TiO2 nanoparticles synthesized by the molten salt method as a dual functional material for dye-sensitized solar cells†
Zhu
Peining
ab,
Wu
Yongzhi
b,
M. V.
Reddy
*c,
A.
Sreekumaran Nair
*b,
Peng
Shengjie
b,
N.
Sharma
d,
V. K.
Peterson
d,
B. V. R.
Chowdari
c and
S.
Ramakrishna
b
aDepartment of Mechanical Engineering, National University of Singapore, Singapore, 117574, Singapore
bHealthcare and Energy Materials Laboratory, Nanoscience and Nanotechnology Initiative and Department of Mechanical Engineering, National University of Singapore, Singapore, 117581, Singapore
cDepartment of Physics, National University of Singapore, Singapore 117542
dThe Bragg Institute, Australian Nuclear Science and Technology Organisation, Locked Bag 2001, Kirrawee DC, NSW 2232, Australia
First published on 13th April 2012
Abstract
We report the simple and versatile molten salt fabrication of mesoporous anatase TiO2 nanoparticles with a high surface area of ∼200 m2 g−1, which show an impressive efficiency of ∼7.5% in dye-sensitized solar cells.
Titanium dioxide (TiO2) is one of the most studied metal oxide semiconductors of the last two decades. TiO2 is used for applications relating to environmental remediation,1 solar cells,2 lithium ion batteries,3 gas sensors,4 photonic crystals,5 and self-cleaning coatings,6 owing to its chemical stability, environmental friendliness, and low cost. The performance of TiO2 in such applications (particularly for solar cells) depends on its crystallinity, crystal phase, surface area, and morphology.
Dye-sensitized solar cells (DSCs) have received prime consideration in renewable energy research owing to factors that include the simple non-vacuum processing conditions used for their fabrication, their low cost as a consequence of the relatively high natural abundance of the raw materials used in manufacturing, and properties that include being light-weight, having high tolerance to impurities, conversion efficiencies comparable to that of amorphous Si solar cells, and suitability for building-integrated photovoltaics.7 High surface area TiO2 is central to the fabrication of DSCs as it supports the sensitizers and transports the photoexcited electrons from the sensitizer to the transparent conducting oxide. Despite different nanostructures of TiO2 being studied for use in DSCs, including fibers,8 tubes,9 rice-like arrangements,10 beads,11 and other morphologies,12 traditional nanoparticles have the best performance.13 However, most synthetic methods to produce anisotropic TiO2 nanostructures involve multi-step reactions, long reaction times, and post-annealing treatments, which often introduce defects and impurities into the TiO2 nanostructures, consequently decreasing the overall device performance. Thus, the search for TiO2 with enhanced surface area and crystallinity for application in high-performance DSCs has become an active research area.
Herein, we report the fabrication of high surface area anatase TiO2 nanoparticles for dual functionality (both as an active and scattering layer) in high performance DSCs. We use the molten salt method (MSM) of synthesis due to its simplicity and versatility in the fabrication of high purity oxide materials, as commercially the MSM has proven effective in the synthesis of LiCoO2,14 ZnCo2O4,15 and Li(Ni1/3Co1/3Mn1/3)O216 materials for lithium ion battery applications. To the best of our knowledge, this is the first application of MSM produced TiO2 nanoparticles in the field of dye-sensitized solar cells. We fabricated TiO2 nanoparticles using the MSM and characterized these using UV-visible spectroscopy, scanning and transmission electron microscopy (SEM and TEM, respectively), X-ray and neutron powder diffraction (XRPD and NPD, respectively), Brunauer–Emmett–Teller (BET) surface area measurements, and their performance as electrodes within DSCs. The dual functional material displays a higher efficiency, 7.5%, than that of commercially available materials (e.g. P25 which shows an efficiency of 6.54% with a scattering layer).
The TiO2 nanoparticles were synthesized as follows: A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.8
8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 molar mixtures of TiOSO4·xH2SO4
1.2 molar mixtures of TiOSO4·xH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiNO3
LiNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCl was heated at 3 °C min−1 to 280 °C for 1 h in an Alumina crucible in air. The mixture was cooled at the same rate as the mixture was heated and the excess Li-salts were washed, filtered, and dried under vacuum at 70 °C for 12 h. The as-obtained TiO2 nanoparticles were mixed with ethyl cellulose and α-terpineol17 to form a paste, and fabricated into electrodes by screen printing/doctor-blading on clean, TiCl4-treated conductive fluorine-doped tin oxide (FTO) substrates. The electrodes were then sintered at 450 °C for 30 min, treated with TiCl4, and sintered again. Dye loading was undertaken by immersing these electrodes in N3 dye for 24 h. The electrodes were then sealed with counter electrodes for performance tests. For comparison, electrodes fabricated from commercial P25 TiO2 nanoparticles (with and without a scattering layer) were also investigated (see the ESI†-1 for more fabrication details).
LiCl was heated at 3 °C min−1 to 280 °C for 1 h in an Alumina crucible in air. The mixture was cooled at the same rate as the mixture was heated and the excess Li-salts were washed, filtered, and dried under vacuum at 70 °C for 12 h. The as-obtained TiO2 nanoparticles were mixed with ethyl cellulose and α-terpineol17 to form a paste, and fabricated into electrodes by screen printing/doctor-blading on clean, TiCl4-treated conductive fluorine-doped tin oxide (FTO) substrates. The electrodes were then sintered at 450 °C for 30 min, treated with TiCl4, and sintered again. Dye loading was undertaken by immersing these electrodes in N3 dye for 24 h. The electrodes were then sealed with counter electrodes for performance tests. For comparison, electrodes fabricated from commercial P25 TiO2 nanoparticles (with and without a scattering layer) were also investigated (see the ESI†-1 for more fabrication details).
The TiO2 nanoparticles were characterized by SEM and TEM (Fig. 1). Fig. 1A shows a typical SEM image of the MSM produced TiO2 and reveals the presence of agglomerated nanostructures with diameters in the range 100–300 nm. The sizes of the spherical aggregates determined using TEM (Fig. 1B) were 40–60 nm in diameter and this can be attributed to the sonication-assisted dispersion of the MSM produced TiO2 in methanol for casting onto the TEM grid. The higher magnification TEM image (Fig. 1C) illustrates that the larger, 40–60 nm diameter, particles are composed of smaller nanoparticles with an average diameter of 5 nm. It is these ∼5 nm nanoparticles that are responsible for the very high surface area of the synthesized TiO2 (∼200 m2 g−1, from BET surface area measurements). The high-resolution TEM image (Fig. 1D) indicates that the nanoparticles in this projection have a lattice spacing of 0.35 nm, corresponding to the (101) crystal face indices of anatase.
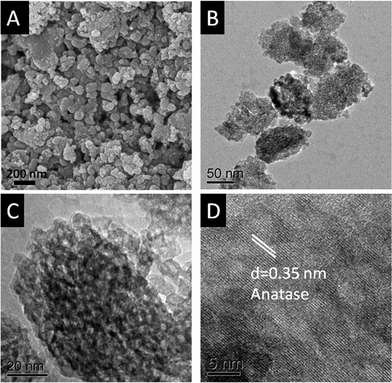 | ||
| Fig. 1 SEM image (a), TEM image in low (b) and high magnification (c), and high resolution TEM image (d) of MSM TiO2 nanoparticles. | ||
Phase-pure anatase was confirmed by XRPD data (ESI†-2, JCPDS No. 04–0477) and shown to be relatively crystalline. The crystal structure of the MSM produced TiO2 was further investigated by Rietveld analysis using neutron powder diffraction (NPD, Fig. 2 and ESI†-1).18 The final Rietveld-refined model had figures of merit that include profile factor (Rp) of 2.01%, the weighted-profile factor, wRp, of 2.59%, and the goodness-of-fit term, χ2, of 1.53 for 16 refinable parameters.
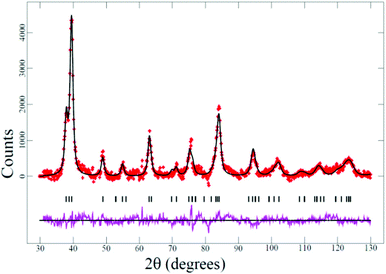 | ||
| Fig. 2 The Rietveld-refined fit for anatase TiO2 using NPD data. The red crosses are the data, the black line through the crosses is the calculated model, the purple line below is the difference between the data and calculated model, and the vertical black lines are reflection markers for anatase TiO2. | ||
The lattice parameters obtained from the NPD data were a = 3.7823(9) Å and c = 9.475(4) Å in the tetragonal I41/amd space-group and these values agree with our XRPD studies (ESI†-2) and literature reports.19 The O atom positional parameter was refined to z = 0.1639(5) and the atomic displacement parameters of Ti and O atoms were found to be 0.0144(26) and 0.0142(12) Å2, respectively. No statistically significant evidence was found for long-range ordered O atom vacancies.
Our synthesis of pure anatase TiO2 with high surface area prompted the investigation of the material's performance as an electrode in DSCs, for comparison with the photovoltaic (PV) parameters of commercially available P25 TiO2. The photocurrent density (Jsc) vs. voltage characteristics of the DSCs are shown in Fig. 3A (see ESI†-3 for details on the PV parameters based on the test results on 5 devices each from MSM and P25 TiO2 nanoparticles). The working area and electrode thickness of both the electrodes were 0.25 cm2 and 13 μm, respectively (see ESI†-4 for thickness-dependent PV parameters). The PV parameters were an open-circuit voltage (OCV) of 0.765 V for MSM TiO2 compared with 0.75 V for P25, a fill factor of 67.5% for MSM TiO2 compared with 65% for P25, and a Jsc of 14.48 mA cm−2 for MSM TiO2 compared with 12.36 mA cm−2 for P25. The consistently higher PV parameters of MSM TiO2 result in a higher efficiency (η) of 7.48% for MSM TiO2 compared with 6.03% for P25 under the same conditions.
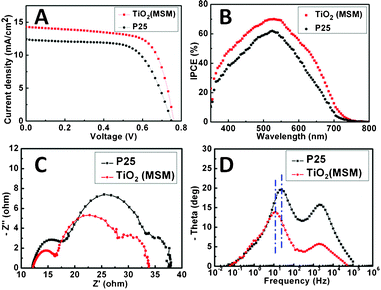 | ||
| Fig. 3 Photocurrent–photovoltage characteristic curves (A), incident photon conversion efficiency (IPCE) spectra (B), Nyquist plots (C), and Bode phase plots (D) of MSM produced and P25 TiO2. | ||
Our MSM produced TiO2 enables both a higher Jsc20 and OCV. This is in part because anatase TiO2 has a larger band-gap than that of rutile TiO221 and phase pure anatase TiO2 eliminates the recombination that can occur at grain boundaries of different TiO2 crystalline forms (P25 is a mixture of ∼75% anatase and ∼25% rutile TiO2). The higher Jsc of the MSM produced TiO2, relative to P25 TiO2, is enabled by its relatively high surface area (200 m2 g−1 in comparison to 55 m2 g−1 for P25 TiO2) that allows a higher dye-loading, 2.57 × 10−7 mol cm−2 for MSM TiO2 compared with 1.28 × 10−7 mol cm−2 for P25 (see ESI†-5 for the UV-Visible spectra of the desorbed dye solutions), in turn enhancing the light harvesting efficiency (η) of devices that contain this material.22 Further evidence of the effect of dye-loading is provided by the incident photon-to-current conversion efficiency (IPCE) spectra from the devices (Fig. 3B). The peak in the IPCE spectrum from MSM produced TiO2 is 70%, which is 13% higher than that from P25 (62%). It can also be observed that the IPCE of the MSM TiO2 is higher that of the P25 TiO2 in all the wavelengths up to ∼750 nm. The enhanced IPCE in the small wavelength region (<600 nm) is due to the high dye-loading (indicating an efficient light harvesting efficiency of the device, see the dye-loading data above) and that in the long wavelength region (>600 nm) is due to the strong light scattering effect as a consequence of the presence of clustering-type aggregates of ∼100–500 nm diameters (see SEM images in ESI†-6) in the MSM TiO2. These aggregates can function as secondary scattering centers that reflect photons back into the electrode and improve the light harvesting efficiency of the electrodes.23,24 This is further evident from the plot of IPCE(MSM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2)/IPCE(P25)vs. wavelength given in ESI†-7. The enhanced light scattering effect is further confirmed from a comparison of the UV-visible spectra of the MSM produced- and P25 TiO2 electrodes (ESI†-8). To get further insights on the better performance of MSM TiO2, electrochemical impedance spectra (EIS) were also measured (under 1 Sun illumination at an applied bias of OCV). From the Nyquist plots presented in Fig. 3C, it is evident that the second semi-circle related to the charge transport resistance of TiO2/dye/electrolyte interface was lower in the case of the MSM TiO2 electrode than that of the P25 electrode. The characteristic peak of the Bode phase plots (Fig. 3D) shifted to lower frequency in the case of MSM TiO2, indicating suppressed charge transport resistance and higher electron lifetime in the MSM TiO2.23,25 These come as a direct consequence of the phase purity (pure anatase with fewer grain boundaries) and the good connectivity between the particles by the clustering-type aggregations in MSM TiO2.
TiO2)/IPCE(P25)vs. wavelength given in ESI†-7. The enhanced light scattering effect is further confirmed from a comparison of the UV-visible spectra of the MSM produced- and P25 TiO2 electrodes (ESI†-8). To get further insights on the better performance of MSM TiO2, electrochemical impedance spectra (EIS) were also measured (under 1 Sun illumination at an applied bias of OCV). From the Nyquist plots presented in Fig. 3C, it is evident that the second semi-circle related to the charge transport resistance of TiO2/dye/electrolyte interface was lower in the case of the MSM TiO2 electrode than that of the P25 electrode. The characteristic peak of the Bode phase plots (Fig. 3D) shifted to lower frequency in the case of MSM TiO2, indicating suppressed charge transport resistance and higher electron lifetime in the MSM TiO2.23,25 These come as a direct consequence of the phase purity (pure anatase with fewer grain boundaries) and the good connectivity between the particles by the clustering-type aggregations in MSM TiO2.
Recently, scientists have been focussing on the fabrication of mesoporous microspheres with large diameters ∼500 nm with high surface area for high performance DSCs.26,27. The intention is to avoid the use of double layer configurations (high-performance DSCs usually have a double layer structure consisting of an active and a scattering layer), as they are complex and cost-intensive. Furthermore, the packing densities of these nanostructures are known to be relatively low28 owing to their large diameters, which in turn decreases the total mass of active TiO2 and the surface area of the film, lowering the overall photocurrent in a device.22,28 Our approach uses the MSM to produce a dual functional electrode. We compare the functionality of an electrode of the MSM produced TiO2 in a single-layer DSC to a double-layer electrode DSC constructed using a 2 μm thick scattering layer of 200 nm diameter TiO2 nanoparticles on the surface of the P25 TiO2 electrode. The double layer electrode DSC shows an efficiency of 6.54% (ESI†-9). We note that the efficiency of the MSM produced TiO2 DSC is higher (7.48%) than that of the double-layer DSC. Thus, we postulate that TiO2 nanostructures with aggregated particles perform dual functions, that of an active and a scattering layer, to produce high-performance DSCs.11 Such materials also facilitate the fabrication of DSCs by allowing a simpler method to be used that avoids the need for additional scattering layers (normally comprising ∼400 nm diameter particles of TiO2/ZrO2 to achieve high-performance) and the double layer configuration.
In summary, we have fabricated a high surface area, porous, and nanostructured anatase TiO2 by the MSM. The MSM produced TiO2 nanoparticles show better PV performance than that of commercially available P25 TiO2 in DSC applications. The high performance is evident from Jscvs. V and IPCE traces, and the EIS data. We believe that the high-performance MSM TiO2 will have wide applications in other fields such as photocatalysis and self-cleaning materials. The simple and versatile MSM can also be extended for the fabrication of other metal oxides, such as ZnO and SnO2, with wide ranging applications.
Acknowledgements
We thank the Clean Energy Program Office (CEPO) of National Research Foundation (NRF, Grant No. NRF 2007 EWT-CERP 01-0531) and M3TC (Economic Development Board, grant No. R-261-501-018-414), Singapore for financial assistance.References
- S. Horikoshi and H. Hidaka, Environ. Sci. Technol., 2002, 36, 1357 CrossRef CAS.
- B. O'regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- P. Zhu, Y. Wu, M. V. Reddy, A. S. Nair, B. V. R. Chowdari and S. Ramakrishna, RSC Adv., 2012, 2, 531 RSC.
- A. M. Ruiz, G. Sakai, A. Cornet, K. Shimanoe, J. R. Morante and N. Yamazoe, Sens. Actuators, B, 2003, 93, 509 CrossRef.
- S. Kanehira, S. Kirihara and Y. Miyamoto, J. Am. Ceram. Soc., 2005, 88, 1461 CrossRef CAS.
- A. Bozzi, T. Yuranova and J. Kiwi, J. Photochem. Photobiol., A, 2005, 172, 27 CrossRef CAS.
- L. M. GonÇalves, V. de Zea Bermudez, H. A. Ribeiro and A. M. Mendes, Energy Environ. Sci., 2008, 1, 655 Search PubMed.
- P. Zhu, A. S. Nair, S. Yang, S. Peng and S. Ramakrishna, J. Mater. Chem., 2011, 21, 12210 RSC.
- G. K. Mor, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, Nano Lett., 2006, 6, 215 CrossRef CAS.
- (a) A. S. Nair, Y. Shengyuan, Z. Peining and S. Ramakrishna, Chem. Commun., 2010, 46, 7421 RSC; (b) Y. Shengyuan, Z. Peining, A. S. Nair and S. Ramakrishna, J. Mater. Chem., 2011, 21, 6541 RSC.
- F. Sauvage, D. Chen, P. Comte, F. Huang, L. P. Heiniger, Y. B. Cheng, R. A. Caruso and M. Grätzel, ACS Nano, 2010, 4, 4420 CrossRef CAS.
- W. J. Zhou, H. Liu, R. I. Boughton, G. J. Du, J. J. Lin, J. Y. Wang and D. Liu, J. Mater. Chem., 2010, 20, 5993 RSC.
- C. Y. Chen, M. Wang, J. Y. Li, N. Pootrakulchote, L. Alibabaei, C. H. Ngoc-Le, J. D. Decoppet, J. H. Tsai, C. Grätzel and C. G. Wu, ACS Nano, 2009, 3, 3103 CrossRef CAS.
- K. S. Tan, M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, J. Power Sources, 2005, 147, 241 CrossRef CAS.
- M. V. Reddy, K. Kenrick, T. Y. Wei, G. Y. Chong, G. H. Leong and B. V. R. Chowdari, J. Electrochem. Soc., 2011, 158, A1423 CrossRef CAS.
- M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, J. Power Sources, 2006, 159, 263 CrossRef CAS.
- S. Peng, Y. Wu, P. Zhu, V. Thavasi, S. Ramakrishna and S. G. Mhaisalkar, J. Mater. Chem., 2011, 21, 15718 RSC.
- A. J. Studer, M. E. Hagen and T. J. Noakes, Phys. B, 2006, 385–386, 1013 CrossRef CAS.
- M. V. Reddy, R. Jose, T. H. Teng, B. V. R. Chowdari and S. Ramakrishna, Electrochim. Acta, 2010, 55, 3109 CrossRef CAS.
- A. Wahl and J. Augustynski, J. Phys. Chem. B, 1998, 102, 7820 CrossRef CAS.
- C. Di Valentin, G. Pacchioni and A. Selloni, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 085116 CrossRef.
- C. J. Barbe, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Grätzel, J. Am. Ceram. Soc., 1997, 80, 3157 CrossRef CAS.
- K. Parmar, E. Ramasamy, J. Lee and J. S. Lee, Chem. Commun., 2011, 47, 8572 RSC.
- S. Hore, P. Nitz, C. Vetter, C. Prahl, M. Niggemann and R. Kern, Chem. Commun., 2005, 2011 RSC.
- C. P. Hsu, K. M. Lee, J. T. W. Huang, C. Y. Lin, C. H. Lee, L. P. Wang, S. Y. Tsai and K. C. Ho, Electrochim. Acta, 2008, 53, 7514 CrossRef CAS.
- D. Chen, F. Huang, Y. B. Cheng and R. A. Caruso, Adv. Mater., 2009, 21, 2206 CrossRef CAS.
- H. J. Koo, Y. J. Kim, Y. H. Lee, W. I. Lee, K. Kim and N. G. Park, Adv. Mater., 2008, 20, 195 CrossRef CAS.
- H.-E. Wang, L.-X. Zheng, C.-P. Liu, Y.-K. Liu, C.-Y. Luan, H. Cheng, Y. Y. Li, L. Martinu, J. A. Zapien and I. Bello, J. Phys. Chem. C, 2011, 115, 10419 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Details of the synthesis and characterization of the MSM TiO2 nanoparticles, powder XRD data, SEM and UV-Vis comparison of MSM and P25 TiO2 electrodes and current density–voltage trace of P25 DSC with a scattering layer. See DOI: 10.1039/c2ra00041e/ |
| This journal is © The Royal Society of Chemistry 2012 |
