Room temperature solution synthesis of hierarchical bow-like Cu2O with high visible light driven photocatalytic activity†
Xiangying
Meng
,
Guohui
Tian
,
Yajie
Chen
,
Yang
Qu
,
Juan
Zhou
,
Kai
Pan
,
Wei
Zhou
,
Guoliang
Zhang
and
Honggang
Fu
*
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education of the People's Republic of China, Heilongjiang University, Harbin 150080, P. R. China. E-mail: fuhg@vip.sina.com
First published on 10th February 2012
Abstract
Novel hierarchical bow-like Cu2O crystals were successfully synthesized via a facile room temperature solution reaction using PVP as a structure-directing agent in the presence of NaBH4. The morphology evolution of the hierarchical bow-like Cu2O crystals were observed to be tunable as a function of reaction parameters, such as the reaction time, the quality of PVP and the reaction temperature. The possible growth mechanism of hierarchical bow-like Cu2O crystals was investigated. It involves the formation process of the intermediate octahedra Cu2O crystals and subsequent oxidation–erosion process from octahedra to hierarchical bow-like Cu2O crystals. It was found that the octahedra Cu2O crystals are a necessary intermediate for the formation of the bow-like Cu2O crystals. The prepared hierarchical bow-like Cu2O crystals exhibited a higher photocatalytic activity for photodegradation of rhodamine B aqueous solution under visible light illumination than the other prepared Cu2O crystal samples with different morphologies (nanoparticles and octahedra) because of its large surface area and specific hierarchical bow-like structure.
1. Introduction
Over the past few years, TiO2 has been one of the most commonly used semiconductor materials in the fields of photocatalysis and environmental pollution control, owing to its cheapness, strong oxidizing power and non-toxicity.1 Unfortunately, due to its wide band gap (3.2 eV for anatase TiO2), TiO2 can only be excited by ultraviolet or near-ultraviolet radiation, which occupies only about 4% of the solar light spectrum.2 Therefore, in order to effectively utilize solar energy and further meet the requirements for environmental pollutant treatment, a great deal of effort has been made to develop heterogeneous visible light-driven photocatalysts.Cuprous oxide (Cu2O), as one of the new-style p-type semiconducting materials, has a high optical absorption coefficient and a bulk band gap of 2.17, which makes it an excellent candidate in some applications. It also shows its performance in practical applications in solar energy conversion,3–5 hydrogen production by photocatalysis of water,6,7 for gas sensing,8,9 photodegradation of dye molecules,10 CO oxidation,11 and as an electrode material.12 It is well known that the properties of Cu2O were strongly dependent on its morphology and microstructure. Thus, morphology controlled synthesis becomes an important issue. In recent years, Cu2O with various morphologies have been explored, such as nanowires,13 nanotubes,14 hollow spheres,15,16 multi-facet Cu2O,17 nanocubes18–21 and octahedral Cu2O.22,23 However, there are few reports about the synthesis of three dimension hierarchical Cu2O with a large surface area. Hierarchical architectures with large surface areas and porous structures have triggered more and more research enthusiasm in recent years due to their high surface-to-volume ratio and permeability, and showed distinctive physicochemical properties in comparison with conventional nanocrystallites.24–26 The unique properties inherent to well-defined three dimension hierarchical structures may offer extraordinary potential applications in photocatalysis and gas sensors.
In this study, we reported a facile method to synthesize novel hierarchical bow-like Cu2O crystals in the presence of PVP and NaBH4. The evolutionary diversification of the product morphology can be controlled by varying the reaction conditions, such as, the reaction time, the quality of PVP and the reaction temperature. The growth mechanism of hierarchical bow-like Cu2O crystals was investigated in detail based on observations from a time-dependent morphology evolution process. Photocatalytic experiments of these Cu2O crystal nanostructures were investigated using the degradation of rhodamine B under visible light irradiation to see how different shapes effect the photocatalytic activity. The enhanced photocatalytic activity of the bow-like Cu2O is attributed to the special hierarchical bow-like porous structure and large surface area.
2. Experimental section
2.1 Synthesis of hierarchical bow-like Cu2O crystals
Cu2O microcrystals were synthesized using a facile solution reaction at room temperature. In a typical procedure, 1.5 mmol (0.2995 g) Cu(CH3COO)2·H2O was dissolved in 90 mL deionized water with vigorous stirring, and 0.15 g polyvinylpyrrolidone (PVP, K-30; MW = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was added in the solution. Then, 1.2 ml of frozen fresh NaBH4 (0.3 mol L−1) was slowly dropped into the mixed solution with a syringe. The color of the solution changes from blue to orange quickly, and finally to brick-red, indicating the formation of cuprous oxide. The whole reaction process was carried out for varied periods of time (10, 60, 90, 170 and 200 min) under magnetic stirring. The resulting precipitate was washed with distilled water and absolute ethanol five times and dried at 50 °C in a vacuum oven for 5 h.
000) was added in the solution. Then, 1.2 ml of frozen fresh NaBH4 (0.3 mol L−1) was slowly dropped into the mixed solution with a syringe. The color of the solution changes from blue to orange quickly, and finally to brick-red, indicating the formation of cuprous oxide. The whole reaction process was carried out for varied periods of time (10, 60, 90, 170 and 200 min) under magnetic stirring. The resulting precipitate was washed with distilled water and absolute ethanol five times and dried at 50 °C in a vacuum oven for 5 h.
2.2. Characterization
The X-ray diffraction (XRD) of powder samples was examined on a Rigaku-Dmax 2500 diffractometer using Cu Kα radiation (λ = 0.15405 nm, 40 kV, 100 mA). Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images of samples were recorded in a JEOL 2100 microscope with a 200 kV accelerating voltage, scanning electron microscope (SEM, Hitachi, S-4800). Nitrogen adsorption–desorption isotherms were collected on an ASAP 2420 (Micrometrics Instruments) nitrogen adsorption apparatus at 77 K. The pore size distribution plots were obtained using the Barret-Joyner-Halenda (BJH) model. UV-visible diffuse reflectance spectra (DRS) were determined using a UV-vis spectrophotometer (Shimadzu UV-2550).2.3. Measurement of photocatalytic activity
The photodegradation experiments were performed in a slurry reactor containing 50 mL of a 10 mg L−1 solution of rhodamine B (RhB) and 0.05 g of catalyst. A 150 W Xe lamp was used as a visible-light source, and a 420 nm cutoff filter was placed above the reactor to cutoff UV light. Prior to irradiation, the suspension was kept in the dark under stirring for 30 min to ensure the establishment of an adsorption/desorption equilibrium. At given time intervals, 2 mL aliquots were collected from the suspension and immediately centrifuged, the concentration of RhB after illumination was determined at 554 nm using a UV–vis spectrophotometer (Shimadzu UV-2550).3. Results and discussion
3.1. XRD characterization of Cu2O crystals
The composition and phase purity of the products prepared from different reaction times were characterized by XRD, as shown in Fig. 1. The XRD patterns show the expected (110), (111), (200), (220), (311), and (222) diffraction peaks corresponding to crystal planes of the Cu2O crystals. No other peaks are observed, indicating that the products are phase-pure Cu2O crystals in all samples, and there is no impurity such as cupric oxide or metallic copper. It is worth noting that the crystalline degree of the products has influence on the peak intensity as well. The facets with a slower growth rate will be exposed more on the crystal surface and consequently exhibit relatively stronger diffraction intensity in the corresponding XRD patterns. Moreover, each crystal face diffraction peak intensity of Cu2O crystal samples in the XRD patterns gradually increases with increasing reaction time from 10 min to 90 min (Fig. 1a–c). When the reaction time increased further, the diffraction intensity of all the crystal planes decreased (Fig. 1d–f). It indicated the decrease in crystallization and transformation from single-crystal to poly-crystal of cuprous oxide resulting from the oxidation–erosion process, which will be further discussed in the section on the growth mechanism of the Cu2O crystals.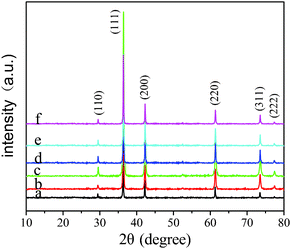 | ||
| Fig. 1 XRD patterns of the Cu2O crystal samples prepared at different reaction times: (a) 10 min, (b) 60 min, (c) 90 min, (d) 150 min, (e) 170 min, and (f) 200 min. | ||
3.2. Growth process of hierarchical bow-like Cu2O crystals
Generally, the growth process of crystals is a kinetically and thermodynamically controlled process that can form different shapes with some degree of shape tunability by changing the reaction parameters.27 In our experiment, we focus on the study of the influence of reaction time, quality of PVP and reaction temperature on the ultimate shapes of the Cu2O crystals.In order to more clearly disclose the growth process of the cuprous oxide, time-dependent morphology evolution experiments were performed. Fig. 2 shows SEM images of the cuprous oxides obtained at different reaction stages. They show the interesting morphology evolution from nanoparticle to bow-like structure of the Cu2O crystals. We intercepted the intermediate products at different reaction times from 10 min to 200 min. At the initial reaction stage (10 min), no nucleation is involved in this process, but only Cu2O nanoparticles with a diameter of about 15 nm are obtained (Fig. 2a). When the reaction time is 60 min, truncate octahedra Cu2O crystals were formed (Fig. 2b). The surface of the truncate octahedra is covered with both {100} and {111} faces, but it is mainly occupied by the {111} faces. While the {100} surfaces constantly decrease in area, concurrently, the {111} planes continually gain in area, eventually leading to an octahedral morphology when the reaction time was prolonged to 90 min (Fig. 2c). The octahedra Cu2O crystals have smooth surfaces with lengths of about 500 nm, which agrees with the TEM investigation (Fig. 3a). The HRTEM image in (Fig. 3b) shows the fringe spacing of 0.25 nm corresponds well to that of the lattice space of {111} of the Cu2O crystals. The corresponding SAED pattern (inset of Fig. 3b) of the octahedra Cu2O crystals shows a single crystal. When the reaction time is extended to 150 min, bow-like Cu2O crystals (Fig. 2d) appear, which result from the oxidation–erosion and sedimentary growth process of the octahedra Cu2O crystals. The length of a bow-like Cu2O crystal is about 2.5 μm. The high magnification SEM (Fig. S1†) and TEM image (Fig. 3c) show that each petal of the bow-like Cu2O crystals is composed of well-defined sheaves of nanoribbons with a lamellar ribbon-like structure about 2.5 μm in length. The nanoribbons are typically 30–100 nm in width and several nanometres in thickness. The fringes in a typical HRTEM image (Fig. 3b) are separated by 0.25 nm, in good agreement with the {111} lattice spacing of the Cu2O crystals. The corresponding SAED pattern (Fig. 3d inset) of a bow-like Cu2O crystal shows its polycrystalline structure.
 | ||
| Fig. 2 SEM images of the Cu2O crystal samples prepared at different reaction times: (a) 10 min, (b) 60 min, (c) 90 min, (d) 150 min, (e) 170 min, and (f) 200 min. | ||
 | ||
| Fig. 3 (a) TEM image of an octahedra Cu2O crystal, (b) HRTEM image of an octahedra Cu2O crystal; (c) TEM image of a bow-like Cu2O crystal, (d) HRTEM image of a bow-like Cu2O crystal. Insets are the corresponding SAED patterns. | ||
3.3. Influence of reaction conditions on the morphologies of Cu2O crystals
As well as reaction time, the role of PVP was found to be critical in morphology control. It is generally thought that the structure-directing agent of PVP can also be added purposely to a solution-phase synthesis to control the shape of nanostructures, because the presence of a structure-directing agent of PVP can change the order of free energies for different crystallographic planes, and thus change their relative growth rates by its chemical interaction with a metallic oxide surface. A crystal surface with a lower growth rate will be more exposed on the nanocrystal surface. To investigate the influence of PVP on the morphological evolution of the Cu2O crystals, control experiments using different amounts of PVP (0 g, 0.05 g, 0.15 g) were carried out, keeping the other experimental conditions identical (the temperature is 20 °C). Only irregular polyhedron Cu2O crystals can be observed without PVP after 90 min (Fig. 4a). When the reaction proceeds for 200 min, the obtained Cu2O crystals are still irregular polyhedrons with an irregular size (Fig. 4d). However, when a small amount of PVP (0.05 g) is added to the reaction system, truncated octahedron Cu2O crystals can be acquired after 90 min (Fig. 4b). It is because with the presence of PVP, the growth rates along the perpendicular direction of these three planes follow a sequence of {110}>{100}>{111}. So the surface area ratio of {111} and {100} faces of the truncated octahedra increases simultaneously, compared with the Cu2O nanoparticles. When extending the reaction time to 200 min, the shape of the truncated octahedron Cu2O crystals remain unchanged (Fig. 4e). It indicates that the influence of low content PVP on Cu2O crystal morphology is weaker or insufficient. Further increasing the amount of PVP to 0.15 g or more, the {111} planes surface energy was further decreased and the surface area of the {111} planes reached a maximum. When the reaction time was 90 min (Fig. 4c), octahedron Cu2O crystals with absolute {111} planes formed. The Cu2O crystals possess perfect octahedral morphology with well-defined edges and sharp corners, and the octahedral Cu2O crystal edge lengths are in the range of 500 nm. When further prolonging the reaction time to 200 min, bow-like Cu2O crystals appear, as shown in (Fig. 4f). However, when non-ionic surfactant PVP was replaced by the anionic surfactant sodium dodecyl sulfate (SDS, 0.1 g), only chaotic structures were formed (Fig. S2†). Similarly, when the cationic surfactant hexadecyl trimethyl ammonium bromide (CTAB, 0.1 g) was substituted instead of PVP, the obtained products were a mixture of nanoparticles and nanospheres with irregular sizes (Fig. S3†). The above results show that the introduction of PVP played an important role in the formation of octahedral Cu2O crystals, which were the necessary intermediates for the formation of bow-like structure Cu2O crystals. | ||
| Fig. 4 SEM images of the Cu2O crystals prepared from different quantities of PVP and different reaction times: (a) 0 g, 90 min, (b) 0.05 g , 90 min, (c) 0.15 g, 90 min, (d) 0 g , 200 min, (e) 0.05 g, 200 min, (f) 0.15 g, 200 min. | ||
It is also worthwhile mentioning that the reaction temperature also affects the appearance and structure of the ultimate product to a certain extent. To prove this point, we performed the experiment under different temperatures (0 °C, 20 °C and 50 °C). When the reaction temperature was 0 °C, only irregular nanoparticles were formed after 90 min (Fig. 5a). This is because the low temperature is not conducive to forming nuclei and regular shapes, leading to the formation of Cu2O nanoparticles. When the reaction time progressed to 200 min, Cu2O nanoparticles were observed (Fig. 5d) with irregular size and morphology. However, when increasing the reaction temperature to 50 °C, only irregular polyhedron Cu2O crystals could be found after 90 min (Fig. 5c). Moreover, there was no further change in morphology after 200 min (Fig. 5f). This is mainly because the high reaction temperature makes the nucleation fast and ruleless. The result shows that the reaction temperature plays an important role in the formation of octahedra Cu2O crystals, and the reaction temperature affects not only the reaction but also the nucleation and growth rates of the particles, so both higher and lower temperatures are worthless.28 Only a moderate reaction temperature (about 20 °C) can form the octahedra Cu2O crystals (Fig. 5b, reaction time is 90 min), which are the necessary intermediates of the final bow-like Cu2O crystals (Fig. 5e, reaction time is 200 min).
 | ||
| Fig. 5 SEM images of the Cu2O crystal samples obtained at different reaction times and reaction temperatures: (a) 0 °C, 90 min, (b) 20 °C, 90 min, (c) 50 °C, 90 min, (d) 0 °C, 200 min, (e) 20 °C, 200 min, (f) 50 °C, 200 min. | ||
3.4. Growth mechanism
Based on the above results, we propose that the bow-like Cu2O crystals can be obtained via a two-step process: firstly, the octahedral Cu2O crystals were formed by reduction of the copper acetate complex with sodium borohydride. Secondly, the bow-like structure Cu2O crystals were formed by an oxidation–erosion process of the octahedral Cu2O crystals using dissolved oxygen in the solution as the oxidant. When the copper acetate was put into deionized water, [Cu(H2O)6]2+ ions and Ac− anions were formed. For [Cu(H2O)6]2+, the six water molecules completely surrounded the Cu2+ ion and shielded it. When NaBH4 solution was added in the mixed solution, chemical reactions occurred and are listed as follows.The first-step process of the reaction: hydrogen, interacting with the hydroxyl ions, causes the production of solvated electrons (2). The Cu2+ ions accept an electron to form Cu+ ions (3). After Cu2+ ions are reduced to Cu+ ions, three sequence reactions can happen. First, cuprous hydroxide (CuOH) is shaped (4). The formed cuprous hydroxide (CuOH) then decomposes to Cu2O nanocrystals (5) (Fig. 2a). With extension of the reaction time, truncated octahedron Cu2O crystals were formed (Fig. 2b). Truncated octahedron Cu2O crystals contain both the {100} and {111} faces. As is known to all, the equilibrium shape that corresponds to a given γ-plot (γ is the anisotropic interfacial free energy per unit area) is given by the Wulff construction, according to which the shape is an inner convex hull bound by planes (Wulff planes).29 However, for the cubic phase, a sequence of γ {111}<γ {100}<γ {110} can be easily deduced from the distances between these three faces and the central Wulff's point.30 For the cuprite structure of cuprous oxide, both the {100} and {111} faces are very easily retained. With the increase of reaction time, the surface energy reduces further. The growth ratio R of the surface energy reached 1.73, which represents the growth rate along <100> to the <111> direction reaching the maximum R and the surface area of the {111} face also reaching the maximum.22 Simultaneously, the structure-directing agent PVP can also be added purposely to the solution to control the shape of the Cu2O nanostructures. The presence of the structure-directing agent PVP can change the order of free energies for different crystallographic planes and transform their relative growth rates by its chemical interaction with a metal surface, which is derived from the structural unit of PVP which holds the easy-to-polarization functional group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The "O" of the C
O. The "O" of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O holds negative charge, which tends to interact with the "Cu" holding positive charge. In the Cu2O crystal lattice, the surface atom structures of {110}, {100} and {111} facets are different.31–37 The {110} and {111} facets are formed by both Cu and O atoms, and surface Cu atoms with dangling bonds on {110} and {111} facets can make them positively charged, so {110} and {111} facets can be strongly protected by a negatively charged agent in the reaction environment.31–37 However, the distance between two “Cu” atoms of the {110} facets is about half of that in the {111} facets, implying the number and density of “Cu” dangling bonds in the {110} facets are higher than that of the {111} facets, so the {110} facets possess more dangling bonds and higher surface energy than that of {111} facets.33 Thus, the low surface energy planes guide the formation of well-faceted octahedra Cu2O crystals (Fig. 2c) when the reaction time is increased to 90 min. The change to color and copper ion concentration of the solutions before and after the reaction also prove the reaction process from Cu2+ to Cu2O(s) crystals (chemical reactions from ((3) to (5)). When the reaction time increased from 0 min to 90 min, the solution color changed from pale blue to colorless (a, c in Fig. S4†). It indicates that octahedron Cu2O (s) is the product of the reactions in first-step process. However, in this system, reactions ((3)→(6)) are simultaneous. With a further increase in the reaction time, the second-step of oxidation–erosion and sedimentary growth process is also under way. The octahedron Cu2O crystals are gradually oxidized by dissolved O2 in the weak acid solution (chemical reaction (6)), and the produced Cu2+ from reaction (6) form Cu2O (s) nanoparticles again through reactions (3)→(5). The produced Cu2O nanoparticles deposit on the surface of the octahedra Cu2O. This is a gradual and slow process, and the bow-like Cu2O was only partly formed when the time was 150 min (Fig. 2d). The bow-like Cu2O crystals dominated when the reaction time increased to 170 min (Fig. 2e). At last, perfect bow-like Cu2O crystals could be observed when the reaction time was increased to 200 min. It should be pointed out that dissolved O2 played a leading role in this process. If the solution is purged with N2 to remove dissolved O2 prior to oxidizing, the oxidation–erosion and sedimentary growth process can be effectively prevented, and Cu2O crystals with different morphologies were obtained (Fig. S5†). Therefore, the dissolved oxygen plays an indispensable role in forming the hierarchical bow-like Cu2O crystals. At this time (200 min), the solution color changed to pale blue (e in Fig. S4†) with the gradual increase of the Cu2+ ion concentration. It should be mentioned that, in the whole oxidation–erosion and sedimentary growth process, the Cu2O oxidation–erosion rate (6) is faster than that of the sedimentary growth of Cu2O ((3)→(5)). Finally, the bow-like Cu2O crystals disappear completely, and the Cu2+ ion content obviously increases, when the reaction time is increased to 420 min, which can be seen from the results of the atomic absorption spectrometry (Table S1†).
O holds negative charge, which tends to interact with the "Cu" holding positive charge. In the Cu2O crystal lattice, the surface atom structures of {110}, {100} and {111} facets are different.31–37 The {110} and {111} facets are formed by both Cu and O atoms, and surface Cu atoms with dangling bonds on {110} and {111} facets can make them positively charged, so {110} and {111} facets can be strongly protected by a negatively charged agent in the reaction environment.31–37 However, the distance between two “Cu” atoms of the {110} facets is about half of that in the {111} facets, implying the number and density of “Cu” dangling bonds in the {110} facets are higher than that of the {111} facets, so the {110} facets possess more dangling bonds and higher surface energy than that of {111} facets.33 Thus, the low surface energy planes guide the formation of well-faceted octahedra Cu2O crystals (Fig. 2c) when the reaction time is increased to 90 min. The change to color and copper ion concentration of the solutions before and after the reaction also prove the reaction process from Cu2+ to Cu2O(s) crystals (chemical reactions from ((3) to (5)). When the reaction time increased from 0 min to 90 min, the solution color changed from pale blue to colorless (a, c in Fig. S4†). It indicates that octahedron Cu2O (s) is the product of the reactions in first-step process. However, in this system, reactions ((3)→(6)) are simultaneous. With a further increase in the reaction time, the second-step of oxidation–erosion and sedimentary growth process is also under way. The octahedron Cu2O crystals are gradually oxidized by dissolved O2 in the weak acid solution (chemical reaction (6)), and the produced Cu2+ from reaction (6) form Cu2O (s) nanoparticles again through reactions (3)→(5). The produced Cu2O nanoparticles deposit on the surface of the octahedra Cu2O. This is a gradual and slow process, and the bow-like Cu2O was only partly formed when the time was 150 min (Fig. 2d). The bow-like Cu2O crystals dominated when the reaction time increased to 170 min (Fig. 2e). At last, perfect bow-like Cu2O crystals could be observed when the reaction time was increased to 200 min. It should be pointed out that dissolved O2 played a leading role in this process. If the solution is purged with N2 to remove dissolved O2 prior to oxidizing, the oxidation–erosion and sedimentary growth process can be effectively prevented, and Cu2O crystals with different morphologies were obtained (Fig. S5†). Therefore, the dissolved oxygen plays an indispensable role in forming the hierarchical bow-like Cu2O crystals. At this time (200 min), the solution color changed to pale blue (e in Fig. S4†) with the gradual increase of the Cu2+ ion concentration. It should be mentioned that, in the whole oxidation–erosion and sedimentary growth process, the Cu2O oxidation–erosion rate (6) is faster than that of the sedimentary growth of Cu2O ((3)→(5)). Finally, the bow-like Cu2O crystals disappear completely, and the Cu2+ ion content obviously increases, when the reaction time is increased to 420 min, which can be seen from the results of the atomic absorption spectrometry (Table S1†).
N2 adsorption–desorption isotherms (Fig. 6) and the corresponding BJH pore size distribution plots (inset) of the as-obtained octahedron Cu2O crystals, Cu2O nanoparticles and hierarchical bow-like Cu2O crystals were performed. It can be seen that the hierarchical bow-like Cu2O crystals have a relative narrow pore size compared to the other two samples. Moreover, the BET surface area of the prepared hierarchical bow-like Cu2O crystals (85 m2 g−1) is larger than that of octahedron Cu2O crystals (3 m2 g−1) and Cu2O nanoparticles (70 m2 g−1). The larger BET surface area of the hierarchical bow-like Cu2O crystals can be attributed to the slice layer structure and interconnected pores in the structure of hierarchical bow-like crystals. The hierarchical bow-like structure with large BET surface area could facilitate more efficient contact with organic contaminants and enhance light-harvesting and thus improve its photocatalytic activity.
 | ||
| Fig. 6 N2 adsorption–desorption isotherm curves and pore size distribution (inset) of the different samples. | ||
The optical absorption of the bow-like Cu2O crystals was measured with an UV-vis absorption spectrometer. As shown in Fig. 7, the hierarchical bow-like Cu2O crystals show photo-absorption properties from the UV light region to the visible light region with wavelengths shorter than 548 nm, and the steep shape of the spectrum indicates that the visible light absorption is due to the intrinsic band-gap transition of nanomaterials.38,39 For a crystalline semiconductor, the optical absorption near the band edge follows the equation ahv = A(hv−Eg)n/2, where a, v, Eg and A are the absorption coefficient, the light frequency, the band gap and a constant, respectively. Among them, n decides the characteristics of the transition in a semiconductor.40 The band gap (Eg) of the bow-like Cu2O crystals is calculated to be about 2.26 eV from the onset of the absorption edge (inset of Fig. 7). Compared to that of bulk Cu2O crystals at 2.17 eV, the band gap energy of bow-like Cu2O crystals is enlarged slightly, which is attributed to the influence on the quantum confinement. This indicates that the hierarchical bow-like Cu2O crystals have a suitable band gap for photocatalytic decomposition of organic contaminants under visible light irradiation.
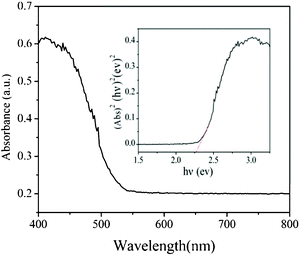 | ||
| Fig. 7 The UV-vis absorption spectrum of the hierarchical bow-like Cu2O crystal sample. | ||
3.5. Photocatalytic characterization
Rhodamine B (RhB), which is one of the most commonly used dyes, was chosen as a representative pollutant to evaluate the photocatalytic performance of the as-prepared Cu2O crystal photocatalysts with different morphologies under visible light illumination. Here, we select the representative morphologies for comparison: nanoparticles (Fig. 1a), octahedral (Fig. 1c), and perfect bow-like Cu2O crystals (Fig. 1f). Temporal changes in the concentration of RhB as monitored by the maximal absorption in UV-vis spectra at 554 nm over the bow-like Cu2O crystals are shown in Fig. 8a. It is found that the intensity of the absorption peak of RhB decreases gradually as the irradiation time increases, accompanied by a slight shift of in the main absorption peak to a lower wavelength and a color change from the initial pink to light yellow. This hypsochromic shift of the major absorption peak corresponds to a step-by-step de-ethylation of RhB.41,42 | ||
| Fig. 8 (a) The temporal evolution of the absorption spectra of the RhB solution in the presence of hierarchical bow-like Cu2O crystals under visible-light irradiation. (b) The adsorption and photocatalytic degradation of RhB by catalysts with different morphologies under visible-light irradiation. | ||
Fig. 8b displays the adsorption and photodegradation spectra of an aqueous solution of RhB photodegraded by different photocatalysts. It shows that the adsorption ability and the photocatalytic activity of the bow-like Cu2O crystals are much higher than the Cu2O nanoparticles and octahedral Cu2O crystals. The enhanced photocatalytic activity of the bow-like Cu2O crystals is attributed to the special hierarchical bow-like structure and large surface area. The increase in the surface area can provide more active catalytic sites.43,44 The intermeshed nanoribbons of the hierarchical bow-like Cu2O crystals can allow multiple reflections of visible light, which enhances light-harvesting and thus increases the quantity of photogenerated electrons and holes available to participate in the photocatalytic decomposition of RhB. It is also indicated that the photocatalytic activity of Cu2O nanoparticles is higher than octahedral Cu2O crystals due to the relatively large surface area of the exposed {111} surfaces. For comparison purposes, we also selected nitrogen-doped Degussa P25 and nitrogen-doped anatase TiO2 nanoparticles as reference substances, which were prepared according to our previous report.45 The results are shown in Fig. S6† and Table S2.† It was found that the prepared hierarchical bow-like Cu2O exhibited much higher visible-light driven photocatalytic activity than the nitrogen-doped anatase TiO2 and nitrogen-doped Degussa P25 under the same photocatalytic experimental conditions. This indicates once again that the hierarchical bow-like Cu2O crystals show highly visible-light driven photocatalytic activity.
The repeated photocatalytic experiments on the hierarchical bow-like Cu2O crystal composites were also examined. After the fifth consecutive cycle of the degradation experiments under visible light irradiation, the photocatalytic activity only showed a slight decrease, due to the amount of hierarchical bow-like Cu2O crystals in the reactor being slightly reduced, as the slurry had been sampled for each measurement of RhB concentration (Fig. S7†). XRD analysis (Fig. S8†) showed that only pure Cu2O was detected, and no other impurified phases could be found. The morphology of the Cu2O (Fig. S9†) has also undergone no obvious change before and after the photocatalytic tests. After the photocatalytic experiments, the solid was centrifuged and the supernatant was analyzed using flame atomic absorption spectrometry, and no copper ions were detected. The above analysis showed that our prepared bow-like Cu2O has relatively high photostability under our experimental conditions.
4. Conclusions
In summary, we report a facile method for the synthesis of hierarchical bow-like Cu2O crystals in the presence of PVP and NaBH4. Experimental results proved that reaction time, reaction temperature and the quality of PVP played an important role in the formation of hierarchical bow-like Cu2O crystals. The morphologies of the Cu2O crystal samples also influenced their photocatalytic property, and hierarchical bow-like Cu2O crystals showed the highest photocatalytic activity for the photodegradation of rhodamine B under visible-light illumination, due to their special hierarchical bow-like structure and large surface area.Acknowledgements
We gratefully acknowledge the support of the Key Program Projects of the National Natural Science Foundation of China (No. 21031001), the National Natural Science Foundation of China (No. 20971040, 91122018, 21101060 and 21101061), the Cultivation Fund of the Key Scientific and Technical Innovation Project, the Knowledge Innovation Program of Chinese Academy of Sciences, Ministry of Education of China (No. 708029), the China Postdoctoral Science Foundation (20110490154), and the Youth Foundation of Heilongjiang Province of China (QC2010021).References
- Y. Park, S. -H. Lee, S. O. Kang and W. Choi, Chem. Commun., 2010, 46, 2477 RSC.
- Z. Y. Liu, X. T. Zhang, S. Nisimoto, T. Murakami and A. Fujishima, Environ. Sci. Technol., 2008, 42, 8547 CrossRef CAS.
- N. Zhang, Y. L. Du, Y. Zhang and C. M. Wang, J. Mater. Chem., 2011, 21, 5408 RSC.
- M. Diab, B. Moshofsky, I. J-L. Plante and Taleb Mokari, J. Mater. Chem., 2011, 21, 11626 RSC.
- H. Y. Zhao, Y. F. Wang and J. H. Zeng, Cryst. Growth Des., 2008, 8, 3731 CAS.
- M. J. Siegfried and K.-S. Choi, J. Am. Chem. Soc., 2006, 128, 10356 CrossRef CAS.
- M. J. Siegfried and K.-S. Choi, Adv. Mater., 2004, 16, 1743 CrossRef CAS.
- J. Zhang, J. Liu, Q. Peng, X. Wang and Y. Li, Chem. Mater., 2006, 18, 867 CrossRef CAS.
- H. Zhang, Q. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766 CrossRef CAS.
- H. Xu, W. Wang and W. Zhu, J. Phys. Chem. B, 2006, 110, 13829 CrossRef CAS.
- B. White, M. Yin, A. Hall, D. Le, S. Stolbov, T. Rahman, N. Turro and S. O'Brien, Nano Lett., 2006, 6, 2095 CrossRef CAS.
- S. X. Wu, Z. Y. Yin, Q. Y. He, G. Lu, X. Z. Zhou and H. Zhang, J. Mater. Chem., 2011, 21, 3467 RSC.
- W. Wang, G. Wang, X. Wang, Y. Zhan, Y. Liu and C. Zheng, Adv. Mater., 2002, 14, 67 CrossRef CAS.
- M. H. Cao, C. W. Hu, Y. H. Wang, Y. H. Guo, C. X. Guo and E. B. Wang, Chem. Commun., 2003, 1884 RSC.
- Y. Chang, J. J. Teo and H. C. Zeng, Langmuir, 2005, 21, 1074 CrossRef CAS.
- L. S. Xu, X. H. Chen, Y. R. Wu, C. S. Chen, W. H. Li, W. Y. Pan and Y. G. Wang, Nanotechnology, 2006, 17, 1501 CrossRef CAS.
- S. D. Sun, H. Zhang, X. P. Song, S. H. Liang, C. C. Kong and Z. M. Yang, CrystEngComm, 2011, 13, 6040 RSC.
- C.-H. Kuo, C.-H. Chen and M. H. Huang, Adv. Funct. Mater., 2007, 17, 3773 CrossRef CAS.
- C.-H. Kuo and M. H. Huang, J. Phys. Chem. C, 2008, 112, 18355 CAS.
- L. Gou and C. J. Murphy, Nano Lett., 2003, 3, 231 CrossRef CAS.
- M. H. Kim, B. Lim, E. P. Lee and Y. Xia, J. Mater. Chem., 2008, 18, 4069 RSC.
- Y. G. Sui, W. Y. Fu, H. B. Yang, Y. Zeng, Y. Y. Zhang, Q. Zhao, Y. G. Li, X. M. Zhou, Y. Leng, M. H. Li and G. T. Zou, Cryst. Growth Des., 2010, 10, 99 CAS.
- S.D. Sun, H. J. You, C. C. Kong, X. P. Song, B. J. Ding and Z. M. Yang, CrystEngComm, 2011, 13, 2837 RSC.
- Y. Li, T. Sasaki, Y. Shimizu and N. Koshizaki, J. Am. Chem. Soc., 2008, 130, 14755 CrossRef CAS.
- X. J. Dai, Y. S. Luo, W. D. Zhang and S. Y. Fu, Dalton Trans., 2010, 39, 3426 RSC.
- C. X. Wang, L. W. Yin, L. Y. Zhang, Y. X. Qi, N. Lun and N. N. Liu, Langmuir, 2010, 26, 12841 CrossRef CAS.
- Z. W. Quan, C. X. Li, X. M. Zhang, J. Yang, P. P. Yang, C. M. Zhang and J. Lin, Cryst. Growth Des., 2008, 8, 2384 CAS.
- C. C. Li, W. P. Cai, B. Q. Cao, F. Q. Sun, Y. Li, C. X. Kan and L. D. Zhang, Adv. Funct. Mater., 2006, 16, 83 CrossRef CAS.
- (a) R. F. Sekerka, Cryst. Res. Technol., 2005, 40, 291 CrossRef CAS; (b) G. Z. Wulff, Krystallogr, 1901, 34, 449 CAS; (c) D. Xu, D. Xue and H. Ratajczak, J. Mol. Struct., 2005, 740, 37 CrossRef CAS; (d) D. Xu and D. Xue, Phys. B, 2005, 370, 84 CrossRef CAS; (e) D. Xu and D. J. Xue, J. Cryst. Growth, 2006, 286, 108 CrossRef CAS.
- J. S. Xu and D. Xue, Acta Mater., 2007, 55, 2397 CrossRef CAS.
- S. D. Sun, C. C. Kong, S. C. Yang, L. Q. Wang, X. P. Song, B. J. Ding and Z. M. Yang, CrystEngComm, 2011, 13, 2217 RSC.
- S. D. Sun, F. Y. Zhou, L. Q. Wang, X. P. Song and Z. M. Yang, Cryst. Growth Des., 2010, 10, 541 CAS.
- Y. Zhang, B. Deng, T. R. Zhang, D. M. Gao and A. W. Xu, J. Phys. Chem. C, 2010, 114, 5073 CAS.
- S. D. Sun, H. J. You, C. C. Kong, X. P. Song, B. J. Ding and Z. M. Yang, CrystEngComm, 2011, 13, 2837 RSC.
- Y. M. Sui, W. Y. Fu, Y. Zeng, H. B. Yang, Y. Y. Zhang, H. Chen, Y. X. Li, M. H. Li and G. T. Zou, Angew. Chem., Int. Ed., 2010, 49, 4282 CrossRef CAS.
- J. Y. Ho and M. H. Huang, J. Phys. Chem. C, 2009, 113, 14159 CAS.
- C. H. Kuo and M. H. Huang, Nano Today, 2010, 5, 106 CrossRef CAS.
- Y. Shimodaira, H. Kato, H. Kobayashi and A. Kudo, J. Phys. Chem. B, 2006, 110, 17790 CrossRef CAS.
- A. Kudo, I. Tsuji and H. Kato, Chem. Commun., 2002, 1958 RSC.
- J. Zhang, F. J. Shi, D. F. Chen, J. M. Gao, Z. X. Huang, X. X. Ding and C. C. Tang, Chem. Mater., 2008, 20, 2937 CrossRef CAS.
- X. J. Dai, Y. S. Luo, W. D. Zhang and S. Y. Fu, Dalton Trans., 2010, 39, 3426 RSC.
- J. H. Bi, L. Wu, J. Li, Z. H. Li, X. X. Wang and X. Z. Fu, Acta Mater., 2007, 55, 4699 CrossRef CAS.
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. (David) Lou, X. R. Xing and G. Q. (Max) Lu, Chem. Commun., 2011, 47, 12578 RSC.
- J. Liu, S. Z. Qiao, H. Liu, J. Chen, A. Orpe, D.Y. Zhao and G. Q. (Max) Lu, Angew. Chem., Int. Ed., 2011, 50, 5947 CrossRef CAS.
- G. H. Tian, Y. J. Chen, K. Pan, D. J. Wang, W. Zhou, Z. Y. Ren and H. G. Fu, Appl. Surf. Sci., 2010, 256, 3740 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra01197b |
| This journal is © The Royal Society of Chemistry 2012 |

