Amine-intercalated montmorillonite matrices for enzyme immobilization and biosensing applications
Muharrem
Seleci
a,
Didem
Ag
a,
Esra Evrim
Yalcinkaya
b,
Dilek Odaci
Demirkol
a,
Cetin
Guler
b and
Suna
Timur
*a
aEge University Faculty of Science Biochemistry Department, 35100, Bornova-Izmir/Turkey. E-mail: suna.timur@ege.edu.tr; Fax: +902323115485; Tel: +902323112455
bEge University Faculty of Science Chemistry Department, 35100, Bornova-Izmir/Turkey
First published on 18th January 2012
Abstract
Clay based biosensors were developed using montmorillonite (Mont) modified with methyl (M) and dimethylamine (DM). X-ray diffraction, Fourier transform infrared spectroscopy, zeta potential and thermal gravimetric measurements were used to characterize the modified clays. After immobilization of glucose oxidase (GOx) via clay on the glassy carbon electrode, its application as a glucose biosensor was investigated in detail. The best response characteristics were obtained by DM-Mont and optimization of enzyme amount, reproducibility of biosensor fabrication, repeatability of measurements and operational stability were all evaluated. The optimized biosensor showed a very good linearity between 0.05 mM and 1.0 mM, a 7 s response time and a limit of detection to glucose of 0.038 mM. Also, kinetic parameters and stabilities were determined. Apparent Km and Imax values were found as 0.73 mM and 2.955 μA, respectively. As well as batch configuration, the DM-Mont/GOx biosensor was successfully applied in the flow injection analysis mode. Finally, the performance of the DM-Mont/GOx biosensor to analyze glucose in a wine sample was compared with HPLC.
Introduction
To ensure stability with complete retention of enzymes' biological activity and good diffusional properties for substrates and/or co-substrates, biomolecule immobilization in biosensor construction is important. Clay modified electrodes are an alternative to improve the analytical characteristics of biosensors.1 Due to the large specific surface area, good adsorbance ability, high cationic exchange capacity and stand out adhesiveness, clays are used as an immobilization matrix.2 Smectite clays (laponite, montmorillonite, and nontronite) and layered double hydroxides (LDHs) are popular clays coupled with proteins such as hemoglobin,3polyphenol oxidase,4–7glucoamylase,8α-amylase and invertase,9glucose oxidase10 and horseradish peroxidase,11lactate oxidase12 in an original and inexpensive immobilization approach.Montmorillonite (Mont) is a naturally occurring cationic phyllosilicate, composed of two external tetrahedral silica groups surrounding internal octahedral alumina groups in a tetrahedral octahedral tetrahedral (TOT) structure.13 These sheets retain a negative charge which is balanced by the introduction of cations such as Na+ or Ca2+ into the layers.2 Numerous studies have been directed at using Mont in different biotechnological applications. For instance; Ozdemir et al. tested the antibacterial effects of Cu2+-, Zn2+-, Ag+-, Ag0- and cetylpridinium-exchanged Mont on pathogenic bacteria, highly resistant to antibiotics such as Pseudomonas aeruginosa ATCC 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 853 and Staphylococcus aureus ATCC 29
853 and Staphylococcus aureus ATCC 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213.14 In another study, Lin et al. tried to prepare Mont intercalated with 5-fluorouracil as a drug carrier and to treat colorectal cancer.15 Moreover Zhou et al. prepared phosphatidyl choline/Mont dispersed it polydimethylsiloxane, and the antithrombogenic property of the composite tested.16 Additionally, electrochemical studies with Mont-modified electrodes have been a very attractive research area.1 Clay-modified electrodes has been applied to detect some analytes such as Pb2+, Fe3+, Cu2+, Hg2+, Ca2+, Ru(NH3)3+, nitrophenol, ascorbic acid, dopamine and uric acid using differential pulse voltammetry (DPV), potentiometric ion sensitive electrode (PISE) and square wave voltammetry (SWV) methods.17–26
213.14 In another study, Lin et al. tried to prepare Mont intercalated with 5-fluorouracil as a drug carrier and to treat colorectal cancer.15 Moreover Zhou et al. prepared phosphatidyl choline/Mont dispersed it polydimethylsiloxane, and the antithrombogenic property of the composite tested.16 Additionally, electrochemical studies with Mont-modified electrodes have been a very attractive research area.1 Clay-modified electrodes has been applied to detect some analytes such as Pb2+, Fe3+, Cu2+, Hg2+, Ca2+, Ru(NH3)3+, nitrophenol, ascorbic acid, dopamine and uric acid using differential pulse voltammetry (DPV), potentiometric ion sensitive electrode (PISE) and square wave voltammetry (SWV) methods.17–26
Herein we described a simple and accurate electrochemical method using clay-modified glassy carbon electrodes. Methyl (M)- and dimethylamine (DA)-exchanged Mont was used as an immobilization matrix. Glucose oxidase (GOx) was chosen as a model enzyme and immobilized on the GCE via clays in the presence of bovine serum albumine (BSA) and glutaraldehyde. During glucose oxidation by GOx, the amount of oxygen is decreased in the bioactive layer and consumed oxygen affects the electrode signals, it was proportional to substrate concentration and followed at −0.7 V vs.Ag/AgCl. To develop clay modified biosensors, two configurations were studied; batch and FIA. After testing the influence of working pH and matrix composition, GOx based clay biosensors were characterized and applied to detect glucose in a wine sample.
Results and discussion
Recently, intercalation of various molecules into Mont has attracted growing interest due to very efficient and promising applications of biomolecule–clay complexes in biotechnological areas. Among them, aliphatic amine-intercalated Mont is used in different industrial approaches such as thixotropic agents, adsorbents and chromatography materials.27 Snircova et al. prepared Ni–exchanged Mont with methyl-, dimethyl-, trimethylamine and tested the steric effects of these alkylamines on the type of interactions with Ni–Mont.27 Herein, amine-functionalized Monts were prepared via intercalation of methyl and dimethylamine into the clay. Amine groups played an important role in the stabile immobilization of the enzyme by crosslinking with glutaraldehyde. The intercalation of methyl and dimethylamine to clay were characterized by XRD, FT-IR zeta potential and TGA measurements. The addition of amine groups to the structure was initially confirmed by FT-IR. As shown in the FT-IR spectra (Fig. 1), pure Na–Mont showed a typically broad O–H stretching band at 3633 cm−1. Absorption bands of adsorbed molecular water appeared at 1642 and 3451 cm−1. They showed a broadly similar pattern of adsorption at 1047 cm−1 arising from Si–O stretching vibrations. In addition to these bands, the bands around 3400 and 1400 cm−1 are attributed to the presence of N–H on the structure. Also, C–H stretching bands of methyl group are assigned at about 2900 cm−1. | ||
| Fig. 1 FT-IR spectrum of Mont (a), M-Mont (b) and DM-Mont (c). | ||
The structures of Mont, M-Mont and DM-Mont were characterized by XRD. The X-Ray diffractogram of Na–Mont is presented in Fig. 2. The basal spacing value (d001) of Mont was calculated as 11.4 Å. For the M-Mont and DM-Mont, the basal spacing was expanded to 12.71 and 13.14 Å, respectively, as the sodium cations in the interlayer galleries are replaced by methylamine and dimethylamine. It is interesting that the XRD peaks sharpen after intercalation, suggesting the increased ordering by exchanging with the organic modifiers. From this diffractogram, the 001 diffraction peak on the XRD spectra was slipped in low angles. This slipping indicated that the sodium cations in the interlayer galleries were replaced by methylamine and dimethylamine salts. Due to the increasing interlayer distance of the Mont, increasing basal spacing values of Mont were clearly seen. It is evidence that the methylamine and dimethylamine salts had entered the interlayer of the clay.
 | ||
| Fig. 2 Typical XRD patterns of Mont (a), M-Mont (b) and DM-Mont (c). | ||
The TGA thermograms of the Mont and modified Monts (M-Mont and M-Mont) were shown in Fig. 3. Mont exhibited about 8.4 wt% weight loss at 1000 °C. The weight loss of M-Mont was found as 21.80 wt% due to the presence of organic materials such as the amine salt modifier. In the case of DM-Mont, this value was calculated as 16.4 wt%. Hence, it can be concluded that 13.4 and 8.0 wt% modifier held on to the Mont for M-Mont and DM-Mont structures, respectively. Application of various characterization techniques provided strong evidence that amine groups are distributed into the structure of Mont.
 | ||
| Fig. 3 TG thermograms of Mont (a), DM-Mont (b) and M-Mont (c). | ||
The zeta potential (ZP) is an indicator of the surface charge properties of a colloid or a particle in solution and varies depending on the surface potential and the thickness of the electric double layer. ZPs were calculated as −42.0, −34.5 ± 3.33 and −32.1 ± 2.24 mV for Mont, M-Mont and DM-Mont from the Smoluchowski equation, respectively. After modification of Mont with methylamine and dimethylamine salts, due to the adsorption at the surface or interlayer of the mineral, the ZPs were changed to less-negative values by virtue of the positively charged amine salts.
In order to investigate electron transfer mechanism between the electrode surface and species in solution, cyclic voltammetry (CV) experiments were carried out in the presence of 5.0 mM [Fe(CN)6]3−/4−. At the bare and clay modified glassy carbon electrodse, oxidation–reduction peaks of [Fe(CN)6]3−/4− were observed and displayed in Fig. 4a. Because of modification of the electrode surface with clay and GOx, the peak currents decreased. Peak to peak separations were obtained with the bare, Mont (non-modified)/GOx, M-Mont/GOx and DM-Mont/GOx modified electrodes as 108, 237, 210 and 134 mV, respectively. Also peak currents were observed Ianodic = 18.40 μA, Icathodic = 12.71 μA for bare; Ianodic = 2.92 μA, Icathodic = 3.55 μA for Mont/GOx; Ianodic = 4.49 μA, Icathodic = 6.92 μA for M-Mont/GOx; Ianodic = 11.84 μA, Icathodic = 11.14 μA for DM-Mont/GOx modified electrodes. Higher current values obtained with DM-Mont/GOx can be attributed to more efficient electron transfer properties than other clays. Fig. 3b displays CVs of DM-Mont/GOx at the different scan rates (5; 10; 25; 50; 75; 100; 125; 150; 175; 200 mV s−1). As shown in the inset of Fig. 4b the peak current increased linearly with the increasing square root of scan rate potential (v1/2), which suggests that the reactions on the DM-Mont/GOx-modified electrode were reversible and the mass transport phenomenon is mainly diffusion controlled.
![(a) Cyclic voltammograms of bare and clay modified electrodes (in 5.0 mM [Fe(CN)6]3−/4− at a scan rate of 25 mV s−1). (b) Cyclic voltammograms of DM-Mont/GOx modified electrodes at the different scan rates, Inset: Peak currents as a function of scan rate (in pH 4.0, 50 mM sodium acetate buffer; 25 °C; electrode composition: 18.4 Unit GOx, 1.0 mg mL−1 BSA and 5.0% glutaraldehyde).](/image/article/2012/RA/c2ra01225a/c2ra01225a-f4.gif) | ||
| Fig. 4 (a) Cyclic voltammograms of bare and clay modified electrodes (in 5.0 mM [Fe(CN)6]3−/4− at a scan rate of 25 mV s−1). (b) Cyclic voltammograms of DM-Mont/GOx modified electrodes at the different scan rates, Inset: Peak currents as a function of scan rate (in pH 4.0, 50 mM sodium acetate buffer; 25 °C; electrode composition: 18.4 Unit GOx, 1.0 mg mL−1 BSA and 5.0% glutaraldehyde). | ||
Optimization studies
The pH of the acetate buffer as a working solution was optimized in the range 3.5–5.0 using batch system. For the Mont, M-Mont and DM-Mont based GOx biosensors; there was an increase of the biosensor response at pH 4.0 (Fig. 5). However, the current decreased rapidly for higher pHs. Then, acetate buffer solution (50 mM pH 4.0) was chosen for subsequent experiments. It is known that the optimum pH of the GOx for free enzyme is 5.5.28 In our case, the optimum working pH of GOx shifted to an acidic pH due to the presence of positive groups in the structure of Mont. It is noted that even though the enzyme immobilization on clay matrices caused a shift in the optima, the same optimum pH values were obtained when either M- or DM-Mont was used. | ||
| Fig. 5 Effect of pH on the response of clay biosensors (in 50 mM sodium acetate buffers; 25 °C; electrode composition: 18.4 Unit GOx, 5.0% glutaraldehyde and 2.0 mg mL−1 BSA for Mont/GOx and M-Mont biosensors and 1.0 mg mL−1 BSA for DM-Mont/GOx biosensor). | ||
Regarding the influence of the enzyme amount on the amperometric response of DM-Mont based biosensor, an increase of the current occurred for more enzyme amounts than 0.25 mg up to 1.0 mg. As shown in Fig. 6, the amperometric response was decreased with the further increase in the amount of enzyme.
 | ||
| Fig. 6 Effect of enzyme amount on the response of DM-Mont/GOx biosensor (in pH 4.0, 50 mM sodium acetate buffer; 25 °C; electrode composition: 18.4 Unit GOx, 1.0 mg mL−1 BSA and 5.0% glutaraldehyde). | ||
Analytical characterization
The amperometric responses of the M-Mont/GOx and DM-Mont/GOx biosensors (including 2.0 mg mL−1 BSA) were linear in the range 0.05–1.0 mM with the equations of y = 1.638× + 0.002 (R2 = 0.999) and y = 2.295 + 0.116 (R2 = 0.997), respectively. According to the results, it can be said that the DM-Mont/GOx biosensor showed higher current values than M-Mont/GOx. Also, the effect of the amount of BSA on the DM-Mont/GOx biosensor was tested using 1.0 and 2.0 mg mL−1 of BSA. The current response shows a linear relationship with the increase of glucose concentration in the ranges of 0.05–1.0 mM (y = 2.361× + 0.094, R2 = 0.999) when 1.0 mg mL−1 BSA was used. The obtained slope was similar to that of DM-Mont containing 2.0 mg mL−1 of BSA. Therefore, due to the higher response signals 1.0 mg mL−1 BSA and DM-Mont clays were used in further experiments. Fig. 7 shows the chronoamperometric response to glucose with the DM-Mont/GOx biosensor. As shown the Figure, the DM-Mont/GOx biosensors were sensitive to the changes in the concentration of glucose and responded within 7 s, (Fig. 7). The limit of detection (LOD) was also calculated as 0.038 mM using S/N = 3. Also, the kinetic parameters (Michaelis–Menten constant (Kmapp) and Imax) calculated from the Lineweaver–Burk plot, are 0.73 mM and 2.955 μA. To evaluate the analytical performance of the DM-Mont/GOx biosensors in FIA configuration (Scheme 1), amperometric responses were recorded with successive injections of 5.0 mM glucose (at −0.7 V vs.Ag/AgCl), (Inset of Fig. 8). FIA- based DM-Mont/GOx biosensors exhibited a linear range between 1.0–10.0 mM (Fig. 8B) with 0.47 mM LOD. | ||
| Fig. 7 Linear range for glucose (Inset: current–time curves obtained at the DM-Mont biosensor with the successive addition of 0.5 mM glucose; in pH 4.0, 50 mM sodium acetate buffer; 25 °C; electrode composition: 18.4 Unit GOx, 1.0 mg mL−1 BSA and 5.0% glutaraldehyde). | ||
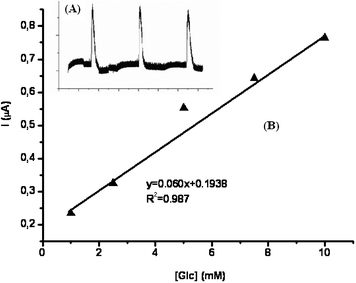 | ||
| Fig. 8 Linear range obtained at the DM-Mont biosensor for glucose (Inset: time dependent current (FIA peaks) with the successive addition of 10.0 mM glucose; in pH 4.0, 50 mM sodium acetate buffer; 25 °C; electrode composition: 18.4 Unit GOx, 1.0 mg mL−1 BSA and 5.0% glutaraldehyde). | ||
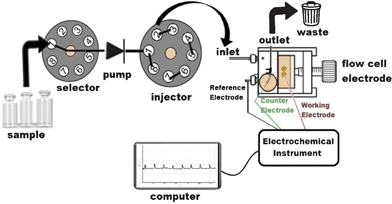 | ||
| Scheme 1 The schematic representation of FIA-biosensor measurement system. | ||
The highly hydrophilic character of the clays supplies a high enzymatic activity of enzymes after immobilization in clays. Following this approach, various clay biosensors for the detection of glucose based on GOx within laponite,29–30laponite gel doped by graphite nanoparticles (immobilization of GOx with horseradish peroxidase to prepare bienzymatic biosensor)10 and alginate/layered double hydroxides hybrid membrane31 were also reported. The analytical performance of some GOx/Mont biosensors in literature is given in Table 1. When DM-Mont/GOx is compared with previous studies32–33, a lower LOD and better linearity has been observed in the batch system.
| CME | Med | Mode | Linearity (mM) | LOD (mM) | Ref |
|---|---|---|---|---|---|
| CME, clay modified electrode; BSA, bovine serum albumin; GA, glutaraldehyde; GOx, glucose oxidase; Pt, platinum nanoparticles; DENs, PAMAM G4; Med, Mediator; TTF, tetrathiafulvalene. | |||||
| DM-Mont/GA/BSA/GOx | — | Batch | 0.05–10 | 0.038 | This work |
| DM-Mont/GA/BSA/GOx | — | FIA | 1–10 | 0.47 | This work |
| Mont/GA/GOx | — | FIA | 0.1–10 | 0.1 | 32 |
| Mont/GA/BSA/GOx | TTF | Batch | 0.1–8 | 0.1 | 33 |
| Mont/Pt-DENs/GOx | — | Batch | 0.01–16 | 0.004 | 34 |
Repeatability and reproducibility and operational stability of the DM-Mont biosensor were also investigated in FIA mode. For the repeatability, after 10 successive measurements using 5.0 mM glucose, the standard deviation (S.D) and variation coefficient (c.v) were calculated as 5.72 ± 0.26 mM and 4.55%, respectively. Electrode to electrode reproducibility was tested using three different biosensors prepared on different days, and the S.D and c.v after successive addition of 5.0 mM glucose were found as 5.40 ± 0.25 mM and 4.63%, respectively. As for the stability, the DM-Mont/GOx biosensor showed no decrease even after 75 injections during 130 min. Additionally, the selectivity of the DM-Mont/GOx biosensor was evaluated by using FIA and some potential interfering compounds (3-acetamidophenol, ethanol and malic acid; 5.0 mM) were injected, current signals were followed and evaluated as relative responses. It was observed that 3-acetamidophenol gave rise to only 10% response signals at the working potential but other tested compounds did not cause any interference effect.
Moreover, the surface morphologies of the microstructured DM-Mont and DM-Mont layer after the membrane formation imaged by SEM are shown in Fig. 9. Image (A) exhibits the typical morphology of pure Mont. The membrane structure was formed after dispersion and crosslinked with the bifunctional crosslinker in the presence of the biomolecules on the electrode surfaces.
 | ||
Fig. 9 The surface structures of DM-Mont (A) and DM-Mont membrane (Composition: 1.0 mg mL−1 clay in PBS containing BSA (1.0 mg mL−1) and 5.0% glutaraldehyde), (B) with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 7500× magnification. 000 and 7500× magnification. | ||
Sample application
The proposed biosensor was utilized for the glucose analysis in a white wine sample. After dilution with carrier buffer, the sample was injected and the glucose content of the wine was calculated from the linear graph. HPLC was used as a reference method to calculate the glucose content of the real sample and a linear graph for the glucose was obtained between 0.25–5.0 mg mL−1 with the equation of y = 173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200× (R2 = 0.999). The responses were noted down and calculated from the corresponding calibration curves. The glucose content of wine was calculated as 18.09 and 18.0 mg mL−1 using DM-Mont/GOx and HPLC system, respectively. Data were calculated as the average of 3 or 4 replicates of trials ±S.D. According to data it can be said that the use of the clay biosensor provided very similar results to the HPLC data (with the recovery of 99.5%) which indicated the analysis without any sample matrix effect.
200× (R2 = 0.999). The responses were noted down and calculated from the corresponding calibration curves. The glucose content of wine was calculated as 18.09 and 18.0 mg mL−1 using DM-Mont/GOx and HPLC system, respectively. Data were calculated as the average of 3 or 4 replicates of trials ±S.D. According to data it can be said that the use of the clay biosensor provided very similar results to the HPLC data (with the recovery of 99.5%) which indicated the analysis without any sample matrix effect.
Experimental
Materials
The Mont was obtained from West Anatolia. It was purified from bentonite before use. Particles <1.5 mm in diameter were obtained by sedimentation (ion-exchange capacity = 92 meq/100 g).35Methylamine and dimethylamine were purchased from Fluka. D(+)-glucose, glucose oxidase (GOx, EC.1.1.3.4, from Aspergillus niger, 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 Units/g) bovine serum albumine (BSA), malic acid, 3-acetamidophenol and glutaraldehyde solution (25%, v/v) were from Sigma-Aldrich. All other chemicals were of analytical grade.
800 Units/g) bovine serum albumine (BSA), malic acid, 3-acetamidophenol and glutaraldehyde solution (25%, v/v) were from Sigma-Aldrich. All other chemicals were of analytical grade.
Apparatus
A PalmSens Potentiostat (Palm Instruments, Houten, Netherlands) was used for amperometric measurements as well as cyclic voltammetric measurements. A three electrode cell with Mont/GOx electrodes as the working electrode, Ag/AgCl electrode with 3.0 M KCl as a reference electrode and platinum (Pt) as the counter electrode were used.Flow injection mode of analysis (FIA) was performed using a single line flow injection manifold with an electrochemical flow through the cell of the cross-flow type with glassy carbon working, Ag/AgCl reference and Pt auxiliary electrodes (CHI130, Austin, USA).36–38 A peristaltic pump (FIAtron, Oconomovoc, WI, USA) equipped with silicon tubing (0.89 mm inner diameter) pumped the working buffer solution as the carrier into the flow line with a flow rate of 1.6 mL min−1. The flow line was made of Teflon tubing (0.5 mm inner diameter). A 50 μL sample solution containing substrate was injected into the carrier stream via an eight-port injection valve (FIAtron, Oconomovoc, WI, USA). The FIA system was connected to a PalmSens potentiostat for the electrochemical measurements. The FIA system was assisted by a software program written in C++ which was developed at the Institute for Technical Chemistry, Leibniz University (Hannover, Germany).
A HPLC (HP1100, Hewlett Packard, USA) with a refractive index detector controlled by a HP-Chemstation from Agilent (Karlsruhe,Germany) was used as a reference method.36–37
A Zeta-Meter 3.0+ (with Zeiss DR microscope, GT-2 type quartz cell, molybdenum-cylinder anode, and platinum-rod cathode electrode) was used for the zeta-potential analysis using sodium acetate buffer (pH 4.0; 50 mM) as the measuring medium. The value of the zeta potential assigned to the dispersions was the average of the data obtained from four experiments. The zeta potential of acid-activated Mont suspensions was calculated automatically from measured electrophoretic mobilities employing the Smoluchowski eqn (1).
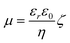 | (1) |
Where ζ is zeta potential, η is viscosity of the medium, μ is electrophoretic mobility at the actual temperature, εr and ε0 are the dielectric constants of the medium and free space, respectively.
Characterization of the Mont mineral and determination of the interlayer spacing of the silicate layers for unmodified Mont and modified Mont were performed by X-Ray diffraction spectrometers (Philips E'xpert Pro; Cu-Kα ray, λ = 1.54056 Å). The basal spacing values of Mont mineral calculated by Bragg's Law. FTIR spectra of the clays were taken with a Perkin–Elmer Pyris 1 FTIR Spectrometer on KBr plates.
The surface morphology of the DM-Mont and DM-Mont membrane were imaged by scanning electron microscopy (Quanta FEG 250, Fei). The DM-Mont sample was mounted on a holder with carbon tape. For the preparation of the DM-Mont membrane was prepared as follows: clay solution (1.0 mg mL−1 in PBS) containing 2.5 μL of BSA (1.0 mg mL−1) and 2.5 μL of glutaraldehyde (5.0%) were mixed. Then, 10 μL of this mixture was dropped on the ITO glass. All samples were then coated with gold and viewed at an accelerating voltage of 5.0 keV.
Clay modifications
Sodium Mont (Cloisite Na+) was intercalated using methylamine and dimethylamine salts to obtain methyl-dopped Mont (M-Mont) and dimethyl-dopped Mont (DM-Mont). For this purpose, sodium Mont was dispersed in distilled water for 24 h at room temperature. An equivalent amount of cation exchange capacity (CEC) of Mont, 0.02 mmol methylamine or dimethylamine, was stirred in distilled water at room temperature. This solution was slowly poured into the Mont dispersion. The final dispersion was stirred for 24 h at room temperature. Then the mixture was separated by filtration and washed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of distilled water–methanol for several times. After this washing no further chloride can be detected in the washing solution with a silver nitrate solution. Modified clay samples (M-Mont and DM-Mont) were dried overnight under vacuum.
1 ratio of distilled water–methanol for several times. After this washing no further chloride can be detected in the washing solution with a silver nitrate solution. Modified clay samples (M-Mont and DM-Mont) were dried overnight under vacuum.
Construction of mont/GOx biosensors
Initially, glassy carbon electrodes were cleaned by polishing with 0.5 μm alumina slurry, followed by ultrasonic cleaning for about 2–3 min with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol distilled water. Then, 5.0 μL of Mont solution (1.0 mg mL−1 in PBS), 2.5 μL of BSA (1.0 mg mL−1 in PBS), 0.5 mg GOx and 2.5 μL of glutaraldehyde (5.0% in PBS) were mixed. Finally, 10 μL of this mixture was dropped on the electrode surface and allowed to dry at room temperature for 1 h. To prepare the clay modified electrodes for FIA the same procedure was applied. A Clay–BSA–GOx–glutaraldehyde mixture was spread over the glassy carbon electrode (GCE).
1 ethanol distilled water. Then, 5.0 μL of Mont solution (1.0 mg mL−1 in PBS), 2.5 μL of BSA (1.0 mg mL−1 in PBS), 0.5 mg GOx and 2.5 μL of glutaraldehyde (5.0% in PBS) were mixed. Finally, 10 μL of this mixture was dropped on the electrode surface and allowed to dry at room temperature for 1 h. To prepare the clay modified electrodes for FIA the same procedure was applied. A Clay–BSA–GOx–glutaraldehyde mixture was spread over the glassy carbon electrode (GCE).
Electrochemical measurements
All experiments were carried out in 10 mL buffer solution. Electrodes were washed with distilled water and kept in 50 mM pH 4.0 sodium acetate for 2 min after each measurement. The enzyme electrodes were initially equilibrated in buffer and glucose as a substrate was added to the reaction cell. During this measurement, an enzymatic reaction proceeds on two half reactions namely a reduction and a following oxidative step. In the first step, GOx catalyzes the oxidation of β -D-glucose to D-glucono-δ-lactone, which is spontaneously converted to gluconic acid and in the second step, the reduced GOx is reoxidized by oxygen to yield H2O2. The decrease in the amount of oxygen is monitored at −0.7 V with respect to Ag/AgCl electrode as a result of enzymatic activity. The biosensor responses were registered as the current signal (μA). Buffer was refreshed after each measurement.Sample application
The clay based GOx biosensor was used to analyze the glucose concentration in a white wine sample. The sample was also analyzed with HPLC-RID. The glucose concentration in the real sample was measured by direct injection of proper amount of beverage samples into buffer, instead of glucose. The amount of glucose in samples was calculated from corresponding calibration curves obtained with the standard glucose solutions.Conclusions
Immobilization of biomolecules in clay matrices is inexpensive, practical and a faster way compared to some other methods in the literature.36–38 In the present study, clay was used as an electrode material and GOx was chosen as a model enzyme to prepare clay based biosensors. After optimization of preparation and working conditions, the Mont/GOx biosensor was calibrated for glucose in batch and FIA systems. The proposed method was applied for glucose determination in real samples. The obtained data for the clay biosensor were in good agreement with HPLC-RID analysis demonstrating that the bioelectrochemical devices are suitable for the determination of analytes in real samples.References
- C. Mousty, Anal. Bioanal. Chem., 2010, 396, 315–325 CrossRef CAS.
- M. Xia, Y. Jiang, L. Zhao, F. Li, B. Xue, M. Sun, D. Liu and X. Zhang, Colloids Surf., A, 2010, 356, 1–9 CrossRef CAS.
- J. Pang, C. Fan, X. Liu, T. Chen and G. Li, Biosens. Bioelectron., 2003, 19, 441–445 CrossRef CAS.
- D. Shan, S. Cosnier and C. Mousty, Anal. Chem., 2003, 75, 3872–3879 CrossRef CAS.
- Q. Fan, D. Shan, H. Xue, Y. He and S. Cosnier, Biosens. Bioelectron., 2007, 22, 816–821 CrossRef CAS.
- D. Shan, C. Mousty, S. Cosnier and S. Mu, Electroanalysis, 2003, 15, 1506–1512 CrossRef CAS.
- D. Shan, C. Mousty and S. Cosnier, Anal. Chem., 2004, 76, 178–183 CrossRef CAS.
- G. Sanjay and S. Sugunan, Catal. Commun., 2005, 6, 525–530 CrossRef CAS.
- S. Gopinath and S. Sugunan, Appl. Clay Sci., 2007, 35, 67–75 CrossRef CAS.
- S. Cosnier, F. Lambert and M. Stoytcheva, Electroanalysis, 2000, 12, 356–360 CrossRef CAS.
- P. Wu, Z. Cai, J. Chen, H. Zhang and C. Cai, Biosens. Bioelectron., 2011, 26, 4012–4017 CrossRef CAS.
- V. P. Zanini, B. López de Mishima and V. Solis, Sens. Actuators, B, 2011, 155, 75–80 CrossRef.
- R. I. Iliescu, E. Andronescu, G. Voicu, A. Ficai and C. I. Covaliu, Appl. Clay Sci., 2011, 52, 62–68 CrossRef CAS.
- G. Ozdemir, M. Hoşgör Limoncu and S. Yapar, Appl. Clay Sci., 2010, 48, 319–323 CrossRef CAS.
- F. H. Lin, Y. H. Lee, C. H. Jian, J. M. Wong, M. J. Shieh and C. Y. Wang, Biomaterials, 2002, 23, 1981–1987 CrossRef CAS.
- N. Zhou, S. Fang, D. Xu, J. Zhang, H. Mo and J. Shen, Appl. Clay Sci., 2009, 46, 401–403 CrossRef CAS.
- J. Wang and T. Martinez, Electroanalysis, 1989, 1, 167–172 CrossRef CAS.
- P. Kula and Z. Navratilova, Fresenius'J. Anal.Chem., 1996, 354, 692–695 CAS.
- W. Huang, C. Yang and S. Zhang, Anal. Bioanal. Chem., 2002, 374, 998–1001 CrossRef CAS.
- S. H. Wang and T. C. Chou, Electroanalysis, 2000, 12, 468–470 CrossRef CAS.
- T. Wieglos and A. Fitch, Electroanalysis, 1990, 2, 449–454 CrossRef.
- S. Hu, C. Xu, G. Wang and D. Ciu, Talanta, 2001, 54, 115–123 CrossRef CAS.
- P. W. Faguy, W. Ma, J. A. Lowe, W. P. Pan and T. Brown, J. Mater. Chem., 1994, 4, 771–772 RSC.
- Y. Liju, C. F. Jang, P. Tuzhi, Y. Hangsheng, G. Cong and L. Guoqing, Electroanalysis, 1999, 11, 438–442 CrossRef.
- B. Chen, L. Wang, X. Huang and P. Wu, Microchim. Acta, 2011, 172, 335–341 CrossRef CAS.
- R. G. B. Bouwe, I. K. Tonle, S. Letaief, E. Ngameni and C. Detellier, Appl. Clay Sci., 2011, 52, 258–265 CrossRef CAS.
- S. Snircova, E. Jona, L. Lajdova, V. Jorik, M. Drabik, M. Pajtasova, D. Ondrusava and S. C. Mojumdar, J. Therm. Anal. Calorim., 2009, 96, 63–66 CrossRef CAS.
- http://www.brenda-enzymes.org. .
- S. Poyard, N. Jaffrezic-Renault, C. Martelet, S. Cosnier, P. Labbe and J. L. Besombes, Sens. Actuators, B, 1996, 33, 44–49 CrossRef.
- D. Shan, J. Zhang, H.-G. Xue, S. N. Ding and S. Cosnier, Biosens. Bioelectron., 2010, 25, 1427 CrossRef CAS.
- S. N. Ding, D. Shan, H. G. Xue, D. B. Zhu and S. Cosnier, Anal. Sci., 2009, 25, 1421–1425 CrossRef CAS.
- Ping Yi Liang, Pei Wen Chang and Chong Mou Wang, J. Electroanal. Chem., 2003, 560, 151–159 CrossRef CAS.
- C. Lei, Z. Zhang, H. Liu and J. Deng, J. Electroanal. Chem., 1996, 419, 93–98 CrossRef CAS.
- X. Han, Y. Zhu, X. Yang and C. Li, Microchim. Acta, 2010, 171, 233–239 CrossRef CAS.
- E. E. Saka and C. Guler, Clay Miner., 2006, 41, 853–861 CrossRef CAS.
- M. Akin, M. Yuksel, C. Geyik, D. Odaci, A. Bluma, T. Höpfner, S. Beutel, T. Scheper and S. Timur, Biotechnol. Prog., 2009, 26, 896–906 CrossRef.
- M. Yuksel, M. Akin, C. Geyik, D. Odaci Demirkol, C. Ozdemir, A. Bluma, T. Höpfner, S. Beutel, S. Timur and T. Scheper, Biotechnol. Prog., 2011, 27, 530–538 CrossRef CAS.
- M. Akin, A. Prediger, M. Yuksel, T. Höpfner, D. Odaci Demirkol, S. Beutel, S. Timur and T. Scheper, Biosens. Bioelectron., 2011, 26, 4532–4537 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
