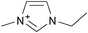
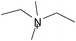
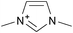Cellulose solubilities in carboxylate-based ionic liquids†
Bin
Zhao
a,
Lasse
Greiner
*ab and
Walter
Leitner
*ac
aInstitut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringerweg 1, 52056, Aachen, Germany
bDECHEMA e. V. Karl-Winnacker-Institut, Theodor-Heuss-Allee 25, 60486, Frankfurt am Main, Germany. E-mail: greiner@dechema.de; Fax: + 49-(0)697564-388; Tel: + 49-(0)697564-428
cMax-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany
First published on 2nd February 2012
Abstract
The dissolution of cellulose allows easier processing of this important biogenic feedstock. For this, ionic liquids have been proposed. Carboxylate-based ionic liquids were identified as the most promising lead towards a high dissolution property. Three homologous series of all 27 combination of the three cations: 1-ethyl-3-methylimidazolium, 1,3-dimethylimidazolium, and N,N-diethyl-N,N-dimethylammonium with nine carboxylates as anions were synthesised. The cellulose solubilities of the 17 ionic liquid compounds (liquid below 373 K) were measured. Up to 18 wt% for 1-ethyl-3-methyl-imidazolium propionate was achieved, slightly higher than when using acetate as the anion. Generally, the solubilities determined for carboxylate-based ionic liquids with imidazolium cations were found to be in the same range, whereas those with quaternary ammonium cations were found to be poor solvents for cellulose. Dicarboxylates gave higher solubilities compared to monocarboxylates. Regenerated ionic liquids had no apparent difference to fresh ones.
Introduction
Cellulose is the major carbohydrate produced by plant photosynthesis and therefore an important biogenic feedstock.1–8 However, poor solubility in conventional solvent systems limits its processing. Ionic liquids (IL) with their low volatility, low flammability, high thermal stability and tunable physicochemical properties are considered an alternative to dissolve cellulose.3–15To foster understanding of the structure activity relationship, a systematic approach towards the synthesis and experimental determination was carried out. This data is mandatory for the future design of novel and superior solvent systems. Therefore, by surveying the current literature2,4–8,11,15–25 and extrapolating possible trends, the synthesis of a set of IL was carried out and used for the experimental determination of cellulose solubility at different temperatures.
Experimental
The α-cellulose and anion exchange resins IRA-400(OH) were purchased from Sigma-Aldrich. All other chemicals were obtained from Alfa Aesar.1H NMR and 13C NMR spectra were recorded on a AV400 MHz NMR (Bruker BioSpin). Water content was determined by coulometric Karl–Fischer titration (Metrohm). Ion chromatography was carried out using ICS-1500 (Dionex). The detection threshold is 10 ppm for all halides. Melting and glass transition temperatures were recorded with differential scanning calorimetry (DSC) (NETZSCH DSC 204). Samples were placed in a sealed aluminum pan with a pinhole. An empty pan was used as the reference. Measurements were carried out by heating from 173 K to 423 K at a rate of 10 K min−1 under nitrogen atmosphere. Decomposition temperatures were measured with NETZSCH TG 209. All samples were run in aluminum oxide pans under nitrogen atmosphere at a heating rate of 10 K min−1.
Three homologous series of cation structures: 1,3-dimethylimidazolium carboxylate ([DMIm] carboxylate, 1a–i), 1-ethyl-3-methylimidazolium Carboxylate ([EMIm] carboxylate, 2a–i) and N,N-diethyl-N,N-dimethylammonium Carboxylate ([DEDMN] carboxylate, 3a–i) were synthesised (Table 1). For experimental details and the characterisation of these structures see the ESI.†
| Cation | 1,3-Dimethylimidazolium | 1-Ethyl-3-methylimidazolium | N,N-Diethyl-N,N-Dimethylammonium |
|---|---|---|---|
| Anion |
|
|
|
| Formate: | white solid | white solid | white solid |
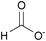
|
1a [DMIm] formate | 2a [EMIm] formate | 3a [DEDMN] formate |
| Acetate: | yellowish solid | yellowish liquid | white solid |
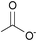
|
1b [DMIm] acetate | 2b [EMIm] acetate | 3b [DEDMN] acetate |
| Propionate: | yellowish liquid | yellowish liquid | yellowish solid |
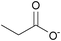
|
1c [DMIm] propionate | 2c [EMIm] propionate | 3c [DEDMN] propionate |
| n-Butyrate: | colorless liquid | colorless liquid | white solid |
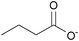
|
1d [DMIm] butyrate | 2d [EMIm] butyrate | 3d [DEDMN] butyrate |
| iso-Butyrate: | colorless liquid | colorless liquid | white solid |
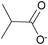
|
1e [DMIm] iso-butyrate | 2e [EMIm] iso-butyrate | 3e [DEDMN] iso-butyrate |
| mono-Maleate: | white solid | colorless liquid | colorless liquid |

|
1f [DMIm] mono-maleate | 2f [EMIm] mono-maleate | 3f [DEDMN] mono-maleate |
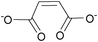
| Maleate: | white solid | colorless liquid | white solid |
|
1g Bis[DMIm] maleate | 2g Bis[EMIm] maleate | 3g Bis[DEDMN] maleate |
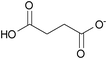
| mono-Succinate: | white solid | colorless liquid | white solid |
|
1h [DMIm] mono-succinate | 2h [EMIm] mono-succinate | 3h [DEDMN] mono-succinate |
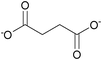
| Succinate: | white solid | white solid | white solid |
|
1i Bis[DMIm] succinate | 2i Bis[EMIm] succinate | 3i Bis[DEDMN] succinate |
[DMIm] carboxylates (1a–1i): The intermediate 1,3-Dimethylimidazolium-2-carboxylate was prepared according to the literature.26 Then with water as solvent, a stoichiometric amount of the corresponding acid was added. After 3 h reaction at 343 K, the mixture was dried under reduced pressure to obtain the product.
[EMIm] carboxylates (2a–2i): The 1-ethyl-3-methylimidazolium carboxylates were prepared in analogy via 1-ethyl-3-methylimidazolium-2-carbonate by the procedure described above or according to literature.27,28 It also can be prepared by using 1-ethyl-3-methylimidazolium hydrogen carbonate as the intermediate.
[DEDMN] carboxylates (3a–3i): A modified literature procedure was used.29 Under argon atmosphere, N,N-diethylammonium chloride and dimethylcarbonate were added into a 75 mL autoclave and stirred at 383 K overnight. After drying under reduced pressure and washing with acetone, the crude intermediate [DEDMN] chloride was obtained in 90% yield. By anion exchange (IRA-400 (OH)) the corresponding hydroxide was obtained. The hydroxide was neutralised with a stoichiometric amount of the corresponding acid. After removal of water under reduced pressure, the product was obtained.
α-Cellulose was dried at 373 K and IL at 333 K under reduced pressure of 5 Pa for 12 h. 1.0 g IL was kept in a Schlenk tube immersed in an oil bath. Starting at 333 K, α-cellulose was added in increments of 10 mg under argon atmosphere. After stirring for 30 min the solution was either clear and another 10 mg cellulose was added. Otherwise the temperature was increased by 20 K, up to the maximum temperature of 373 K.
Results and discussion
To avoid impurities and their impact on the physical properties of ionic liquids,9,30 we chose a straightforward route to obtain imidazolium carboxylates. A two step approach via the carboxylate–type zwitterion and subsequent neutralisation with acid was used which allows the avoidance of halides and other impurites.26,31,32 Care has to be taken with chlorinated compounds as we found them to be susceptible to dehalogenation by carboxylate anions.33 As this access is not possible for quaternary ammonium carboxylates we used the three-step method via alkylation with ammonium halide, anion exchange, and acid neutralisation. Three homologous series with 27 combinations of the three cations in total: [EMIm], [DMIm] and [DEDMN] with nine carboxylates as anions were synthesised (Table 1). Of them seventeen compounds actually turned out to be ILs as they are fluids below 373 K. For all [EMIm] carboxylates and [DMIm] carboxylates, the halide content is less than 10 ppm. To the best of our knowledge, fifteen compounds 1d, 1e, 1g, 1h, 1i, 2e, 2h, 2i, 3a, 3c, 3d, 3e, 3g, 3h, and 3i have not been reported before.Thermal properties of the synthesised structures
Differential scanning calorimetry (DSC) and thermogravity analysis (TGA) techniques were used to characterise phase behaviour and thermostability respectively. The results are summarised in Table 2.| 1a | 1b | 1c | 1d | 1e | 1f | 1ge | 1h | 1ie | |
|---|---|---|---|---|---|---|---|---|---|
| a T m: melting temperature. b T g: onset glass transition temperature. c T d: onset decomposition temperature. d In line with results reported previously.34 e no phase transition within the inspected temperature range. f All these phase transitions are most probably crystal structure rearrangements. | |||||||||
| T m /K | 363 | 296 | — | — | — | 394 | — | 384 | — |
| T g /K | — | — | — | 197 | 215 | — | — | — | — |
| T d /K | 494 | 490 | 486 | 487 | 485 | 472 | 441 | 508 | 495 |
| 2a | 2b | 2ce | 2d | 2e | 2f | 2g | 2h | 2ie | |
| T m /K | 329 | — | — | — | 209 | — | — | — | — |
| T g /K | — | 203d | 200 | 195 | — | 193 | 216 | 236 | — |
| T d /K | 495 | 492 | 488 | 511 | 499 | 468 | 438 | 511 | 499 |
| 3af | 3b | 3cf | 3d | 3ef | 3f | 3ge | 3hf | 3if | |
| T m /K | — | 357 | — | 356 | — | 277 | — | — | — |
| T g /K | — | — | — | — | — | — | — | — | — |
| T d /K | 450 | 459 | 455 | 454 | 452 | 501 | 463 | 485 | 464 |
For 18 salts no melting temperature can be given. According to the DSC and TGA results, eight of them show glass transition at temperatures of 190 to 240 K. Decomposition temperatures for all compounds are above 430 K.
The cellulose solubilities of these compounds are given in Fig. 1. Mono-succinate or mono-maleate effectively blocks the hydrogen bond formation between cellulose and the anions, resulting in no detectable cellulose solubility (2f, 2h, 3f). In comparison, 2g and 2i with two carboxylate groups show some solubility for cellulose at elevated temperature. Generally, bigger anions give lower solubility. Comparing succinate to maleate, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond also negatively affects solubility.
C double bond also negatively affects solubility.
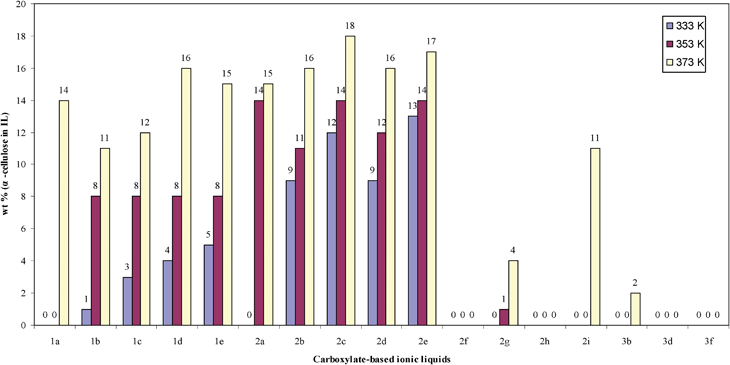 | ||
| Fig. 1 α-cellulose solubilities of carboxylate-based ionic liquids as a function of structure at 333, 353, 373 K. | ||
Alternative to commonly used cellulose solvent [EMIm]Ac (2b), other carboxylate-based ionic liquids (2a, 2c, 2d, 2e) have similar cellulose solubility. Considering their similar β value, which describes the anion basicity and correlates with the ability of anion to expand and dissolve pine lignocellulose, from Kamlet–Taft parameters (the β values of formate, acetate, propionate and n-butyrate are 1.01, 1.09, 1.10, 1.10, respectively.),6,18 and different anion concentrations per gram IL (the anion concentrations of 2a, 2b, 2c, 2d and 2e are: 6.4, 5.9, 5.4, 5.0, 5.0 mmol g−1, respectively), the similar results with [EMIm]Ac are not too surprising. With the same anion, [EMIm] as the cation has a higher cellulose solubility than [DMIm]. Quaternary ammonium as a cation has lower cellulose solubility. Although the influence of the cation on cellulose solubility is less important than the anion, it cannot be neglected.
Water content and halide content influence
Water content in ILs significantly affects cellulose solubility.19,20,35 Swatloski et al. reported that 1 wt% water can dramatically decrease the cellulose solubility and make cellulose precipitate from its ionic liquid solution.7 In order to avoid the water influence, the dissolution was carried out under argon atmosphere, and cellulose and IL were dried for at least 12 h under reduced pressure. Due to the high hydrophilicity of carboxylate-based ionic liquids, a typical water content of 103 ppm was obtained.Our results for [EMIm]Ac (2b) for the dissolution of cellulose showed that a water content of less than 2500 ppm does not affect cellulose solubility (Table 3).
| Entry | Compounds | 333 K/wt (%) | 353 K/wt (%) | 373 K/wt (%) | Water Content/ppm | Chloride Content/ppm |
|---|---|---|---|---|---|---|
| a Chloride method detection threshold of ion chromatography is 10 ppm. b Purchased from Iolitec Co. c EMImAcetate was synthesised according to the literature.36 d EMImAcetate was synthesised according to the synthetic route described. | ||||||
| 1 | [EMIm]Acetateb | 9 | 11 | 16 | 1262 | 1192 |
| 2 | [EMIm]Acetateb | 10 | 12 | 16 | 1423 | 1192 |
| 3 | [EMIm]Acetateb | — | 11 | — | 889 | 1192 |
| 4 | [EMIm]Acetatec | — | 11 | — | 1981 | 44 |
| 5 | [EMIm]Acetated | 9 | 13 | 15 | 2513 | < 10a |
Since chloride ions may severely affect the properties of ionic liquids even in trace amounts,21,30 we investigated the effect of chloride content on dissolution. Results showed that chloride content less than 1192 ppm do not influence on cellulose solubility (Entry 3–5).
We chose several cellulose-ionic liquids solutions (1b, 1d, 2b, 2d, 2i) as representatives to investigate the changes of ionic liquids through dissolving cellulose. After adding water to precipitate the cellulose, the IL were regenerated. After removing water under reduced pressure and drying overnight at 333 K the 1H NMR and 13C NMR showed no apparent differences.
Conclusions
Three series of ILs based on carboxylate anions and [EMIm], [DMIm] and [DEDMN] as cations were synthesised. All imidazolium based ILs were obtained with a straightforward halide-free method in high purity. Cellulose solubilities revealed similar solubilities to those reported for the best systems of up to 18 wt%. Generally, imidazolium based systems showed higher solubility than quaternary ammonium based [DEDMN] ILs. These findings broaden the number of applicable ILs for cellulose processing.Acknowledgements
This work was performed as part of the Cluster of Excellence “Tailor-Made Fuels from Biomass”, which is funded by the Excellence Initiative of the German federal and state governments to promote science and research at German universities. We also would like to thank Prof. Dr Crisan Popescu from DWI RWTH Aachen for thermal property measurements.References
- T. R. Dawsey and C. L. McCormick, J. Macromol. Sci., Part C, 1990, 30, 405–440 CrossRef.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44–46 RSC.
- W. Leitner, P. G. Jessop, C.-J. Li, P. Wasserscheid and A. Stark, Handbook of Green Chemistry—Green Solvents: 2, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2010 Search PubMed.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS.
- M. g. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Energy Fuels, 2010, 24, 737–745 CrossRef CAS.
- H. Ohno and Y. Fukaya, Chem. Lett., 2009, 38, 2–7 CrossRef CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS.
- T. F. Liebert, T. J. Heinze and K. J. Edgar, Cellulose Solvents: For Analysis, Shaping and Chemical Modification, American Chemical Society, 2010 Search PubMed.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, 2nd Ed., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007 Search PubMed.
- J. Kahlen, K. Masuch and K. Leonhard, Green Chem., 2010, 12, 2172–2181 RSC.
- Z. Chen, S. Liu, Z. Li, Q. Zhang and Y. Deng, New J. Chem., 2011, 35, 1596–1606 RSC.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi and I. Kilpeläinen, Angew. Chem., Int. Ed., 2011, 50, 6301–6305 CrossRef CAS.
- N. Sun, H. Rodriguez, M. Rahman and R. D. Rogers, Chem. Commun., 2011, 47, 1405–1421 RSC.
- P. Mäki-Arvela, I. Anugwom, P. Virtanen, R. Sjöholm and J. P. Mikkola, Ind. Crops Prod., 2010, 32, 175–201 CrossRef.
- A. Pinkert, K. N. Marsh and S. Pang, Ind. Eng. Chem. Res., 2010, 49, 11121–11130 CrossRef CAS.
- H. Garcia, R. Ferreira, M. Petkovic, J. L. Ferguson, M. C. Leitao, H. Q. N. Gunaratne, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Green Chem., 2010, 12, 367–369 RSC.
- A. A. Rosatella, L. C. Branco and C. A. M. Afonso, Green Chem., 2009, 11, 1406–1413 RSC.
- A. Brandt, J. P. Hallett, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2010, 12, 672–679 RSC.
- A. Ostlund, D. Lundberg, L. Nordstierna, K. Holmberg and M. Nyden, Biomacromolecules, 2009, 10, 2401–2407 CrossRef.
- J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC.
- Q. Liu, M. H. A. Janssen, F. v. Rantwijk and R. A. Sheldon, Green Chem., 2005, 7, 39–42 RSC.
- H. Zhang, J. Wu, J. Zhang and J. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- A. Xu, J. Wang and H. Wang, Green Chem., 2010, 12, 268–275 RSC.
- H. Liu, K. L. Sale, B. M. Holmes, B. A. Simmons and S. Singh, J. Phys. Chem. B, 2010, 114, 4293–4301 CrossRef CAS.
- J. Guo, D. Zhang, C. Duan and C. Liu, Carbohydr. Res., 2010, 345, 2201–2205 CrossRef CAS.
- J. D. Holbrey, W. M. Reichert, I. Tkatchenko, E. Bouajila, O. Walter, I. Tommasi and R. D. Rogers, Chem. Commun., 2003, 28–29 RSC.
- C. Rijksen and R. D. Rogers, J. Org. Chem., 2008, 73, 5582–5584 CrossRef CAS.
- B. Bantu, G. M. Pawar, K. Wurst, U. Decker, A. M. Schmidt and M. R. Buchmeiser, Eur. J. Inorg. Chem., 2009, 2009, 1970–1976 CrossRef.
- Z. Q. Zheng, J. Wang, T. H. Wu and X. P. Zhou, Adv. Synth. Catal., 2007, 349, 1095–1101 CrossRef CAS.
- K. R. Seddon, A. Stark and M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275 CrossRef CAS.
- M. Smiglak, J. D. Holbrey, S. T. Griffin, W. M. Reichert, R. P. Swatloski, A. R. Katritzky, H. F. Yang, D. Z. Zhang, K. Kirichenko and R. D. Rogers, Green Chem., 2007, 9, 90–98 RSC.
- N. J. Bridges, C. C. Hines, M. Smiglak and R. D. Rogers, Chem.–Eur. J., 2007, 13, 5207–5212 CrossRef CAS.
- B. Zhao, L. Greiner and W. Leitner, Chem. Commun., 2011, 47, 2973–2975 RSC.
- S. Troshenkova, E. Sashina, N. Novoselov, K. Arndt and S. Jankowsky, Russ. J. Gen. Chem., 2010, 80, 106–111 CrossRef CAS.
- M. Zavrel, D. Bross, M. Funke, J. Büchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS.
- M. Maase, K. Massone and L. Szarvas, US Pat. 2007/0142646, 2007 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: characterisation of carboxylate-based ionic liquids, and their appearances. See DOI: 10.1039/c2ra01224c/ |
| This journal is © The Royal Society of Chemistry 2012 |