prolonging the reaction time indicates an increase of crystallization and/or the size of the Fe3O4 crystals.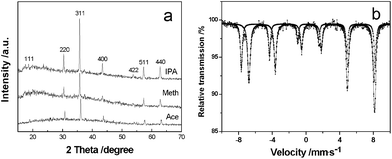 | ||
| Fig. 1 (a) XRD patterns of magnetite prepared in different solvents. (b) Room temperature 57Fe Mössbauer spectrum of typical crystals produced in methanol. | ||
Representative FESEM images of 3D Fe3O4 microcrystals with the shape of hierarchical hexapods synthesized in isopropanol (labeled as h-hexapods) are shown in Fig. 2(a, b). It is clear that every microcrystal has six symmetric arms in three-dimensions which are perpendicular to each other with the scale of 2–5 μm. Observations of a magnified individual hexapod (Fig. 2b) find well-defined secondary growth perpendicular to the trunk pod along four symmetric directions; moreover, these secondary branches are in a line on the trunk pod, which results in the formation of branched radial structures on the trunk pods, and it reveals that the hierarchical structures are cross-shaped in an image plane orthogonal to the primary trunk and comb-like in an image plane parallel to the primary trunk. The length of the trunk pod is about 1.5–2.5 μm, and its diameter decreases gradually from the bone-core to the tip along the pod. Similarly, the diameter and length of the secondary pods vary with their growth position on the trunk and also become shorter and thinner from bottom to tip, which reflect the growth process of hierarchical magnetite.
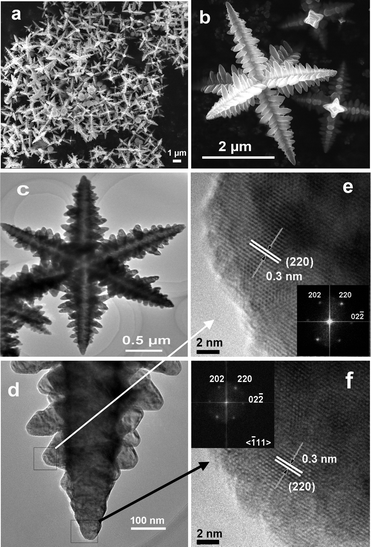 | ||
| Fig. 2 (a) A general view of representative FESEM images of Fe3O4 hierarchical hexapods prepared in isopropanol at 350 °C for 12 h. (b) Higher magnification image of an individual h-hexapod in (a). (c) TEM image of Fe3O4h-hexapods prepared at 350 °C for 6 h in isopropanol. (d) Magnified TEM image of one arm from (c). (e, f) HRTEM images of the sub-branch (e) and the tip (f) of one arm from (d). The insets in (e) and (f) are their corresponding FFT patterns. | ||
To determine the crystalline structure of the hierarchical hexapod magnetite, TEM and high-resolution TEM (HRTEM) were employed to characterize these samples. Fig. 2(c–f) display the tip details of one arm in an individual branched h-hexapod from the observations of TEM and HRTEM. It is distinct that the distance between the adjacent fringes within the tip of the trunk and sub-branch (Fig. 2(e, f)) is measured to be around 0.30 nm, close to the (220) lattice spacing of spinel-structured Fe3O4, indicating that the side of each branch is enclosed by four equivalent planes of {110}.8 Integrated by the perfect symmetry of both primary and secondary pods of the h-hexapod in three-dimensional space observed in Fig. 2b, it is clear that the whole star-like h-hexapod crystal grows preferentially along the six <100> directions of the cubic Fe3O4,6,7,21 resulting in each terminate of the trunk pod and the sub-branch with a pyramidal tip. A schematic representation of the crystal growth direction of the primary and secondary branches is indicated in Fig. S2 (ESI†). Combined with the corresponding FFT spots due to the [111] zone axis of the sample in two insets in Fig. 2, the continuous lattice in the HRTEM images of both the trunk tip and the secondary pod distinctly reveal that the as-prepared hierarchical magnetite is single crystalline in nature.
Influence of temperature and solvent
A series of control experiments have been conducted to optimize the formation conditions of the hierarchical shaped Fe3O4. Table S1 (ESI†) summarizes the product major morphologies as functions of solvent composition and dosage, reaction temperature, the concentration of precursor and reaction time.In the case of isopropanol, the initial volume of solvent and the concentration of ferrocene could notably influence the product morphology (Table S1). Microspheres were readily harvested either at a high filling amount of solvent or concentrated precursor. For the formation of symmetric h-hexapod magnetite, the reaction temperature seems to be the most important parameter in the present work. It is found that h-hexapod magnetite is the dominant product in the temperature range of 350–450 °C, however, the secondary structures on the main stems change obviously with the variation of temperature (Fig. S3, ESI†). In addition to temperature, the composition and property of the solvent also strongly affect the formation of hierarchical-shape magnetite, as reported previously.10,22 Magnetite with the six-pod feature can be easily formed in oxygen-containing solvents, such as methanol, acetone and isopropanol, accompanied by subtle variations in the shape of the arms (Fig. 2 and 3). Whereas in non-oxygen-containing hydrocarbon solvents (i.e. benzene or cyclohexane), only irregular nano- to micrometre sized particles emerge (Fig. S4(a, b), ESI†). Investigation of the direct pyrolysis of pure ferrocene in the absence of any solvent under similar conditions also shows that quasi-spherical particles about 1 μm in diameter, rather than star-shaped, become the dominant product (Fig. S4c).
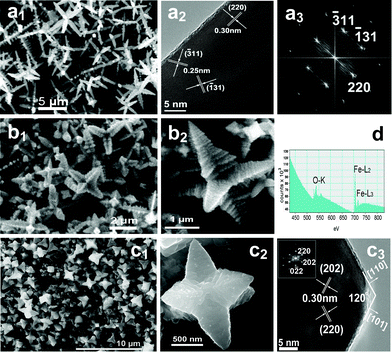 | ||
| Fig. 3 FESEM and HRTEM images of magnetite with various morphologies prepared in different solvents. (a1) h-hexapods prepared at 400 °C for 24 h in acetone, (a2, a3) HRTEM image and the corresponding FFT pattern of one pod of an individual h-hexapod in (a1); (b1, b2) p-hexapods synthesized at 400 °C for 24 h in methanol and their higher magnification image; (c1, c2) p-hexapods synthesized at 300 °C for 30 h in methanol and their higher magnification image, as well as (c3) a HRTEM image and the corresponding FFT pattern of the top of one pod in (c2); (d) EELS spectrum of Fe3O4h-hexapods in acetone with atomic ratios of Fe (43.6%) and O (56.4%), indicating the O K shell ionization edge and the Fe L2 and Fe L3 shell ionization edges. | ||
Fig. 3 provides representative FESEM images of hexapod magnetite prepared in acetone and methanol. Surprisingly, uniform h-hexapod magnetite generated in acetone (Fig. 3a1) is very similar to that produced in isopropanol (Fig. 2), only with relatively larger aspect ratio for both the primary and secondary pods. Whereas in the case of methanol, the hexapod magnetite produced is evidently different from the product from isopropanol (Fig. 3b1, c1). From the high-resolution observations of an individual hexapod (Fig. 3(b2, c2)), one can see clearly that the main stems of the hexapod crystals are much thicker and stronger than those formed in isopropanol/acetone under similar conditions, exhibiting a typical pyramid shape with a sharp diameter decreasing from the base to the tip (labeled as p-hexapods). This implies the relative growth rates along the axes of the six stems and their lateral growth are sensitive to the concentration of reactant, comparable at the early stage with a high concentration of reactant and a relatively fast elongation rate at the late stage with a low concentration of reactant. It is also found that increasing the reaction temperature (from 300 °C to 450 °C) is very propitious to the formation of p-hexapod morphology in methanol, six trunk arms grow in a step-by-step mode with the adjacent layer varying from tightly stacked along the trunk to a broadening one, as shown in Fig. 3(b2, c2) and Fig. S5 (ESI†). The single-crystallinity and composition of the hexapods obtained in acetone/methanol are also confirmed by HRTEM observations and EELS spectrum detection (Fig. 3(a2, a3, c3, d)).
Generally, in the case of the hydrothermal synthesis of magnetite with ferrous or ferric salts as precursors, the polarity of the solvent is an essential factor and significantly influences the solubility of the precursor,23 and a high polarity solvent could favor the formation of large size Fe3O4 nanoparticles. Whereas in the present work, an iron organic compound, ferrocene, is introduced as the iron source, and it would sublimate and form a gaseous mixture with the solvent at the reaction temperature (>300 °C), which is in the scope of the supercritical fluids of these solvents used; therefore, the effect of different pure solvent on the solubility of the precursor might be ignored. The similarity of the h-hexapod magnetites prepared in acetone and isopropanol might be owing to the similar intermediate species, such as olefinic alcohol,24 derived from these two kinds of solvent during the reaction under high temperature (300–450 °C). For the diverse morphologies of Fe3O4 from isopropanol and methanol, it is reasonable to imagine that different intermediate species derived from different solvent molecules selectively attach on the specific crystal planes of the newly formed Fe3O4 particles,23,25 leading to oriented anisotropic growth and the eventual formation of h-hexapod or p-hexapod magnetite. However, more detailed characterization and direct evidence are needed to clarify the growth process.
Growth mechanism
To understand the formation mechanism of hexapod Fe3O4 microcrystals, the morphological evolution process was investigated as a function of reaction time in isopropanol at 350 °C, as shown in Fig. 4. The digital image (Fig. 4a) of the products obtained at different reaction times clearly demonstrates the Fe3O4 output increases with prolonged reaction time, accompanied by the colour degradation of the residual liquid resulting from the increasing consumption of dissolved ferrocene. The morphologies of the corresponding products were observed by SEM and TEM. As can be clearly seen, the primary particles initially formed are 20–40 nm polyhedral or truncated octahedron-shaped nanoparticles revealed by TEM observation (Fig. 4b). Then, the spherical aggregates deplete and the hierarchical hexapods gradually become dominant with increasing time (Fig. 4c, d), and are harvested in high yield after 24 h (Fig. 4e). The polycrystalline nature of the spherical aggregates formed at early reaction time has been revealed by TEM characterization (Fig. 4c1, c2), which shows the spherical aggregates turn out to be assembled from a large quantity of Fe3O4 nanoparticles. | ||
| Fig. 4 (a) A digital photograph of the products obtained in isopropanol at different reaction stage at 350 °C. (b–e) TEM or SEM images of the products obtained at reaction times of 0.5 h (b), 2 h (c), 6 h (d) and 24 h (e). (c1, c2) TEM image (c1) of one spherical aggregate in (c) and its SAED pattern (c2). | ||
From the crystallographic point of view, magnetite with octahedra are generally formed, as reported in the literature.21,26 Obviously, the hierarchical structures in the present work originate from the much faster growth rate of (100) than that of (111) for the newly grown truncated octahedral nanoparticles. The anisotropic Fe3O4 nanoparticles behave like magnetic dipoles,27 and the nanocrystals exhibit the greatest attraction along six <100> directions.28 As a result, small truncated octahedral seeds can be further used as building blocks for the construction of hierarchical magnetite crystals. The aspect ratio of stem in the architecture (Fig. 2b) also suggests some information about the relationship between the connection and subsequent growth of Fe3O4 nanoparticles. Therefore, the formation of branched hexapod Fe3O4 may be attributed to the oriented attachment mechanism.16,26,29,30
The growth mechanism of the hierarchical hexapods can probably be described as following. Firstly, ferrocene decomposes at high temperature to give rise to Fe species which react with the residue oxygen in the reactor (Note: careful exclusion of air in the reaction system did not give rise to star-shaped product) to form Fe3O4 nanocrystallites. Then, the primary Fe3O4 nanoparticles grow into small truncated octahedron-shaped seed particles, which act as the building blocks for the formation of ordered assembly-micrometre crystals. It should be noted that, with highly anisotropic Fe3O4 nanoparticles, the effect of interparticle forces must be particularly significant, and the nanocrystals in the sample exhibit relatively strong attraction along the six <100> directions as mentioned above.10,28 This feature distinguishes Fe3O4 essentially from isotropic metal or semiconductor nanoparticles.31 Therefore, as the truncated octahedra continue to grow, polarity attraction increases with the size of the Fe3O4 crystals,27 the nanoparticles would preferentially self-assemble along six <100> directions to form the initial hexapod pattern. The oriented attachment and subsequent growth of Fe3O4 nanoparticles lead to the hierarchical branch-hexapod crystals.
Magnetic properties
Compared with semiconductor and metallic nanocrystals, magnetic nanoparticles with non-spherical shapes demonstrate more appealing anisotropic magnetic properties.32 The room temperature ferrimagnetic properties of three magnetites with different shapes (p-hexapods, h-hexapods, and microspherical crystals synthesized in isopropanol, Table S1, ESI†) are shown in Fig. 5, and the corresponding magnetic parameters are listed in Table S2 (ESI†). It can be seen that the difference between p-hexapods and h-hexapods is significant although the hysteresis loops for p-hexapods and microspheres overlap to some degree (Fig. 5a). The relatively higher specific saturation magnetization (σs, 128 emu g−1) and coercivity (Hc, 238 Oe) values of Fe3O4h-hexapods compared with bulk magnetite13,19a might be due to their shape anisotropy and magneto-crystalline anisotropy, as well as their single-crystalline structures.16 The magnified low field hysteresis curves (Fig. 5b) reveal that the low specific remanent magnetization (σr) and high Hc of the samples vary with the crystal size and morphology (Table S2).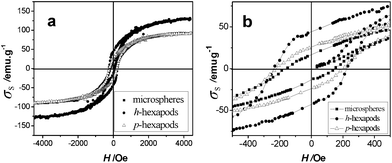 | ||
| Fig. 5 Field dependence of magnetization (a) and the expanded low field hysteresis curves (b) for the as-prepared magnetites measured at room temperature. (■ Microspheres prepared in 4 mL isopropanol at 350 °C for 6 h, ● h-hexapods prepared in 2 mL isopropanol at 350 °C for 24 h, △ p-hexapods prepared in 2 mL methanol at 350 °C for 24 h). | ||
Self-assembly of magnetite hexapods and microspheres
Various Fe3O4 order assemblies have been reported, including body-centered cubic (bcc) superlattices constructed from crystallographically aligned monodisperse magnetite nanoparticles with truncated octahedral shape,14 3D hexagonal closed-packed superlattices upon recrystallization of monodisperse spherical nanocrystals of Fe3O4,33a and microspheres from Fe3O4 nanoparticle aggregates with oriented attachment structure.33b In addition, the formation of an anisotropic chain-like and ring structure resulting from strong dipolar–dipolar attractions between magnetic dipoles has also been demonstrated.26,33cGenerally, nanosize magnetite crystals with ball-like shape readily self-organize to give a well-defined assembly, however, the self-assembly of non-ball-like crystals is hardly reported. In the present study, an evaporation-mediated self-assembly process was adopted for the assembly of 3D magnetite microcrystals. Fig. 6 shows the “hand-in-hand” arrangement of Fe3O4 star-shaped hexapods (Fig. 6(a1, a2)) and the long range organization of quasi-spherical Fe3O4 microcrystals (Fig. 6b) from tens to hundreds of particles with the length exceeding tens of micrometres. Moreover, the necklace-like self-assembled Fe3O4 microspheres are also obtained on the TEM grids (Fig. 6(c–e)), and the evident tendency of flux-closure ring self-assembly in microspheres can be clearly shown.
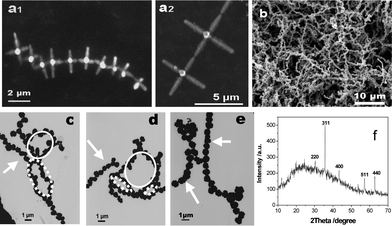 | ||
| Fig. 6 SEM and TEM images of the self-assembly of magnetite microcrystals without an external field. (a1, a2) Star-shaped hexapods, (b) microspheres, (c, d, e) ellipse-like (⋯), circle-like (—), and linear-like (→) assemblies of microspheres with close or open-mode. (f) XRD pattern of microspheres in (b). | ||
It is reasonable that self-assembly is much more difficult for hexapods than microspheres owing to their high shape-related assembly barrier.34 To the best of our knowledge, this is the first time the self-assembly of micrometre star-shape magnetite, which can be ascribed to its magnetic properties, has been observed and reported. For fcc magnetite, the highest probability and the largest magnitude of the dipole moment are predicted along the <100> direction.27,35 The driving force from dipoles remarkably becomes stronger with increasing crystal size,12,13,36 and promotes the preference of hexapods for a head-to-tail arrangement along their <100> direction33a,35 to form chain-like clusters. The exceptional self-assembly of these star-like architectures is consistent with the oriented attachment mechanism proposed for hexapod magnetite growth, in other words, the post-synthesis self-assembly behavior of hexapod magnetite provides additional evidence for the occurrence of ordered assembly of the nanoparticles during crystal growth.
Conclusions
In summary, single-crystalline Fe3O4 hexapod microcrystals with various morphologies have been fabricated via a facile solvothermal route without any additional surfactant. The size, shape, and magnetic properties of the microcrystals can be truly controlled by tailoring the experimental parameters. The observed post-synthesis self-assembly behavior of Fe3O4 hexapods provides evidence for their growth mechanism. Our approach offers tremendous opportunities for the design of Fe3O4 assemblies with varying shapes, structures, and functions. Furthermore, the simple large-scale synthesis of hierarchical hexapods enables the study of their magnetization properties and applications in many fields.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos: 51072028, 20725619, 20876026 and 21176043). We thank Prof. Jun-hu Wang at Dalian Institute of Chemical Physics in the Chinese Academy of Sciences, and Prof. Xu-guang Liu from Taiyuan University of Technology for 57Fe Mössbauer spectra measurements and FESEM observations, respectively.References
- (a) J. Park, J. Joo, S. G. Kwon, Y. Jang and T. Hyeon, Angew. Chem., Int. Ed., 2007, 46, 4630 CrossRef CAS; (b) S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204 CrossRef CAS.
- (a) H. J. Kim, K. Choi, A. Pan, D. Kim, H. R. Kim, K. M. Kim, C. W. Na, G. Cao and J. H. Lee, J. Mater. Chem., 2011, 21, 6549 RSC; (b) M. R. Gao, S. R. Zhang, J. Jiang, Y. R. Zheng, D. Q. Tao and S. H. Yu, J. Mater. Chem., 2011, 21, 16888 RSC; (c) Z. Y. Wang, D. Y. Luan, S. Madhavi, C. M. Li and X. W. Lou, Chem. Commun., 2011, 47, 8061 RSC.
- Y. W. Jun, J. S. Choi and J. Cheon, Angew. Chem., Int. Ed., 2006, 45, 3414 CrossRef CAS.
- Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664 CrossRef CAS.
- Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- S. M. Lee, S. N. Cho and J. Cheon, Adv. Mater., 2003, 15, 441 CrossRef CAS.
- S. M. Lee, Y. W. Jun, S. N. Cho and J. Cheon, J. Am. Chem. Soc., 2002, 124, 11244 CrossRef CAS.
- X. Liu, R. Huang and J. Zhu, Chem. Mater., 2008, 20, 192 CrossRef CAS.
- T. Haxhimali, A. Karma, F. Gonzales and M. Rappaz, Nat. Mater., 2006, 5, 660 CrossRef CAS.
- Y. Chang and H. C. Zeng, Cryst. Growth Des., 2004, 4, 273 CAS.
- N. R. Jana, Y. Chen and X. Peng, Chem. Mater., 2004, 16, 3931 CrossRef CAS.
- H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef.
- R. M. Cornell and U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, Wiley-VCH, Germany, 2003 Search PubMed.
- R. Zheng, H. Gu, B. Xu, K. K. Fung, X. Zhang and S. P. Ringe, Adv. Mater., 2006, 18, 2418 CrossRef CAS.
- (a) Q. Zhang, S. J. Liu and S. H. Yu, J. Mater. Chem., 2009, 19, 191 RSC; (b) R. L. Penn and J. F. Banfield, Science, 1998, 281, 969 CrossRef CAS.
- D. Kim, N. Lee, M. Park, B. H. Kim, K. An and T. Hyeon, J. Am. Chem. Soc., 2009, 131, 454 CrossRef CAS.
- Y. Xiong, J. Ye, X. Gu and Q. Chen, J. Phys. Chem. C, 2007, 111, 6998 CAS.
- (a) J. Wan, X. Chen, Z. Wang, X. Yang and Y. Qian, J. Cryst. Growth, 2005, 276, 571 CrossRef CAS; (b) J. Wang, Q. Chen, C. Zeng and B. Hou, Adv. Mater., 2004, 16, 137 CrossRef CAS; (c) C. Jia, L. Sun, F. Luo, X. Han, L. Heyderman, Z. G. Yan and C. H. Yan, J. Am. Chem. Soc., 2008, 130, 16968 CrossRef CAS.
- (a) B. Geng, J. Ma and J. You, Cryst. Growth Des., 2008, 8, 1443 CrossRef CAS; (b) S. Xuan, L. Hao, W. Jiang, L. Song, Y. Hu, Z. Chen, L. Fei and T. Li, Cryst. Growth Des., 2007, 7, 430 CrossRef CAS; (c) G. Xi, C. Wang and X. Wang, Eur. J. Inorg. Chem., 2008, 425 CrossRef CAS.
- (a) M. Hu, J. Jiang and X. Li, Cryst. Growth Des., 2009, 9, 820 CrossRef CAS; (b) G. Zou, K. Xiong, C. Jiang, H. Li, T. Li, J. Du and Y. Qian, J. Phys. Chem. B, 2005, 109, 18356 CrossRef CAS.
- (a) Y. Ding, J. R. Morber, R. L. Snyder and Z. L. Wang, Adv. Funct. Mater., 2007, 17, 1172 CrossRef CAS; (b) Z. L. Wang, Adv. Mater., 1998, 10, 13 CrossRef CAS.
- T. Zhu, J. S. Chen and X. W. Lou, J. Mater. Chem., 2010, 20, 7015 RSC.
- X. Liang, X. Wang, J. Zhuang, Y. Chen, D. Wang and Y. Li, Adv. Funct. Mater., 2006, 16, 1805 CrossRef CAS.
- T. Luo, L. Gao, J. Liu, L. Chen, J. Shen, L. Wang and Y. Qian, J. Phys. Chem. B, 2005, 109, 15272 CrossRef CAS.
- (a) J. Yang and T. Sasaki, Cryst. Growth Des., 2010, 10, 1233 CrossRef CAS; (b) J. Chen, F. Wang, K. Huang, Y. Liu and S. Liu, J. Alloys Compd., 2009, 475, 898 CrossRef CAS.
- K. S. Cho, D. V. Talapin, W. Gaschler and C. B. Murray, J. Am. Chem. Soc., 2005, 127, 7140 CrossRef CAS.
- F. X. Redl, C. T. Black, G. C. Papaefthymiou, R. L. Sandstrom, M. Yin, H. Zeng, C. B. Murray and S. P. O’Brien, J. Am. Chem. Soc., 2004, 126, 14583 CrossRef CAS.
- L. Meng, W. Chen, C. Chen, H. Zhou, Q. Peng and Y. Li, Cryst. Growth Des., 2010, 10, 479 CAS.
- J. F. Banfield, S. A. Welch, H. Zhang, T. T. Ebert and R. L. Penn, Science, 2000, 289, 751 CrossRef CAS.
- (a) H. C. Zeng, Int. J. Nanotechnol., 2007, 4, 329 CrossRef CAS; (b) K. X. Yao, X. M. Yin, T. H. Wang and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 6131 CrossRef CAS.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS.
- (a) M. Chen, J. Kim, J. P. Liu, H. Y. Fan and S. H. Sun, J. Am. Chem. Soc., 2006, 128, 7132 CrossRef CAS; (b) L. Li, Y. Yang, J. Ding and J. Xue, Chem. Mater., 2010, 22, 3183 CrossRef CAS.
- (a) Q. Song, Y. Ding, Z. L. Wang and Z. J. Zhang, J. Phys. Chem. B, 2006, 110, 25547 CrossRef CAS; (b) Y. Zhu, W. Zhao, H. Chen and J. Shi, J. Phys. Chem. C, 2007, 111, 5281 CrossRef CAS; (c) S. L. Tripp, S. V. Pusztay, A. E. Ribbe and A. Wei, J. Am. Chem. Soc., 2002, 124, 7914 CrossRef CAS.
- (a) B. Grzybowski, Science, 2002, 295, 2418 CrossRef; (b) T. D. Clark, R. Ferrigno, J. Tien, K. E. Paul and G. M. Whitesides, J. Am. Chem. Soc., 2002, 124, 5419 CrossRef CAS; (c) K. J. M. Bishop, C. E. Wilmer, S. Soh and B. A. Grzybowski, Small, 2009, 5, 1600 CrossRef CAS.
- (a) H. Qi, Q. Chen, M. Wang, M. Wen and J. Xiong, J. Phys. Chem. C, 2009, 113, 17301 CrossRef CAS; (b) R. Bardhan, O. Neumann, N. Mirin, H. Wang and N. J. Halas, ACS Nano, 2009, 3, 266 CrossRef CAS.
- (a) E. Kim and G. M. Whitesides, Chem. Mater., 1995, 7, 1257 CrossRef CAS; (b) S. Disch, E. Wetterskog, R. P. Hermann, G. S. Alvarez, P. Busch, T. Bruckel, L. Bergstrom and S. Kamali, Nano Lett., 2011, 11, 1651 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD profiles, SEM and TEM images, EDX spectrum, a list of control experiments and analysis of the magnetic properties at room temperature of the products obtained under various conditions. See DOI: 10.1039/c2ra01270g |
| This journal is © The Royal Society of Chemistry 2012 |
