Facile preparation of a robust and flexible antioxidant film based on self-polymerized dopamine in a microporous battery separator†
Manthiriyappan
Sureshkumar
ab,
Pin-Ni
Lee
b and
Cheng-Kang
Lee
*b
aDepartment of Chemistry, The University of Suwon, Hwasung-shi, Kyonggi-do 445-743, Korea
bDepartment of Chemical Engineering, National Taiwan University of Science and Technology, Taipei-106, Taiwan. E-mail: cklee@mail.ntust.edu.tw; Tel: +886-2-2737-6629
First published on 20th March 2012
Abstract
The microslits of a microporous polyethylene (PE) separator membrane were filled with polydopamine (Pdop) via auto-oxidation and self-polymerization of dopamine to generate a robust, antioxidant, and free standing flexible thin film. Multifunctional groups present on the Pdop act as strong anti-oxidative agents and can induce in situ formation of noble metal nanoparticles on the film surface. The superior antioxidant property of the film was well maintained even after harsh ultrasonic irradiation, tape peeling, and extreme pH treatment.
Dopamine or mussel-inspired catechol amine based simple and mild multifunctional surface coating has attracted enormous attention over the past few years. Under alkaline pH conditions, dopamine is known to auto-oxidize and self-polymerize to form a multifunctional nanometer thick polydopamine (Pdop) layer on various kinds of solid surface through strong intermolecular interactions such as hydrogen-bonding, metal chelation, and π–π interactions.1,2 The thickness of the self-coated layer can be easily tuned by adjusting the duration of auto-oxidation and concentration of dopamine. Hydrophobic membranes such as polyethylene (PE), poly(vinylidene fluoride) (PVDF) and polytetrafluoroethylene (PTFE) have been surface modified with Pdop to increase the filtration flux.3 Recently, many applications based on the coated Pdop surface have been carried out.4–13 The Pdop surface can not only be electrolessly metallized but also used for biomolecule immobilization.14–16 However, Pdop generally formed on solid surfaces as an ad-layer. Its adhesion to a flexible polymer surface has been demonstrated to be unsatisfactory due to its glassy nature.17 The solubility of synthetic dopamine-melanin, which has a similar chemical structure as Pdop, has been reported to be markedly increased by increasing the pH to 13.18 This shows that in addition to its mechanical strength, the chemical stability of Pdop at higher alkaline conditions should also be considered if wider applications of the existing hydrophilic, biocompatible, reductive, and other functional properties of Pdop were pursued. Therefore, an ideal solid surface platform is urgently required to support the formation of Pdop with good mechanical strength and chemical stability. Such an approach will also be an advantage for the integration of robustness with multifunctional properties of the easily generated bioinspired surfaces.
In this work, the low cost, high porosity PE microporous membrane of a suitable mechanical strength was used as an ideal template for robust Pdop film preparation. The microporous membrane is commonly used as a lithium ion battery separator with a thickness of about 20 μm and ∼40% porosity (Coin Nanotech, Taipei, Taiwan). The membrane separator is mainly composed of 0.1 μm × 0.5 μm slit-like through-pores (Fig. 1a). Through Pdop ad-layer formation on the lumen surface of the microslits, the antioxidant function of Pdop is expected to be well-preserved under harsh chemical and physical treatment because the microslits through-pore structure can provide a very high specific surface area for Pdop to interact with. Thus, we decided to place Pdop into the microslits of a microporous PE battery separator with the aim of verifying its potential performance as a robust antioxidant film. Two schemes for preparing the anti-oxidative Pdop film were designed (Scheme 1). One is to wet microporous PE with pure ethanol before immersing it into pH 8.5 dopamine solution (2 mg ml−1) and the other one is without the ethanol wetting pretreatment. As shown in Fig. 1b, the microslits observed in the pristine membrane disappeared after 24 h incubation in dopamine solution for both ethanol wetted and nonwetted samples. But, the color that appeared on the ethanol wetted sample was darker than that of the non-wetted one (ESI†, Fig 1S). As reported in previous studies,19 the self-polymerization of oxidized dopamine will result in spontaneous deposition of a thin adherent layer on the solid surface as indicated by the surface color changes into black-brown. Evidently, the blackish color observed on both samples indicates that Pdop was coated onto the microporous PE membrane. Since the hydrophobic microslits can only be wetted by dopamine solution after ethanol pretreatment, apparently the darker film obtained from ethanol wetting pretreatment can be attributed to Pdop formation inside all the microslits. In contrast, Pdop can only form on the top surface of the non-ethanol-wetted PE membrane that leads to the formation of a lighter color film. As shown in Fig. 1d, the Pdop on the top surface can easily be peeled off by Scotch tape to expose the original microslits structure. The peeling test can easily remove the Pdop ad-layer not only on the porous surface of the PE membrane separator but also on the non-porous surface of a flat PE film (ESI†, Fig 1S). In contrast, the original microslits structure could not be observed by SEM when the peeling test was applied to the ethanol pretreated sample (Fig. 1c). The much lighter color intensity observed on the Scotch tape peeled from the ethanol pretreated sample (ESI†, Fig 2S) also demonstrates that Pdop was securely preserved in the microslits of the microporous PE membrane when ethanol wetting was applied.
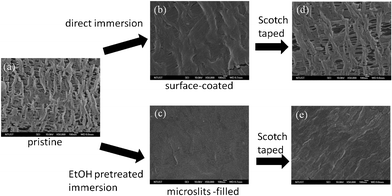 | ||
| Fig. 1 FE-SEM images of (a) pristine PE (b–d) surface coated Pdop and microslits-filled Pdop PE films before and after peeling by Scotch tape. | ||
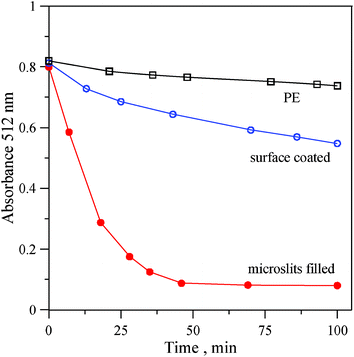 | ||
| Fig. 2 DPPH radical scavenging assay of original PE, surface coated and microslits-filled Pdop PE films. | ||
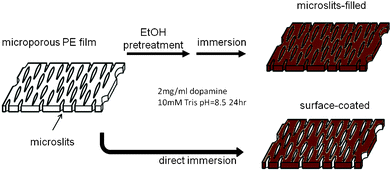 | ||
| Scheme 1 Preparation of flexible antioxidant films based on a microporous PE membrane with Pdop coating. | ||
Recently, melanin-like nanoparticles synthesized from oxidized dopamine have been reported to have excellent free-radical scavenging properties.20 DPPH radical scavenging assay is one of the commonly used methods to evaluate the antioxidant activity of phenolic compounds. The purple color (512 nm) of the DPPH radical in solution will disappear as the free radical is reduced by the phenolic compounds. Therefore, the DPPH radical scavenging activity was employed to evaluate the antioxidant performance of the Pdop–PE films. As shown in Fig. 2 and Fig 3S, ESI†, with the potent antioxidant nature of surface-coated and microslits-filled Pdop films, the absorbance at 512 nm quickly decreased over 5–10 min as a result of the DPPH radicals being reduced by Pdop on PE membranes. Microslits-filled Pdop film has an approximately 4-fold faster absorbance decay rate featuring a higher antioxidant activity than that of surface-coated Pdop film. Total phenolic contents of these two Pdop film preparations were measured using a spectrophotometric technique,21 based on the Folin-Ciocalteau reagent and expressed as gallic acid equivalents (GAE). The microslits-filled Pdop film possesses a 2-fold higher total phenolic content (12.3 ± 10 mg GAE per g) than the surface-coated Pdop film (6.36 ± 6 mg GAE per g). Evidently, the higher phenolic content of microslits-filled Pdop film is mainly responsible for its higher antioxidant activity.
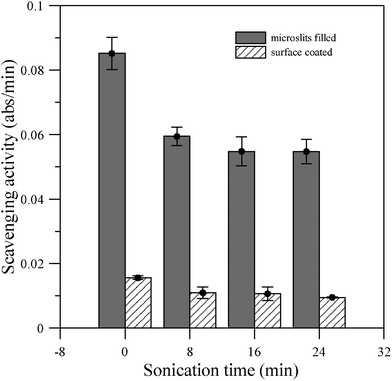 | ||
| Fig. 3 DPPH radical scavenging activity of Pdop modified microporous PE membranes after ultrasonic irradiation treatment for different durations. | ||
The mechanical stability of Pdop film under ultrasonic irradiation treatment was further investigated by measuring its remaining DPPH free radical scavenging activity, which was defined as the first 10 min absorbance decreasing rate. As shown in Fig. 3, there was a ∼25% and ∼15% activity decrease after 8 min ultrasonic irradiation (Qsonica Ultrasonic Processor Q700, amplitude: 5 and power: 12–15 W) on microslits-filled and surface-coated Pdop films, respectively. However, with further ultrasonication (16 and 24 min) only slight activity decreases were noticed. This indicates that a small fraction of Pdop will be detached from Pdop–PE film by ultrasonic irradiation.
So far, the chemical structure of Pdop is not well elucidated but is believed to be similar to eumelanins, one major type of melanins.20 Since some natural melanins can be isolated by extraction with NaOH or NH4OH solution22,23 and the solubility of synthetic melanins at pH 13 has also been reported,18 it is worthy to study the stability of the as-prepared Pdop film in strong alkaline conditions by measuring its antioxidant activity. Relative free radical scavenging activity of the Pdop–PE films was calculated according to eqn (1),
| Relative scavenging activity = At = t/At = 0 × 100% | (1) |
Where, At = t is the DPPH scavenging activity of Pdop–PE film after incubation in 0.1 N NaOH for a certain time and At = 0 is the activity before NaOH incubation. As shown in Fig. 4, the relative scavenging activity of microslits-filled Pdop film decreased to almost half after 2 h incubation in 0.1 N NaOH solution. When the incubation was prolonged to 21 h, the microslits-filled Pdop film still exhibited about 23% of its original activity. On the other hand, the relative activity of the surface-coated Pdop film decreased to less than 10% after only 2 h incubation. Evidently, the microslits-filled Pdop film is much more chemically stable than the surface-coated Pdop film. This result is in accordance with a recent report24 that upon exposure to 1 N NaOH for 24 h the Pdop coated on the PE separator still maintains its adhesion via XPS analysis. The activity of Pdop film will be degraded by the strong alkaline solution but the microslits can prevent the alkaline solution from easily accessing the Pdop in the microslits which prolongs the antioxidant activity of microslits-filled Pdop film. This robust antioxidant film may be used for food packaging applications. It can also easily be used to make various nanometal particles including silver deposited on its surface from their salt solutions as reported previously19 (ESI†, Fig 4S), which can be used for antimicrobial and catalytic reaction applications. A new robust and flexible antioxidant thin film has been designed and developed, consisting of a microporous PE film and Pdop. Both mechanical (peeling and ultrasonic irradiation) and strong alkaline tests showed that microslits can effectively protect and support the filled Pdop.
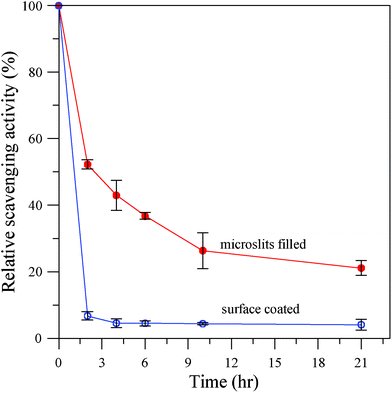 | ||
| Fig. 4 Relative DPPH radical scavenging activity of Pdop modified microporous PE membranes after incubation in 0.1 N NaOH for different durations. | ||
References
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS.
- Z. Y. Xi, Y. Y. Xu, L. P. Zhu, Y. Wang and B. K. Zhu, J. Membr. Sci., 2009, 327, 244–253 CrossRef CAS.
- K. J. Jeong, L. Wang, C. F. Stefanescu, M. W. Lawlor, J. Polat, C. H. Dohlman, R. S. Langer and D. S. Kohane, Soft Matter, 2011, 7, 8305–8312 RSC.
- L. Zhang, J. Shi, Z. Jiang, Y. Jiang, S. Qiao, J. Li, R. Wang, R. Meng, Y. Zhu and Y. Zheng, Green Chem., 2011, 13, 300–306 RSC.
- T. S. Sileika, H. D. Kim, P. Maniak and P. B. Messersmith, ACS Appl. Mater. Interfaces, 2011, 3, 4602–4610 CAS.
- S. H. Ku, J. S. Lee and C. B. Park, Langmuir, 2010, 26, 15104–15108 CrossRef CAS.
- B. Yu, D. A. Wang, Q. Ye, F. Zhou and W. Liu, Chem. Commun., 2009, 6789–6791 RSC.
- B. Çelen, D. Ekiz, E. Pişkin and G. Demirel, J. Mol. Catal. A: Chem., 2011, 350, 97–102 CrossRef.
- Y. Liu, B. Yu, J. Hao and F. Zhou, J. Colloid Interface Sci., 2011, 362, 127–134 CrossRef CAS.
- K. Sun, L. Song, Y. Xie, D. Liu, D. Wang, Z. Wang, W. Ma, J. Zhu and X. Jiang, Langmuir, 2011, 27, 5709–5712 CrossRef CAS.
- B. Li, W. Liu, Z. Jiang, X. Dong, B. Wang and Y. Zhong, Langmuir, 2009, 25, 7368–7374 CrossRef CAS.
- J. Ryu, S. H. Ku, H. Lee and C. B. Park, Adv. Funct. Mater., 2010, 20, 2132–2139 CrossRef CAS.
- H. Lee, J. Rho and P. B. Messersmith, Adv. Mater., 2009, 21, 431–434 CrossRef CAS.
- J. H. Jiang, L. P. Zhu, X. L. Li, Y. Y. Xu and B. K. Zhu, J. Membr. Sci., 2010, 364, 194–202 CrossRef CAS.
- L. P. Zhu, J. H. Jiang, B. K. Zhu and Y. Y. Xu, Colloids Surf., B, 2011 Search PubMed.
- F. K. Yang and B. Zhao, Open Surf. Sci. J., 2011, 3, 115–112 CAS.
- F. Bernsmann, O. Ersen, J. C. Voegel, E. Jan, N. A. Kotov and V. Ball, ChemPhysChem, 2010, 11, 3299–3305 CrossRef CAS.
- M. Sureshkumar, P. N. Lee and C. K. Lee, J. Mater. Chem., 2011, 21, 12316–12320 RSC.
- K. Y. Ju, Y. Lee, S. Lee, S. B. Park and J. K. Lee, Biomacromolecules, 2011, 12, 625–632 CrossRef CAS.
- Y. Velioglu, G. Mazza, L. Gao and B. Oomah, J. Agric. Food Chem., 1998, 46, 4113–4117 CrossRef CAS.
- H. Wang, Y. Pan, X. Tang and Z. Huang, LWT–Food Sci. Technol., 2006, 39, 496–502 CrossRef CAS.
- V. M. Sava, B. N. Galkin, M. Y. Hong, P. C. Yang and G. S. Huang, Food Res. Int., 2001, 34, 337–343 CrossRef CAS.
- M. H. Ryou, Y. M. Lee, J. K. Park and J. W. Choi, Adv. Mater., 2011, 23, 3066–3070 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra01264b |
| This journal is © The Royal Society of Chemistry 2012 |
