Silver nanoparticles in a polyether-block-polyamide copolymer towards antimicrobial and antifouling membranes
Grace M.
Nisola
,
Joon Soek
Park
,
Arnel B.
Beltran
and
Wook-Jin
Chung
*
Energy and Environment Fusion Technology Center (E 2FTC), Department of Environmental Engineering and Biotechnology, Myongji University, Yongin, South Korea. E-mail: wjc0828@gmail.com (W.-J. Chung); Fax: +82-31-337-2902; Tel: +82-31-330-6687
First published on 2nd February 2012
Abstract
The potential of hydrophilic polyether-block-polyamide copolymer (PEBA) with antimicrobial silver nanoparticles (nano-Ag) to alleviate membrane biofouling was investigated. PEBA solutions of different nano-Ag content were prepared as dense films and as coating materials for ultrafiltration polysulfone (PSf) membranes. Disc diffusion and surface contact tests revealed the capability of the PEBA/nano-Ag films to inhibit the growth of Escherichia coli (E. coli). Contact angle measurements confirmed the hydrophilisation of the PSf surface after coating with PEBA. Field emission scanning electron microscopy, atomic force microscopy (AFM) and fourier transform infrared spectroscopy were performed to confirm the surface modification of PSf. As a proof-of-concept, filtration performances of bare PSf, PEBA coated-and PEBA/nano-Ag coated PSf were compared using a simulated solution inoculated with E. coli as the feed. The results revealed that the hydrophilisation of PSf by coating with PEBA improved the fouling resistance of the membrane as indicated by the retarded flux reduction rate and higher flux recovery. However, PSf exhibited the highest antifouling resistance when coated with PEBA/nano-Ag. The AFM images of used membranes showed that PEBA/nano-Ag minimized the attachment and growth of E. coli on the membrane which abated irreversible biofouling, a problem that was most severe on bare PSf.
1. Introduction
With tight wastewater discharge regulations, membrane filtration is one of the very few reliable technologies available that can produce effluent water with superior quality.1–4 However, the gradual water flux decline during operation often limits the performance of this process.5–7 This predicament is caused by membrane fouling which arises from the mutual interactions between the membrane and various components present in water.8,9 Special attention is given to biofouling, which occurs due to the adhesion, deposition and accumulation of microbiological matter on the membrane surface.10 Under prolonged operation, the irreversible formation of stable biofilm networks contributes to the membrane resistance which becomes a challenge for maintaining the filtration efficiency.Typical biofoulants, like suspended microorganisms, extra-cellular polymeric substances and proteins, are hydrophobic, and so are most of the commercially available filtration membranes.9 As a surface phenomenon, biofouling can be alleviated by weakening the hydrophobic–hydrophobic interactions between the biofoulants and the membrane surface.9,10 One way to achieve this is through membrane surface hydrophilisation.11–15
Among the hydrophilisation techniques available, coating a hydrophilic material on a commercial membrane is a convenient strategy for faster and wider application.
Polyether-block-polyamide copolymer (PEBA) is a thermoplastic elastomer with tunable hydrophilic character and good film-forming ability.16 Most investigations on PEBA are related to gas separation and pervaporation while very few studies have employed this polymeric material for membrane filtration.17,18 High polyether containing (50–60%) PEBA has been used in previous studies as a nonporous water-permeable coating on polyvinylidene fluoride (PVDF) membranes.11,16Filtration of oil-in-water emulsions revealed that fouling was successfully minimized in PEBA coated PVDF membranes. As an antifoulant, PEBA reduced the adsorption of foulants on the surface and restricted foulant access to the membrane pores.11 For the purification of water with biological components, it is anticipated that PEBA can be an effective membrane coating material to minimize biofouling.
Additionally, accumulation and formation of biofilms can be alleviated if the coating material possesses an antimicrobial property. Aside from improving the microbiological quality of water, microbial growth suppression in membrane systems could lower the incidence of biofouling.
Silver (Ag) is one of the well-studied materials with broad-spectrum biocidal activity towards various types of microorganisms while exhibiting lower toxicity to mammalian cells.19,20 Generally as nanoparticles, nano-Ag has been widely tested for disinfection, longevity of biomaterials and medical applications.21–23 Previous studies have successfully incorporated nano-Ag into various solid supports like paper, capsules, mats, nanofibers and membranes.21,24–26 Particularly for the development of antimicrobial membranes, nano-Ag has been immobilized in cellulose, polyethersulfone and PVDF by physical entrapment like dispersion and blending or by in situ formation which involves Ag+ capture followed by reduction to nano-Ag.27–29
So far as is known, the combination of hydrophilic PEBA and nano-Ag as a composite material with augmented anti-biofouling property has not been reported.
In this study, the feasibility of combined PEBA and nano-Ag to alleviate membrane biofouling was investigated. The antimicrobial properties of PEBA and PEBA/nano-Ag were elucidated through disc diffusion and membrane surface contact tests using Escherichia coli (E. coli) as the test microorganism. The applicability of PEBA and PEBA nano-Ag as coating materials was determined by modifying a commercial ultrafiltration (UF) polysulfone (PSf) flat sheet membrane. A series of characterization techniques was carried out to confirm the surface modification of PSf. As a proof-of-concept, filtration experiments and membrane inspections were performed to observe the biofouling on the modified membranes. The flux reduction rate and flux recovery were investigated using a synthetic feed solution inoculated with E. colicells.
2. Materials and methods
2.1. Reagents
Hydrophilic PEBA resin (Pebax MH 1657, 60 wt% polyether block content, ρ = 1.14 g cm−3) was purchased from Arkema Technical Polymers (Colombes, France). Stock solution of nano-Ag (1000 mg in 1 L ethanol, ρ = 1.03 g mL−1, primary particle diameter = 5–10 nm) was supplied by Miji-Tech (Korea). The UFPSf membrane was kindly provided by the Kolon Company (Korea). The solvent n-butanol was acquired from Junsei Chemicals (Japan).2.2. Membrane preparation
Stock PEBA solution (1.25 wt%) was prepared by dissolving the resin in a 500 mL 3-neck round bottom flask using n-butanol as the solvent.11 The mixture was stirred constantly at 150 rpm while being heated under reflux at 80 °C for 24 h. After which, the solution was cooled, collected and stored in a glass bottle. Appropriate portions of stock nano-Ag solution were added in the polymer solution to attain various nano-Ag loadings with respect to PEBA (1.0 wt%).For PEBA and PEBA/nano-Ag film preparation, 10 mL solutions were poured into Teflon dishes, vacuum-dried at room temperature for 24 h and further cured at 60 °C for 2 h.
For composite membranes, pristine PSf membranes were soaked in water overnight prior to coating. After which, the membranes were fixed on glass plates and the surfaces were quickly dried using lint-free paper to remove excess water. With a sponge roller, the membranes were coated with the prepared PEBA solutions containing different amounts of nano-Ag. After which, the coated PSf membranes were vacuum-dried for 12 h at room temperature then baked for another 2 h at 60 °C. The last step facilitated PEBA cross-linking which resulted to the formation of a thin film layer on the surface of PSf.
2.3. Membrane characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 at 2 cm−1 resolution. Membrane morphology inspection and elemental composition analysis were performed at 15.0 kV acceleration voltage using field emission scanning electron microscopy coupled with energy dispersive spectroscopy (FE-SEM EDS, Jeol JSM-6700F). To investigate the occurrence of biofouling, atomic force microscopy (AFM; Autoprobe CP Research, Veeco, USA) was used to observe the surface topology of the membranes before and after filtration experiments. The AFM was operated in the non-contact mode to prevent damage on the membrane surface during analysis.30 The AFM samples were scanned within a 5 μm × 5 μm area at a 1 Hz scan rate. Contact angles of PEBA coated PSf membranes were measured via the sessile drop method.31
000 cm−1 at 2 cm−1 resolution. Membrane morphology inspection and elemental composition analysis were performed at 15.0 kV acceleration voltage using field emission scanning electron microscopy coupled with energy dispersive spectroscopy (FE-SEM EDS, Jeol JSM-6700F). To investigate the occurrence of biofouling, atomic force microscopy (AFM; Autoprobe CP Research, Veeco, USA) was used to observe the surface topology of the membranes before and after filtration experiments. The AFM was operated in the non-contact mode to prevent damage on the membrane surface during analysis.30 The AFM samples were scanned within a 5 μm × 5 μm area at a 1 Hz scan rate. Contact angles of PEBA coated PSf membranes were measured via the sessile drop method.31
2.4. Microbial cultivation
Escherichia coli (Migula) Castellani strain (ATCC 8739) was cultivated in Difco Becton (Dickinson & Co., France) nutrient broth (per 100 mL solution: 0.3 g beef extract, 0.5 g peptone) at 37 °C. For cell quantification, the viable cell number (as CFU/mL) was initially correlated with the optical density (OD) measured at 600 nm (OD600) using a Hewlett Packard UV-Vis spectrophotometer (HP8453E). The viable cell number measurement was performed as follows: samples with known OD600 were serially diluted, then aliquots were spread on nutrient agar plates. The inoculated plates were then incubated upside down at 37 °C for 24 h prior to CFU counting.2.5. Modified Kirby–Bauer disc diffusion test
The PEBA films were cut into circles (diameter = 20 mm) and were placed on nutrient agar freshly inoculated with 0.1 mL of 1 × 107 CFU mL−1E. colicells by the cotton swab method.32 The plates were incubated for 20 h at 37 °C and afterwards, zone of inhibition (ZoI) diameters were measured.332.6. Minimum inhibition concentration (MIC) assay
To estimate the minimum inhibition concentration (MIC) of silver on E. coli, minuscule amount (approx. 3 mg) of agar samples (agar density ρ = 0.87 g cm−3) in triplicate were picked at the edge of the ZoI using capillary glass tubes (diameter = 1 mm). The weighed samples were analyzed for Ag content using ICP-MS as described in Section 2.9. Another set of experiments was performed to determine the MIC using silver nitrate (AgNO3) as the silver source. In 9.8 mL nutrient broths with different AgNO3 concentrations, 0.2 mL of 5.13 × 108 CFU mL−1E. colicells were inoculated. Aliquots (0.25 mL) in triplicate (n= 3) were loaded in 96-honeycomb wells and the OD600 was measured for 20 h using Bioscreen C (Oy Growth Curves Ab Ltd., Finland). MIC was determined as the Ag+ concentration at which no E. coligrowth was observed (i.e. initial OD600 ≅ OD600 after 20 h).342.7. Antimicrobial membrane surface contact test
The antimicrobial effects of PEBA and PEBA/nano-Ag films on E. coli suspensions were investigated through a series of contact tests. Circular membranes (45 mm diameter) were fixed by O-rings at the bottom of a vessel (50 mL Amicon; Milipore Corp., USA). Each vessel contained 20 mL nutrient broth inoculated with 0.2 mL of 1 × 108 CFU mL−1 of E. colicells. The samples were incubated for 24 h at 37 °C. After incubation, the OD600 was measured. Inoculum concentrations (CFU mL−1) in samples with low OD600 values (<0.1) were estimated through viable cell counting as mentioned in Section 2.4. The antimicrobial properties of the membranes were reported in terms of growth suppression efficiency (%) expressed in eqn (1), where [CFU mL−1] is the E. coli inoculum concentration at the control (sample without membrane) and test (PEBA films with various nano-Ag contents) samples. After incubation, Ag+ concentrations in the broth were measured as described in Section 2.9. | (1) |
2.8. Filtration test
Three stainless steel flat sheet membrane cells in series were installed with feed and retentate pressure gauges to control the trans-membrane pressure. The feed temperature was controlled at 25 °C using a lab-assembled heat exchanger connected to a chiller. The water was fed using Hydra-Cell® pump (Model No. M-03-B, USA). Flux was measured using an analytical mass balance (GF-2000 AND, Japan) equipped with Microsoft Excel interface software.Membrane filtration cells and connections were cleaned prior to operation. The membranes (diameter = 58 mm) were initially compacted at 980 kPa (10 kg f cm−2) for 30 min. Deionized (DI) water was filtered for the next 30 min at 490 kPa (5 kg f cm−2) to measure the pure water flux of the membranes. This was followed by filtration of synthetic feed solution (composition: 0.8 g Difco Becton nutrient powder L−1 DI water, 1.16 mM sodium citrate, 0.94 mM ammonium chloride, 0.45 mM phosphate buffer, 0.5 mM CaCl2·2H2O, 2.0 mM NaCl and 0.6 mM MgSO4·7H2O) and after several hours, the feed was replaced with the same media but inoculated with 1 × 105 CFU mL−1 of suspended E. coli. The total organic carbon (TOC) content of the feed solution, which was analysed using a Shimadzu TOC-V CPH (Japan), was 238 mg TOC L−1. To observe discernable differences between the membranes, filtration was performed until the highest severity of biofouling was attained. The filtration performances of the membranes were recovered by flushing the system with DI water for 10 min. To measure the flux recovery, pure water flux (DI) was again measured after DI flushing.
Since PEBA is a high-water permeable nonporous film which forms on the PSf membrane,11,16 the relative flux reduction (rc) due to the coating layer was calculated using eqn (2). The initial pure water flux of the coated PSf membranes (Fc) was compared to that of the bare PSf (FPSf). Filtration results were presented in terms of normalized flux (NF) as shown in eqn (3), wherein the flux (Ft) was divided by the respective initial flux value. This is to provide better comparisons of the membrane performances despite the differences in their initial flux values.35 Total flux declines (rt) due to fouling were evaluated using eqn (4) to (6), which were classified as reversible (rrev) or irreversible (rirrev).15 In these equations, NFf and NFw are the normalized flux values of the fouled membrane and pure water flux after cleaning, respectively. Flux recovery (FR) was calculated according to eqn (7).
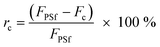 | (2) |
 | (3) |
| rt = (1 − NFf) × 100% | (4) |
| rrev = (NFw − NFf) × 100% | (5) |
| rirrev = (1 − NFw) × 100% | (6) |
| FR = NFw × 100% | (7) |
2.9. Silver analysis
The silver content was measured viaICP-MS (Agilent 7500 series, USA). Sample pre-treatment was performed by microwave acid digestion (MARS-5, CEM, USA) using 10 mL of heavy metal grade (30 wt%) nitric acid (Junsei Chemicals Co., Ltd., Japan). For total Ag content in PEBA, pre-weighed membrane samples in triplicate (1 cm2) were used. Note that the acquired Ag concentrations in all liquid samples refer to the released silvers ions (Ag+), which were determined based on previously reported methods with minor modifications.20,36 All liquid samples were centrifuged at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g (High speed Micro 17R+ Hanil, Korea) for 30 min and were passed through membrane syringe filters before acid pre-treatment and ICP-MS quantification.
000g (High speed Micro 17R+ Hanil, Korea) for 30 min and were passed through membrane syringe filters before acid pre-treatment and ICP-MS quantification.
3. Results and discussion
3.1. PEBA and PEBA/Nano-Ag characterization
The prepared PEBA films with various nano-Ag contents have densities between 0.93 and 0.98 g cm−3. From ICP-MS analysis, total Ag contents in the PEBA films were 0.62, 0.91, 1.38 and 1.95 wt% for nominal nano-Ag loadings of 0.5, 1.0, 1.5 and 2.0 wt%, respectively.3.2. Kirby–Bauer disc diffusion test
The results of disc diffusion tests for the bactericidal property of PEBA and PEBA/nano-Ag are shown in Fig. 1. The optical images in Fig. 1a show the conspicuous formation of ZoIs in all the PEBA/nano-Ag samples while none formed in plain PEBA. From this result, it is apparent that PEBA has no antimicrobial property and that the growth inhibitions observed in PEBA/nano-Ag films are solely attributable to nano-Ag. | ||
| Fig. 1 Antimicrobial test results of PEBA and PEBA/nano Ag by disc diffusion test (n = 5). | ||
While the antimicrobial ability of silver has been known since antiquity, its mode of action has not been completely understood. 36–40 Association of silver toxicity to its nanoparticle or ionic form (Ag+) has been a subject of interest in recent years.20,36 The results from disc diffusion test may provide a perspective on the bactericidal mechanism of nano-Ag in the PEBA film.
In Fig. 1(a), the formation of ZoI in all the PEBA/nano-Ag samples suggests that sufficient amounts of Ag were present to mediate the bactericidal effect on the E. colicells. Optical images reveal the increase in ZoI diameter as nano-Ag content is increased. The relationship between the loaded amount of nano-Ag (Q) in the films and ZoI was elucidated according to a diffusion model expressed in eqn (8), wherein A and C are empirical constants.32 In Fig. 1(b), the high correlation (r2 = 0.96) of ZoI square diameter (ZoI2) with log10Q indicates the diffusive nature of silver. This behaviour is consistent with the theory behind the Kirby–Bauer method wherein ZoIs are formed due to the presence of diffusing antimicrobial compounds through the agar media.41
| (ZoI)2 = AlogQ + C | (8) |
In a previous study, the penetration of gold nanoparticles into the agar was demonstrated.34 It was expected since suspended nanoparticles were directly injected into the constructed agar wells. However in this case, the nano-Ag in PEBA have restricted mobility. Thus considering that nano-Ag did not diffuse through the agar, it is most likely that halos were formed due to the elution of silver ions (Ag+) from the films.
When exposed to oxygen (i.e. in air), the nano-Ag surface is partially oxidized to form an oxide layer that can be a rich source of Ag+.20,42 This might have taken place during membrane preparation and aerobic incubation of agar plates. Previous studies demonstrated the bactericidal properties of partially oxidized nano-Ag and non-activity of zero-valent silver (Ag0).43,44 These works strongly indicated that the antimicrobial ability of silver is linked to its ionic derivative (Ag+) and not to its reduced form (Ag0).
3.3. Minimum inhibition concentration
In the Kirby–Bauer disc diffusion test, MIC pertains to the amount of silver below which no ZoI is formed around the disc. Theoretically, MIC represents the critical concentration at the edge of ZoI.33 In this study, a method was devised to measure MIC using the agar samples from disc diffusion test (see Section 2.6). In Fig. 1(b), MIC values (silver concentration at ZoI boundary) vary between 31 μM and 35 μM. It is noticeable that the values slightly increased with increasing nano-Ag content. This may be attributable to the differences in radial concentration gradients of the diffusing Ag+ in the agar at different nano-Ag contents.From the cultivation experiments of E. coli in nutrient broths containing AgNO3 as silver source, MIC = 37 μM was determined (Table 1). It is evident that the MIC values from the two methods are similar, which confirms the previous assumption that ZoI formation in the disc diffusion test is due to the diffusion of Ag+ through the agar. This also suggests that PEBA, with its hydrophilic property, had no difficulty in releasing Ag+ while confining the nano-Ags within its matrix.16
| Ag+ (μM) | OD600 | E. coli growth | |
|---|---|---|---|
| t = 0 | t = 20 h | ||
| 0 | 0.051 | 1.567 | + |
| 5 | 0.049 | 1.562 | + |
| 9 | 0.055 | 1.548 | + |
| 23 | 0.051 | 1.457 | + |
| 37 | 0.052 | 0.034 | − |
| 46 | 0.053 | 0.015 | − |
| 70 | 0.050 | 0.020 | − |
However, the drawback of using disc diffusion samples is the apparent dependency of MIC on the loaded amount of Ag source (i.e. nano-Ag) in the films. Thus, further research is needed to improve the reliability of this technique. Nonetheless, the devised technique can still be used to roughly estimate MIC values.
3.4. PEBA and PEBA/nano-Ag surface contact test
While disc diffusion test successfully demonstrated the antimicrobial property of PEBA/nano-Ag films, it is inadequate to describe the interaction between the membrane surface and microorganisms. Results of contact tests between the suspended E. colicells and PEBA/nano-Ag films are shown in Fig. 2. After 24 h incubation, the control sample (no membrane) had OD600nm = 3.17 (3.02 × 109 CFU mL−1) while the broth in contact with the PEBA film (Fig. 2 left photo) had OD600nm = 3.12 (2.97 × 109 CFU mL−1). This finding consistently indicates that PEBA has no antimicrobial properties. On the other hand, minimal or no E. coligrowths were observed in the PEBA/nano-Ag films (OD600nm < 0.06). The antimicrobial property of PEBA/nano-Ag was evident even at the lowest loading of 0.5 wt% (right photo) with >99.8% growth suppression. No E. coli grew in PEBA with nano-Ag ≥ 1.0 wt%.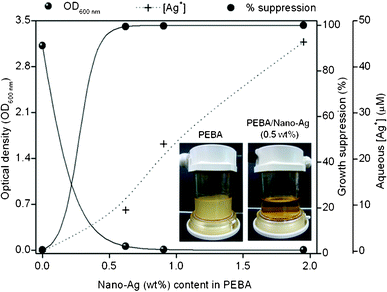 | ||
| Fig. 2 Surface contact test of PEBA and PEBA/nano-Ag with suspended E. colicells. | ||
After incubation, the Ag+ concentrations in the liquid media (i.e. [Ag+]) are shown in Fig. 2. It is evident that [Ag+] < MIC in all samples except for PEBA with 2.0 wt% nano-Ag. Apparently, the growth of E. colicells was still prevented despite the sub-MIC levels of [Ag+] in the liquid media. This finding indicates that aside from the released Ag+ in the broth, the direct contact of PEBA/nano-Ag surface with the liquid media must have contributed to curb the growth of suspended E. colicells.
To confirm this, another series of surface contact tests was performed in such a way that the eluted Ag+ in the broth is ≪MIC level. Different sub-MIC Ag+ concentrations were attained by incubating 1 × 107 CFU mL−1 of E. colicells (150 RPM, 37 °C) at different nutrient media volumes (MV = 25 to 250 mL) containing PEBA films with 1 wt% nano-Ag (diameter = 20 mm). The released Ag+ in the media was monitored during cultivation.
In Fig. 3a, growth curves reveal that E. colicells did not thrive well at all MVs. No E. coli grew at MV ≤ 50 mL while lowest growth suppression (97.8%) was achieved at MV = 250 mL (Table 2). During cultivation, nonlinear increase of Ag+ concentration was observed (Fig. 3b). As expected, highest Ag+ was obtained from the lowest MV = 25 mL whereas Ag+ was lowest at MV = 250 mL due to dilution effect. After incubation, all Ag+ concentrations listed in Table 2 were <10 μM, which are remarkably lower than the obtained MIC level in Section 3.3. Thus, if the antimicrobial activity of nano-Ag is solely imparted by the Ag+ released into the bulk liquid medium, then no growth suppression could have been observed. This finding strongly indicates that aside from the released Ag+, the growth of suspended E. colicells was inactivated by the PEBA/nano-Ag films through its direct contact with the liquid medium.34,45
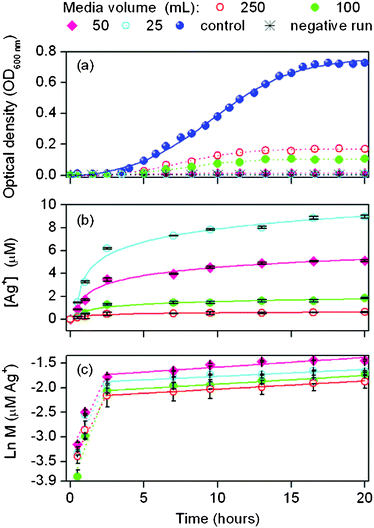 | ||
| Fig. 3 Surface contact test of PEBA/nano-Ag film with sub-MIC levels of Ag+ in the liquid media (n = 3–6). | ||
| Media volume (mL) | Inoculum concentrationa(CFU mL−1) | Growth suppressiona,b (%) | [Ag+] ± SDa (μM) | Ag+ release rate constant (h−1) | Total Ag in film (initial, μg) | Total Ag+ released into the brotha (μg) | |
|---|---|---|---|---|---|---|---|
| k 1 c ± SD | k 2 d ± SD | ||||||
| a After 20 h cultivation. b Calculated based on control sample—PEBA film without nano-Ag. c First phase (t ≤ 2.5 h). d Second phase (t > 2.5 h). | |||||||
| 25 | No growth | Complete | 8.95 ± 0.13 | 0.63 ± 0.38 | 0.014 ± 0.007 | 77.3 | 24.1 |
| 50 | No growth | Complete | 5.12 ± 0.08 | 0.64 ± 0.27 | 0.020 ± 0.009 | 78.2 | 27.6 |
| 100 | 1.04 × 107 | 98.6 | 1.85 ± 0.05 | 0.81 ± 0.36 | 0.018 ± 0.005 | 61.6 | 19.0 |
| 250 | 1.72 × 107 | 97.6 | 0.62 ± 0.08 | 0.58 ± 0.21 | 0.017 ± 0.002 | 64.4 | 19.0 |
| Control | 7.25 × 108 | — | — | — | — | — | — |
To further elucidate the elution behavior of Ag+, the concentration profiles in Fig. 3b were transformed as shown in Fig. 3c. Results reveal that the release of Ag+ from PEBA follows first order kinetics as expressed in eqn (9). Here k is the release rate constant of Ag+, X is an empirical constant while M is the amount of eluted Ag+ at reaction time t. Kinetic results reveal that Ag+ release appeared to occur in two stages. Initially fast Ag+ release occurred during the first 2.5 h (k1) followed by slower and steadier rates (k2). With similar amount nano-Ag added (1 wt%) at all MVs, similar k values were obtained in each stage. No correlation was observed between k and MV. The slight differences in k values may be due to the variation in membrane sample weights which also explains the differences in total initial Ag in films and total released Ag+ after incubation.
| ln(M) = kt + X | (9) |
It is hypothesized that in stage 1, there is a surplus of Ag+ available on the nano-Ag surfaces that were easily released from the film into the solution. At t > 2.5 h, Ag+ might have been depleted and the lower availability of Ag+ for release could explain why k2 < k1. It is shown in Table 2 that the total Ag+ released after incubation ranged from 19.0 to 24.1 μg, which indicates that majority of nano-Ag remained in the films.20,46
It is known that aside from the elutable Ag+ that is released to the liquid media, partially oxidized nano-Ag contain chemically bound Ag+ on its surface (i.e. chemisorbed Ag+).46 Thus at t > 2.5 h, it is possible that the majority of the remaining Ag+ in the film was in this form. Considering that the volume of PEBA film is remarkably small, it can be estimated that the chemisorbed Ag+ concentration in the film is significantly higher than the MIC.45 Thus despite the eventual decline in Ag+ release rate and the sub-MIC levels of Ag+ in the liquid phase, the remaining Ag+ in the film (i.e. chemisorbed Ag+) might have been sufficient to mediate the growth inhibition of E. coli through surface contact.47
Since filtration would involve the direct interaction of the feed water and membrane surface, results suggest that PEBA/nano-Ag could be a good candidate material as a surface coating to improve the biofouling resistance of filtration membranes.
3.5. Surface analysis of bare and coated PSf
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide bond stretch at 1660 cm−1 (3) and secondary amide N–H bend at 1580 cm−1 (4).16 The presence of these peaks in PEBA and PEBA/nano-Ag coated PSf confirms that the coating was performed successfully. Concomitant with the appearance of these peak are the disappearances of the aromatic C
O amide bond stretch at 1660 cm−1 (3) and secondary amide N–H bend at 1580 cm−1 (4).16 The presence of these peaks in PEBA and PEBA/nano-Ag coated PSf confirms that the coating was performed successfully. Concomitant with the appearance of these peak are the disappearances of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond stretch at 1620 cm−1 (a) and the sulfone group’s (O
C bond stretch at 1620 cm−1 (a) and the sulfone group’s (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
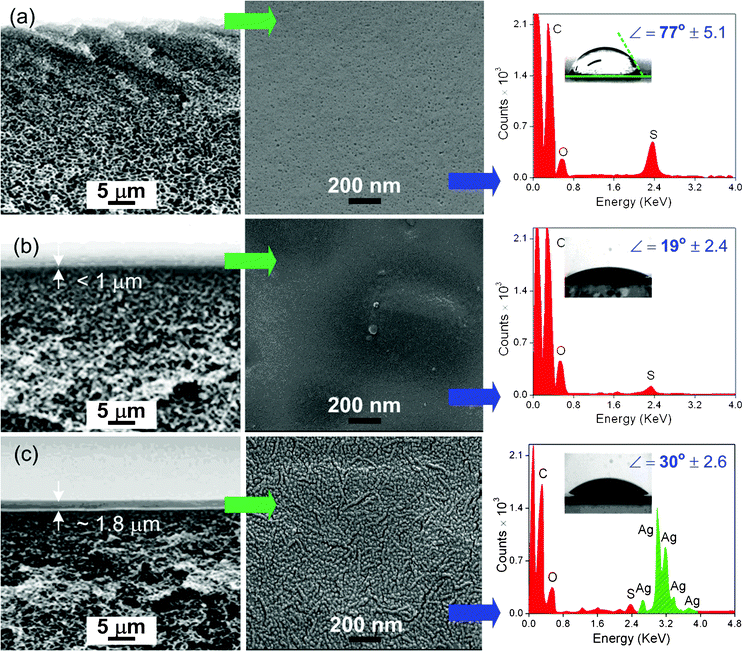
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) symmetric bond stretch at 1050 cm−1 (b), which are characteristic peaks of PSf.48 However, concurrent vibrational bands of both PEBA and PSf are evident in PEBA coated PSf. This may be due to the relatively thin PEBA coating layer (<1 μm) as compared to PEBA/nano-Ag (≅1.8 μm). The IR beam possibly penetrated through the PEBA film and interacted with the PSf material underneath.16
O) symmetric bond stretch at 1050 cm−1 (b), which are characteristic peaks of PSf.48 However, concurrent vibrational bands of both PEBA and PSf are evident in PEBA coated PSf. This may be due to the relatively thin PEBA coating layer (<1 μm) as compared to PEBA/nano-Ag (≅1.8 μm). The IR beam possibly penetrated through the PEBA film and interacted with the PSf material underneath.16
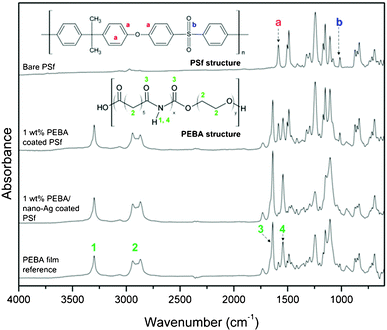 | ||
| Fig. 4 FTIR spectra of bare and modified PSf membranes. | ||
 | ||
| Fig. 6 AFM images of freshly prepared membranes: (a) pristine PSf; (b) PEBA coated PSf; (c) PEBA/nano-Ag coated PSf. | ||
3.6. Flux test
As a proof-of-concept, a 40 h filtration test was performed using bare PSf, PEBA coated PSf and PEBA/nano-Ag coated PSf membranes. Fig. 7 illustrates the filtration results of 1 × 105 CFU mL−1E. coli suspensions. The performance of PSf coated with PEBA/nano-Ag (0.5 wt%) was compared with PEBA coated PSf and bare PSf. Abiotic fouling (rm) or flux decline due to the presence of inorganic and other non-biological components in water was also estimated from the filtration of media solution without the E. colicells.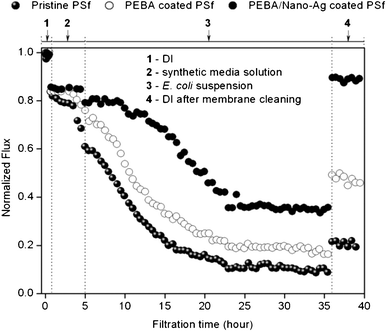 | ||
| Fig. 7 Flux test of the modified PSf membranes using synthetic media inoculated with E.colicells as feed. | ||
The summary of filtration results is shown in Table 3. Pure water flux (1) of PEBA and PEBA/nano-Ag coated PSf were rc = 9% and 18% lower than the bare PSf with FPSf = 649 LMH at 490 kPa. This is mainly attributed to the added membrane resistances of the coating layers. In Fig. 5, it is shown that PEBA/nano-Ag coating layer was ≅2 times thicker than PEBA, which explains its lower Fc value.
| Parameters | r t (%) | r m (%) | r rev (%) | r irrev (%) | FR (%) |
|---|---|---|---|---|---|
| Bare PSf | 89 | 32 | 10 | 79 | 21 |
| PEBA coated PSf | 81 | 22 | 28 | 53 | 47 |
| PEBA/nano-Ag coated PSf | 64 | 16 | 53 | 11 | 89 |
After synthetic media filtration (2), bare PSf had the highest rm = 32%, indicating its high vulnerability to abiotic fouling. The rm values of both coated PSf membranes did not vary remarkably, which suggests that PEBA predominantly reduced abiotic fouling while nano-Ag apparently had little effect.
Upon introduction of suspended E. colicells (3), flux decline in bare PSf was rather instantaneous as compared to both coated PSf membranes. Before the membranes attained steady NFf, flux reduction rates of 0.048 h−1, 0.044 h−1 and 0.035 h−1 were measured from bare PSf, PEBA coated PSf and PEBA/nano-Ag coated PSf, respectively.
Compared to bare PSf, flux declination rates in PEBA and PEBA/nano-Ag coated PSf were retarded by 9% and 27%, respectively. After 23 h, steady NFf values were attained and rt values follow the sequence: PSf > PEBA coated PSf > PEBA/nano-Ag coated PSf.
After flushing with DI water (4), the FR value was highest in PEBA/nano-Ag coated PSf > PEBA coated PSf > bare PSf. These results indicate that among the tested membranes, PEBA/nano-Ag coated PSf showed the highest resistance to biofouling. Flux recovery of PSf was improved in the presence of PEBA but higher improvement was observed with PEBA/nano-Ag coated PSf.
Additionally, the majority of fouling in PEBA/nano-Ag coated PSf was reversible (rrev: PEBA/nano-Ag > PEBA > PSf) while that in bare PSf was mostly irreversible (rirrev: PSf > PEBA > PEBA/nano-Ag).
3.7. Fouling inspection by AFM
The AFM images of used membranes after the operation are shown in Fig. 8. Compared to pristine PSf sample in Fig. 5, fouling of bare PSf was severe as exhibited by asperity elevations as high as 1500 nm. This is mainly attributed to the deposited media components and formation of biofilms which fouled the bare PSf irreversibly.27 On the other hand, only partial accumulation of foulants were observed on PEBA coated PSf while PEBA/nano-Ag showed a relatively clean surface. | ||
| Fig. 8 AFM images of used membranes: (a) fouled PSf; (b) PEBA coated PSf; (c) PEBA/nano-Ag coated PSf. | ||
From these results, it is evident that hydrophilisation of PSfviaPEBA coating improved the resistance of PSf to abiotic and biofouling. The protective coating layer of PEBA prevented the foulants from blocking the pores of the membrane. Additionally, flux decline was retarded and biofouling was reduced by decreasing cell affinity on membrane surface.11,16 On the other hand, PEBA/nano-Ag coated PSf features both the hydrophilic character of PEBA and the antimicrobial properties of nano-Ag. The presence of nano-Ag in PEBA predominantly prevented the formation of a stable biofilm on the PSf surface, which makes PEBA/nano-Ag a more effective anti-biofoulant than pure PEBA. These results clearly indicate that a composite hydrophilic polymer coating with antimicrobial properties like PEBA/nano-Ag is a promising material to improve the resistance of filtration membranes to biofouling.
4. Conclusions
In this study, a hydrophilic polymer like PEBA and an antimicrobial agent like nano-Ag successfully improved the biofouling resistance of an ultrafiltration membrane. PEBA as a hydrophilic, pore-protective layer improved the resistance of PSf to abiotic fouling and to a certain extent, biofouling. But addition of nano-Ag in the PEBA significantly enhanced the anti-biofouling property of PSf. Irreversible biofouling, which was observed on bare PSf, was significantly prevented in the presence of PEBA/nano-Ag.Acknowledgements
This work was supported by the Priority Research Centers Program (No. 2011-0022968) through the National Research Foundation (NRF) of Korea funded by the Ministry of Education Science and Technology (MEST).References
- L. D. Eaux, Water Treatment Membrane Processes, American Water Research Foundation, Water Research Commission of South Africa, McGraw-Hill, South Africa, 1996 Search PubMed.
- M. Mulder, Basic Principles of Membrane Technology, Kluwer Academic Publishers, The Netherlands, 1996 Search PubMed.
- K. Scott and R. Hughes, Industrial Membrane Separation Technology, Blackie Academic & Professional, Glasgow, New Zealand, 1996 Search PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Nature, 2008, 452, 301 CrossRef CAS.
- G. Fane and C. J. D. Fell, Desalination, 1987, 62, 117 CrossRef.
- G. B. Van den Berg and C. A. Smolders, Desalination, 1990, 77, 101 CAS.
- M. F. A. Goosen, S. S. Sablani, H. Al-Hinai, S. Al-Obeidani, R. Al-Belushi and D. Jackson, Sep. Sci. Technol., 2004, 39, 2261 CrossRef CAS.
- S. Jönsson, Sep. Sci. Technol., 1995, 30, 301 CrossRef.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448 CrossRef CAS.
- J. S. Baker and L. Y. Dudley, Desalination, 1998, 118, 81 CrossRef CAS.
- S. P. Nunes, M. L. Sforça and K. V. Peinemann, J. Membr. Sci., 1995, 106, 49 CrossRef CAS.
- K. S. Kim, K. H. Lee, K. Cho and C. E. Park, J. Membr. Sci., 2002, 199, 135 CrossRef CAS.
- S. Krishnan, R. Ayothi, A. Hexemer, J. A. Finlay, K. E. Sohn, R. Perry, C. K. Ober, E. J. Kramer, M. E. Callow and J. A. Callow, Langmuir, 2006, 22, 5075 CrossRef CAS.
- Y. Q. Wang, Y. L. Su, Q. Sun, X. L. Ma and Z. Y. Jiang, J. Membr. Sci., 2006, 286, 228 CrossRef CAS.
- W. Zhao, Y. Su, C. Li, Q. Shi, X. Ning and Z. Jiang, J. Membr. Sci., 2008, 318, 405 CrossRef CAS.
- J. S. Louie, I. Pinnau, I. Ciobanu, K. P. Ishida, A. Ng and M. Reinhard, J. Membr. Sci., 2006, 280, 762 CrossRef CAS.
- P. Sampranpiboon, R. Jiraratananon, D. Uttapap, X. Feng and R. Y. M. Huang, J. Membr. Sci., 2000, 173, 53 CrossRef CAS.
- S. Sridhar, R. Suryamurali, B. Smith and T. M. Aminabhavi, Colloids Surf., A, 2007, 297, 267 CrossRef CAS.
- G. J. Zhao and S. E. Stevens, BioMetals, 1998, 11, 27 CrossRef CAS.
- S. Kittler, C. Greulich, J. Diendorf, M. Köller and M. Epple, Chem. Mater., 2010, 22, 4548 CrossRef CAS.
- A. Melaiye, Z. Sun, K. Hindi, A. Milsted, D. Ely, D. H. Reneker, C. A. Tessier and W. J. Youngs, J. Am. Chem. Soc., 2005, 127, 2285 CrossRef CAS.
- D. Maynard, R. J. Aitken, T. Butz, V. Colvin, K. Donaldson, G. Oberdorster, M. A. Philbert, J. Ryan, A. Seaton, V. Stone, S. S. Tinkle, L. Tran, N. J. Walker and D. B. Warheit, Nature, 2006, 444, 267 CrossRef.
- W. R. Li, X. B. Xie, Q. S. Shi, H. Y. Zeng, Y. S. Ou-Yang and Y.-B. Chen, Appl. Microbiol. Biotechnol., 2010, 85, 1115 CrossRef CAS.
- V. Sambhy, M. M. MacBride, B. R. Peterson and A. Sen, J. Am. Chem. Soc., 2006, 128, 9798 CrossRef CAS.
- H. Kong and J. Jang, Langmuir, 2008, 24, 2051 CrossRef CAS.
- R. Gottesman, S. Shukla, N. Perkas, L. A. Solovyov, Y. Nitzan and A. Gedanken, Langmuir, 2011, 27, 720 CrossRef CAS.
- W. Hu, S. Chen, X. Li, S. Shi, W. Shen, X. Zhang and H. Wang, Mater. Sci. Eng., C, 2009, 29, 1216 CrossRef CAS.
- X. Cao, M. Tang, F. Liu, Y. Nie and C. S. Zhao, Colloids Surf., B, 2010, 81, 555 CrossRef CAS.
- B. De Gusseme, T. Hennebel, E. Christiaens, H. Saveyn, K. Verbeken, J. P. Fitts, N. Boon and W. Verstraete, Water Res., 2011, 45, 1856 CrossRef CAS.
- S.-Y. Kwak, S. G. Jung, Y. S. Yoon and D. W. Ihm, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 1429 CrossRef CAS.
- M. Stamm, Polymer Surfaces and Interfaces: Characterization, modification and applications, Springer-Verlag Berlin Heidelberg, Leipzig, Germany, 2008 Search PubMed.
- G. Kronvall, J. Clin. Microbiol., 1982, 16, 784 CAS.
- Y. Lee, R. E. Cohen and M. F. Rubner, Langmuir, 2005, 21, 9651 CrossRef.
- G. L. Burygin, B. N. Khlebtsov, A. N. Shantrokha, L. A. Dykman, V. A. Bogatyrev and N. G. Khlebtsov, Nanoscale Res. Lett., 2009, 4, 794 CrossRef CAS.
- H. Susanto, Y. Feng and M. Ulbricht, J. Food Eng., 2009, 91, 333 CrossRef CAS.
- E. Navarro, F. Piccapietra, B. Wagner, F. Marconi, R. Kaegi, N. Odzak, L. Sigg and R. Behra, Environ. Sci. Technol., 2008, 42, 8959 CrossRef CAS.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76 CrossRef CAS.
- V. K. Sharma, R. A. Yngard and Y. Lin, Adv. Colloid Interface Sci., 2009, 145, 83 CrossRef CAS.
- M. Sureshkumar, D. Y. Siswanto and C.-K. Lee, J. Mater. Chem., 2010, 20, 6948 RSC.
- J. D. Schiffman, Y. Wang, E. P. Giannelis and M. Elimelech, Langmuir, 2011, 27, 13159 CrossRef CAS.
- M. T. Madigan, J. M. Martinko and J. Parker, Brock Biology of Microorganisms, ninth ed., Prentice Hall Upper Saddle River, New Jersey, USA, 2000 Search PubMed.
- A. Henglein, Chem. Mater., 1998, 10, 444 CrossRef CAS.
- S. S. Djokic and R. E. Burrell, J. Electrochem. Soc., 1998, 145, 1426 CrossRef CAS.
- C. N. Lok, C. M. Ho, R. Chen, Q. Y. He and W. Y. Yu, J. Proteome Res., 2006, 5, 916 CrossRef CAS.
- C. N. Lok, C. M. Ho, R. Chen, Q. Y. He, W. Y. Yu, H. Sun, P. K. H. Tam, J. F. Chiu and C. M. Che, JBIC, J. Biol. Inorg. Chem., 2007, 12, 527 CrossRef CAS.
- R. Kumar, S. Howdle and H. Münstedt, J. Biomed. Mater. Res., Part B, 2005, 75B, 311 CrossRef CAS.
- U. Samuel and J. P. Guggenbichler, Int. J. Antimicrob. Agents, 2004, 23, 75 CrossRef.
- R. Naim, A. F. Ismail, H. Saidi and, E. Saion, Development of Sulfonated Polysulfone Membranes as a Material for Proton Exchange Membrane (PEM), in: Proceedings of Regional Symposium on Membrane Science and Technology, Puteri Pan Pacific Hotel, Johor Bharu, Malaysia, 2004 Search PubMed.
- S. Behrens, J. Wu, W. Habicht and E. Unger, Chem. Mater., 2004, 16, 3085 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |