An investigation of heating rate effects on particle size and concentration: instruction for scale-up†
Ling
Zhang
a,
Hiroyuki
Nakamura
*a,
Chan-Gi
Lee
a and
Hideaki
Maeda
*abc
aMeasurement Solution Research Center, Micro-Space Chemistry Solution Team, AIST, 807-1, Shuku, Tosu, Saga, Japan. E-mail: nakamura-hiroyuki@aist.go.jp; Fax: +81-942-81-3657; Tel: +81-942-81-3650
bDepartment of Molecular and Material Sciences, Interdisciplinary Graduate School of Engineering Sciences, Kyushu University, 6-1 Kasuga-kouen, Kasuga, Fukuoka, Japan
cCREST, Japan Science and Technology Agency, 4-1-8, Hon-cho, Kawaguchi, Japan. E-mail: maeda-h@aist.go.jp; Fax: +81-942-81-3657; Tel: +81-942-81-4675
First published on 2nd March 2012
Abstract
Increasing the synthesis scale is one of the most important issues in nanocrystal synthesis. The main difference between small and large reactors is the thermal transfer rate, which has been reported to have great effects on particle nucleation and growth. According to this viewpoint, the heating rate effects should be studied before designing the scale-up process. In this paper, CdSe quantum dots synthesis was used as a model to investigate the heating rate effects in a microreactor system capable of precisely controlling the temperature and heating rate. The results showed a high dependence of heating rate effects on synthesis parameters. The effects of nucleation and growth kinetics were also investigated. Test experiments to demonstrate the possibility of a scale-up were conducted and results showed that products synthesized by a batch reactor were comparable with microreactor products. Further investigation indicated that heating rate could affect the growth kinetics and subsequently affect the final products at 10% and 20% DDA. Based on these results, the application of a large reactor for large scale synthesis can be considered possible, otherwise a larger reactor cannot be applied and precise heating rate controlling is deemed necessary.
Introduction
Semiconductor quantum dots (QDs) are of great interest for both fundamental research and industrial applications, such as biomedical tags, light emitting diodes, laser and solar cells, due to their size-tunable optical properties.1–3 So far, many processes for QDs syntheses have been developed in the laboratory and QDs with unique properties have been prepared.4–9 A well-controlled synthesis process is a prerequisite to maintaining size uniformity of the formed QDs because of the high size dependence of optical properties. Nucleation and growth kinetics of QDs are the key factors in controlling size uniformity. Unfortunately, full understanding of the nucleation and growth process of a nanocrystal is still a big challenge due to the lack of efficient methods to quantitatively determine the size and concentration of the formed nanocrystal at early stage.One of the most applied theories is the classic nucleation theory (CNT), which predicts that a higher monomer concentration can reduce the critical nucleus size, generate a higher nucleus concentration and narrow the size distribution.10 As a result, rapid heating can achieve a burst nucleation, which will be propitious to give a higher nucleus concentration and narrower size distribution. According to this viewpoint, hot-injection methods can be developed by the injection of a room-temperature precursor into a vigorously-stirred, hot, bulk liquid to achieve the burst nucleation. Unfortunately, these techniques cannot supply the quantities of nanocrystals that will be required for any widespread, practical application.11 In addition, the particle growth kinetics are not always reproducible and attempts to eliminate concentration and temperature gradients within the reaction vessel during the synthesis should be reduced.12 In order to avoid this problem, the microreactor has emerged as an alternative for preparing high-quality QDs due to its enhanced heat and mass transfer, precise and independent control of operating parameters.13–17
Microreactors are normally operated at relatively small flow rates. Although QDs are usually used in small amounts, large scale production is needed in order to meet growing demand for QDs. It has been reported that scaling up of the microreactor synthesis could be achieved by simply increasing the number of capillaries or microchannels.18–21 Although scale-up of microreactors has been realized in some cases, these strategies are still not free from problems, such as difficulty in multi-channel device fabrication and thermal and mass transfer. On the other hand, larger scale reactors (e.g. tube reactors, batch reactors etc.), can be highly efficient candidates for scale-up if they can produce the same products as those produced by microreactors or small reactors. If one considers scale-up of nanocrystal synthesis by large-scale reactors, the difference between large reactors and microreactors should be taken into account, for example, specific surface area, mass and thermal transfer rate, etc. According to the CNT, the nucleation stage has great effects on particle size and size distribution. Consequently, the heating rate has to be controlled during the synthesis process. Recently, a number of studies have investigated the relationship between the chemical kinetics and particle growth kinetics.22–31 Results showed that the nanocrystal formation rate was limited by chemical reaction kinetics22,31 or precursor conversion kinetics8,27,30 during semiconductor nanocrystal synthesis. Here, we thought that this difference could be attributed to the different synthesis parameters and the heating rate effects were highly dependent on synthesis parameters.32 For the purpose of scale-up of the synthesis of QDs using optimized synthesis parameters, which were optimized by the microreactor,33,34 the effects of heating rate should be systematically studied. In the case of the non-existence of heating rate effects, increasing the synthesis scale can be easily realized by directly utilizing large reactors. On the other hand, if the effects of heating rate exist, results on the study of heating rate effects can give researchers important hints in designing the scale-up process.
In this study, using a microreactor system capable of precisely controlling the temperature and heating rate, the heating rate effects were studied with different synthesis parameters. Experiments investigating the scale-up of QD synthesis using a batch reactor were conducted and corresponding results showed that the data on the effects of heating rate, which were obtained by the microreactor setup, have high reliability.
Materials and methods
Materials
Reagent grade Cd(CH3COO)2·2H2O (Sigma-Aldrich Co., Ltd.), Oleic Acid (Wako Pure Chemical Industries, Ltd.), 1-octadecene (ODE; Wako Pure Chemical Industries, Ltd.), Se powder (Soekawa Chemical Co., Ltd.), trioctylphosphine (TOP; Tokyo Chemical Industry Co., Ltd.), and dodecylamine (DDA; Sigma-Aldrich Corp.) were used. Oleic Acid, ODE and DDA were used after vacuum distillation under an argon atmosphere, while other reagents were used as purchased.Methods
The experimental setup is illustrated in Fig. 1. The raw material solution was supplied by a syringe and a syringe pump. The heating section is divided into two parts: the heating part and the aging part. The heating part is a temperature gradient zone which was created by a long copper plate and a plate heater. The end point of the copper plate was placed on the plate heater and then the temperature gradient was formed from the start point to the end point of the copper plate. The aging part is a plate heater on which the temperature is kept constant and homogeneous. The micro-capillary was fitted on the surface of the heater as a reactor. The heating rate was controlled by the variation of flowing rate or temperature gradient length (Supporting Information†). The reaction time was controlled by the variation of capillary length. The raw material preparation methods applied in this study were the same as those reported by Toyota et al.11 Several reaction conditions were selected from Toyota's results. At the beginning of the experiments, three raw material solutions were pre-mixed and loaded into a 10 ml syringe. The UV-vis absorption spectra of the products were measured by online UV-vis spectrometer. The collected products were diluted with toluene for measurement of the UV-vis absorption spectra (UV-570; Jasco Corp., Japan) and PL emission spectra (FP-6600; Jasco Corp., Japan). Online UV-vis measurements were done by a spectrometer (QE65000; Ocean Optics Inc., USA) combined with a Teflon cell that was connected to the capillary outlet. The average particle diameter and particle concentration were estimated by the method that was introduced by Mulvaney et al.23 The particle size distribution was described by the half width at half maximum (HWHM) of the first absorption peak (UV-vis absorption spectrum) and full width at half maximum (FWHM) of the PL peak.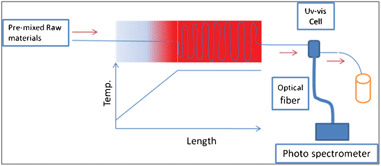 | ||
| Fig. 1 An illustration of the experimental setup. | ||
Results
Additives (DDA, oleic acid etc.) play multiple roles in nanoparticle synthesis; for example, as stabilizing reagents for monomers, capping reagents for small clusters and nanoparticles, coordination reagents for reactants etc. In particular, they have great effects on particle nucleation and growth kinetics.35–38 Nanocrystals with characteristic particle size and morphologies have been prepared by varying the additive type and concentration. In current study, the nucleation and particle growth kinetics under different additive concentrations were determined. The results are shown in Fig. 2. DDA concentration had great effects on the particle size of the final products. The particle diameter development shows that the equilibrium size of the final particle is smaller (4.1 nm) with 2% DDA and a larger particle size (5.6 nm) was produced with a higher DDA concentration. Fig. 2 shows the development of particle concentration during the growth process. The particle concentration increased rapidly in the case of 2% DDA, whereas an almost constant concentration was found for 20% DDA. These results show the obvious effects of DDA on the nucleation and growth of CdSe QDs and demonstrate the preference for low DDA concentration in order to approach the shorter wavelength of the PL (597 nm) and the dependence on high DDA concentration for longer PL wavelength (641 nm). | ||
| Fig. 2 The effects of DDA concentration on particle nucleation and growth rate (280 °C). | ||
The heating rate of the microreactor could reach to as high as c.a. 1000 °C s−1 (direct heating). In the case of the larger reactor, the heating rate was much slower. In a temperature-triggered synthesis process, rapid heating is said to favor rapid nucleation, which could reduce the critical nucleus size, generate a higher nucleus concentration and narrow the size distribution, as described by CNT. Therefore, a study of the heating rate effects on the particle size and size distribution is crucial in designing the scale-up process. In order to quantitatively investigate its effects on products, the heating rate during the synthesis process was precisely controlled and varied from 15 °C s−1 to 0.83 °C s−1. We investigated the heating rate dependency of particle concentration and size distribution based on the reported great effects of nucleation and growth kinetics on particle concentration and size distribution. As shown in Fig. 3, the heating rate effects were highly related to the additive concentration. When the DDA concentration was 2%, the products obtained at a slower heating rate, i.e. 0.83 °C s−1, showed a lower particle concentration and slightly narrower size distribution. In the case of 5% DDA, there was almost no difference in the particle number and size distribution of both 0.83 °C s−1 and 1000 °C s−1 heating rate products. This high consistency showed that the heating rate at this range had no effect on the final product, which indicated that heating rate didn't affect the nucleation and growth process with 5% DDA, even below 1000 times difference of heating rate. However, in the case of 10% DDA, although the particle concentration almost did not change when the heating rate was varied, the size distribution, which was described by HWHM of the UV-vis absorption spectrum, increased upon a decrease of the heating rate. When the DDA concentration was increased to 20%, both the particle concentration and size distribution were different, while the heating rate became slower than 15 °C s−1.
 | ||
| Fig. 3 A study on the effects of heating rate with different DDA concentrations. A) 2% DDA; B) 5% DDA; C) 10% DDA; D) 20% DDA. | ||
These results are consistent with our expectation that the effects of heating rate vary with synthesis parameters. As described above, the main difference between small and large scale reactors lies in the heating rate due to thermal transfer limitation. In a temperature-triggered reaction similar to the current case, in the absence of heating rate effects (for example, the 5% DDA case), small scale methods should be directly applied for larger scale synthesis by using larger reactors; otherwise, particular equipment is needed to control the heating rate to preserve reproducibility (for example, the 20% DDA case). In order to demonstrate the idea, scale-up experiments were conducted. A batch reaction system was employed to test the potential for scale-up. A 100 ml pre-mixed raw material solution was reacted in a 200 ml two-neck flask. After a given reaction time, serial aliquots were taken out and then measured by an offline UV-vis absorption photospectrometer and fluorescence spectrophotometer to detect the nucleating and growing process of the products. The quantum yield of the products, which is one of the most important properties of a fluorescence material, was taken into account to fully judge the applicability of larger reactors.
As shown in Fig. 4, different DDA concentrations were applied and batch reaction data fits perfectly with results on the study of heating rate obtained from the microreactor setup. When the DDA concentration was 2%, the batch reaction products showed a lower particle concentration and slightly narrower size distribution compared with the quickly heated products of the microreactor. These results show the same tendency as the results of the study on heating rate effects shown in Fig. 3B. In the case of 5% DDA, no heating rate effect was observed in the microreactor setup when the heating rate was varied. The batch reaction products showed the same nucleation and growth process as the microreactor products. Both products from the microreactor and the batch reactor had the same development tendency, and the particle concentration–particle size and size distribution–particle size curves almost superimposed. In addition, the products prepared by different reactors had almost the same quantum yields. The same products have been obtained by one-pot batch reactor. On the other hand, as shown in Fig. 4C and D, the 100 ml batch gave different products compared with the microreactor when the DDA concentration was 10% and 20%. As predicted by the results of the study of heating rate effects, the heating rate showed great effects on particle concentration and size distribution. In the case of 10% DDA, batch reaction products had a wider size distribution compared with the directly heated products of the microreactor. Although the final product had almost the same quantum yield, the products from the batch reactor had a wider FWHM. When the DDA concentration was 20%, the batch reactor gave a higher particle concentration of the products. In addition, the quantum yields of products from the batch reactor were much lower than that of products from the microreactor. The STEM images showed that products from the microreactor had a spherical shape with narrow size distribution, while those produced by the batch reactor had folded rod-like and tetrapod morphologies, as shown in Fig. 5. These results indicate that heating rate is quite important in the case of 20% DDA, which was predicted by the results of the study on heating rate effects.
 | ||
| Fig. 4 Particle concentration, FWHM of PL peak and quantum yield of 100 ml batch reactor products and microreactor products: A) 2% DDA; B) 5% DDA; C) 10% DDA; D) 20% DDA. (MRQH: particles synthesized by microreactor with quick heating (1000 °C s−1); 100 ml batch: particles synthesized by 200 ml batch reactor). | ||
 | ||
| Fig. 5 STEM images of final products under different conditions: A) microreactor, 5% DDA; B) 100 ml batch reactor, 5% DDA; C) microreactor, 20% DDA; D) 100 ml batch reactor, 20% DDA. | ||
Discussion
As described above, several models about the formation mechanism of nanocrystals in solution have been proposed. In this study, the heating rate showed different effects on the products when the synthesis parameters were different, which was not consistent with the nucleation limited mechanism. According to Xie's and Owen's viewpoints, the chemical reaction kinetics31 or precursor conversion kinetics30 limited the nanocrystal generation rate. We investigated the heating rate effects on the yield-diameter curve and the results are shown in Fig. 6. The precursor conversion yield was calculated from the absorbance at 350 nm using the size-independent extinction coefficient published by Leatherdale.39 | ||
| Fig. 6 Heating rate effects on the precursor conversion rate and particle growth rate. A) 2% DDA; B) 5% DDA; C) 10% DDA; D) 20% DDA. | ||
As shown in Fig. 2, particle concentration almost didn't change at the early stage, even though the final particle concentration was quite different at different DDA concentrations. This phenomenon could be explained by the ultra-rapid nucleation rate,31 therefore, it is unlikely that the first nucleation stage played a significant role in the formation of the nanocrystal. As described by the precursor conversion limited mechanism, the precursor slowly releases monomer or solute, which is consumed by particle growth or accumulated in the solution until a “critical” concentration is reached and nucleation begins.30 If the particle growth rate is high enough to consume the newly generated monomer or solute, the particle concentration should be kept constant (in the case of 20% DDA) or decrease because of Ostwald Ripening. Otherwise, secondary nucleation would occur and the particle concentration would increase (as can be seen in the case of 2%, 5% and 10% DDA). These results clearly indicated that a chemical reaction kinetics31 or precursor conversion kinetics30 limited growth mechanism occurred in this study.
The development tendency of precursor conversion yields and particle diameters during the synthesis process indicates the precursor conversion kinetics and particle growth kinetics, respectively. As shown in Fig. 6, in the case of 2% and 5% DDA, no obvious variation in yield–diameter curves was observed when different heating rates were applied. This showed that heating rate had consistent effects on the precursor conversion kinetics and particle growth kinetics in this case; as a result, the products were the same, although the heating rate was quite different. When the DDA concentration was 10% and 20%, a slower heating rate decreased the particle growth rate more than precursor conversion rate. Therefore, a slower heating rate gave products with a smaller particle size, wider size distribution and/or higher particle concentration when the precursor conversion yield was the same, compared with the products obtained by rapid heating. Based on the above data, we supposed that the effects of heating rate on the products can be attributed to the heating rate effects on the precursor conversion kinetics and particle growth kinetics. If the heating rate has consistent effects on both precursor conversion kinetics and particle growth kinetics, the product would not be affected by the heating rate. This model implied that controlled synthesis of colloidal nanocrystals is closely related to identifying the precursor conversion kinetics and particle growth kinetics. In order to gain full understanding of these kinetics to predict the heating rate effects, our heating rate investigation setup would be useful for both investigating the effects of heating rate and finding the proper direction for designing the scale-up process.
On the other hand, it has been reported that particle morphology could be varied under different growth kinetics. With proper growth kinetics, anisotropic growth could be observed.10,40,41 When the DDA concentration was 10% and 20%, heating rate had great effects on the morphologies of the products. As shown in Fig. 7, only spherical particles were obtained in the case of rapid heating (microreactor), which indicates that isotropic growth has taken place. When the heating rate was slow (batch reactor), tetrapod-like and folded rod-like products were obtained. These results showed that slow growth kinetics were in favor of anisotropic growth kinetics in the case of 10% and 20% DDA. While in the case of 2% and 5% DDA, only spherical particles were obtained, even with different heating rates, which indicate that growth kinetics has no effect on the particle morphologies. Comparing the microreactor with the batch reactor, another big difference is the specific surface area of the batch reactor, with the exception of heating rate. The larger specific surface area might have easily resulted from the heterogeneous nucleation and affected the particle growth. In order to distinguish the effects of specific surface area from the effects of heating rate, a similar heating curve with the batch reactor was applied to the microreactor. With the similar heating curve, both the products from the microreactor and the batch reactor have similar morphologies and the particles had similar growth tendency (Supporting Information Fig. S1†). Based on this result, the reason for the difference caused by the batch reactor and the microreactor could be particularly attributed to the different heating rates in the case of 20% DDA.
 | ||
| Fig. 7 Heating rate effects on the particle morphologies (20% DDA): A) microreactor 30 s; B) microreactor 10 min; C) batch reactor 4 min; D) batch reactor 12 min. | ||
Conclusion
In conclusion, a microreactor setup was designed to investigate the effects of heating rate on the particle concentration and size distribution in order to find the right direction for scale-up. By using this setup, temperature and heating rate could be precisely controlled and the effects of heating rate could be efficiently investigated. According to the results of our study on the effects of heating rate, one could find the right direction for large scale synthesis. Taking CdSe synthesis as a model, an appropriate instruction for scale-up was found based on the results on the effects of heating rate i.e., a batch reactor is suitable with 2% and 5% DDA, but the micro-fluidic reactor which can achieve a high heating rate is necessary under conditions of 10% and 20% DDA. Further study indicates that the CdSe generation rate is determined by the chemical reaction kinetics or precursor conversion kinetics in this reaction system. A study on the effects of heating rate can give more choices of reactors for large scale synthesis. For example, in the case of the absence of heating rate effects, larger scale reactors can be directly applied for scale-up. This heating rate control setup can be useful not only in designing the scale-up process, but also in the study of nucleation and growth process of particles in nanocrystal synthesis. By combining the study on the effects of heating rate with the combinatorial synthesis system,33 we can find the optimum synthesis condition for practical application and achieve the proper methods for large scale synthesis. Application of this system can greatly reduce the efforts needed to bring laboratory methods to industry.Acknowledgements
Financial support from Japan Science and Technology Agency (JST), CREST is gratefully acknowledged.References
- S. M. Reimann and M. Manninen, Rev. Mod. Phys., 2002, 74, 1283–1342 CrossRef CAS.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS.
- R. S. Dibbell, D. G. Youker and D. F. Watson, J. Phys. Chem. C, 2009, 113, 18643–18651 CAS.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- H. Nakamura, Y. Yamaguchi, M. Miyazaki, H. Maeda, M. Uehara and P. Mulvaney, Chem. Commun., 2002,(23), 2844–2845 RSC.
- E. M. Chan, A. P. Alivisatos and R. A. Mathies, J. Am. Chem. Soc., 2005, 127, 13854–13861 CrossRef CAS.
- D. W. Lucey, D. J. MacRae, M. Furis, Y. Sahoo, A. N. Cartwright and P. N. Prasad, Chem. Mater., 2005, 17, 3754–3762 CrossRef CAS.
- G. Zlateva, Z. Zhelev, R. Bakalova and I. Kanno, Inorg. Chem., 2007, 46, 6212–6214 CrossRef CAS.
- H. W. Yang, W. L. Luan, Z. Wan, S. T. Tu, W. K. Yuan and Z. M. Wang, Cryst. Growth Des., 2009, 9, 4807–4813 CAS.
- Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664–670 CrossRef CAS.
- Y. Song, J. Hormes and C. S. S. R. Kumar, Small, 2008, 4, 698–711 CrossRef CAS.
- S. Marrea and K. F. Jensenb, Chem. Soc. Rev., 2010, 39, 1183–1201 RSC.
- A. M. Nightingale and J. C. D. Mello, J. Mater. Chem., 2010, 20, 8454–8463 RSC.
- E. M. Chan, a. P. Alivisatos and R. a. Mathies, J. Am. Chem. Soc., 2005, 127, 13854–13861 CrossRef CAS.
- S. Marre, J. Park, J. Rempel, J. Guan, M. G. Bawendi and K. F. Jensen, Adv. Mater., 2008, 20, 4830–4834 CrossRef CAS.
- S. Krishnadasan, J. Tovilla, R. Vilar, A. J. deMello and J. C. deMello, J. Mater. Chem., 2004, 14, 2655–2660 RSC.
- S. Krishnadasan, R. J. C. Brown, A. J. Demello and J. C. Demello, Lab Chip, 2007, 7, 1434–1441 RSC.
- U. H. Felcht, Chem. Eng. Technol., 2002, 25, 345–355 CrossRef CAS.
- M. Freemantle, Chem. Eng. News, 2003, 81, 36–37 Search PubMed.
- R. Schenk, V. Hessel, C. Hofmann, J. Kiss, H. Lowe and A. Ziogas, Chem. Eng. J., 2004, 101, 421–429 CrossRef CAS.
- H. D. Jin, A. Garrison, T. Tseng, B. K. Paul and C. H. Chang, Nanotechnology, 2010, 21, 445604 CrossRef.
- C. M. Evans, M. E. Evans and T. D. Krauss, J. Am. Chem. Soc., 2010, 132, 10973–10975 CrossRef CAS.
- J. S. Steckel, B. K. H. Yen, D. C. Oertel and M. G. Bawendi, J. Am. Chem. Soc., 2006, 128, 13032–13033 CrossRef CAS.
- H. T. Liu, J. S. Owen and A. P. Alivisatos, J. Am. Chem. Soc., 2007, 129, 305–312 CrossRef CAS.
- Z. J. Jiang and D. F. Kelley, ACS Nano, 2010, 4, 1561–1572 CrossRef CAS.
- J. Joo, J. M. Pietryga, J. A. McGuire, S. H. Jeon, D. J. Williams, H. L. Wang and V. I. Klimov, J. Am. Chem. Soc., 2009, 131, 10620–10628 CrossRef CAS.
- V. Kloper, R. Osovsky, J. Kolny-Olesiak, A. Sashchiuk and E. Lifshitz, J. Phys. Chem. C, 2007, 111, 10336–10341 CAS.
- S. G. Kwon and T. Hyeon, Small, 2011, 7, 2685–2702 CrossRef CAS.
- R. Garcia-Rodriguez and H. Liu, J. Am. Chem. Soc., 2012, 134, 1400–1403 CrossRef CAS.
- J. S. Owen, E. M. Chan, H. T. Liu and A. P. Alivisatos, J. Am. Chem. Soc., 2010, 132, 18206–18213 CrossRef CAS.
- R. Xie, Z. Li and X. Peng, J. Am. Chem. Soc., 2009, 131, 15457–15466 CrossRef CAS.
- J. S. Han, X. T. Luo, D. Zhou, H. Z. Sun, H. Zhang and B. Yang, J. Phys. Chem. C, 2010, 114, 6418–6425 CAS.
- A. Toyota, H. Nakamura, H. Ozono, K. Yamashita, M. Uehara and H. Maeda, J. Phys. Chem. C, 2010, 114, 7527–7534 CAS.
- S. Krishnadasan, R. J. C. Brown, a. J. DeMello and J. C. DeMello, Lab Chip, 2007, 7, 1434–1441 RSC.
- Z. H. Sun, H. Oyanagi, H. Nakamura, Y. Jiang, L. Zhang, M. Uehara, K. Yamashita, A. Fukano and H. Maeda, J. Phys. Chem. C, 2010, 114, 10126–10131 CAS.
- N. Pradhan, D. Reifsnyder, R. G. Xie, J. Aldana and X. G. Peng, J. Am. Chem. Soc., 2007, 129, 9500–9509 CrossRef CAS.
- J. van Embden and P. Mulvaney, Langmuir, 2005, 21, 10226–10233 CrossRef CAS.
- C. R. Bullen and P. Mulvaney, Nano Lett., 2004, 4, 2303–2307 CrossRef CAS.
- C. a. Leatherdale, W.-K. Woo, F. V. Mikulec and M. G. Bawendi, J. Phys. Chem. B, 2002, 106, 7619–7622 CrossRef CAS.
- L. P. Liu, Z. B. Zhuang, T. Xie, Y. G. Wang, J. Li, Q. Peng and Y. D. Li, J. Am. Chem. Soc., 2009, 131, 16423–16429 CrossRef CAS.
- C. G. Lee, H. Nakamura, M. Uehara and H. Maeda, Chem. Lett., (in preparation) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c2ra01232d |
| This journal is © The Royal Society of Chemistry 2012 |
