Synthesis of a novel cellulose nanowhisker-based drug delivery system
Rajalaxmi
Dash
and
Arthur J.
Ragauskas
*
School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332, USA. E-mail: art.ragauskas@ipst.gatech.edu; Tel: 404-894-4778
First published on 28th February 2012
Abstract
Recently, cellulose nanowhiskers have attracted attention as a promising material in the biomedical field because of their outstanding properties such as hydrophilicity, biocompatibility, biodegradability, and high surface area. In this work, we prepared a novel nanometric carrier molecule for amine-containing biologically active molecules and drugs employing functionalized cellulose nanowhiskers. The nanowhiskers were grafted with a spacer molecule, gamma aminobutyric acid, using a periodate oxidation and Schiff's base condensation reaction sequence. To achieve controlled and rapid delivery of the targeting moiety, syringyl alcohol, a releasable linker, was then attached to it. All the reactions were carried out in aqueous media and the resulting products were characterized by FT-IR, NMR, XPS, and TEM experiments to evaluate the functional groups, structure, and morphology respectively.
Introduction
Development of nanoparticle delivery systems for drugs, proteins, and enzymes remains an important challenge in the field of biomedical research on account of the effective delivery and release of the carrier molecules at the target site. Over the past few decades, extensive studies have been performed on both synthetic and natural polymer-based nano carriers and several of them are already in clinical use.1–3 Of the readily available natural polymers, polysaccharide nanoparticles, especially cellulose nanowhiskers (CNWs) have attracted increasing attention as a promising delivery system for drugs due to their outstanding properties such as nano dimension, high surface area, hydrophilicity, biocompatibility, and biodegradability.4,5Cellulose nanowhiskers are defined as elongated rod-like crystals with a dimension of 100–1000 nm in length and 5–50 nm in diameter, typically obtained by acid hydrolysis of cellulose fibers. Significant efforts have been dedicated to explore the preparation and properties of cellulose nanowhiskers from various cellulosic sources.6–10 It was observed that the dimensions and properties of nanowhiskers vary depending on their sources as well as the hydrolysis conditions employed. Several recent studies on cellulose nanowhiskers and their derivatives indicate the potential biomedical applications of cellulose nanowhiskers. For example, Dong and Roman11 have chemically labeled nanowhiskers with fluorescein isothiocyanate (FITC) fluorophore through a three-step reaction pathway and evaluated it for fluorescence bioassay and bioimaging applications. Cellulose nanowhisker surfaces have also been successfully modified using L-leucine amino acid showing a possible platform for introducing biologically active building blocks.12
A supramolecular hydrogel based on cyclodextrin/polymer inclusion was prepared by Zhang and co-workers13 and it was shown that the incorporation of cellulose nanowhiskers into the hydrogel enhances its gelation, mechanical strength and facilitates sustained release of drugs. Further evaluation of the hydrogel on cell viability did not indicate any additional cytotoxicity implying a potential candidate for smart delivery and sustained release applications. Cellulose nanowhisker surfaces were functionalized with gold nanoparticles by Mahmoud et al. and this templated material was demonstrated as an excellent immobilization support for enzymes with high loading capacity.14 Single-stranded oligonucleotides were effectively grafted on to TEMPO oxidized nanowhisker surfaces, setting a platform for the future development of nanodevices from inexpensive and chemically versatile nanowhiskers.15
The toxicity assessment of cellulose nanowhiskers has been examined by interacting with microvascular endothelial cells, rainbow trout hepatocytes, and other aquatic species.16,17 It was concluded that cellulose nanowhiskers are non-toxic to cells and could be considered for the delivery of biomolecules and therapeutics. Dugan et al. examined the bioactivity of nanowhiskers by interacting them with myoblast cells, which showed that patterned CNWs surfaces actively direct the growth of tissue leading to future applications in tissue engineering, and also that nanowhiskers exhibited non-cytotoxicity and non-immunogenicity even at relatively high concentrations.18,19 Additionally, due to the presence of abundant hydroxyl groups, cellulose nanowhiskers are hydrophilic in nature, and thus exhibit low protein adsorption, prompting a long blood circulation half-life and delaying initial clearance from the blood stream.20
Besides these biomedical properties, cellulose nanowhiskers are easy to prepare, form a colloidal suspension in water and mild reaction conditions can be followed to functionalize CNWs, which motivated us to examine the synthesis of a nanowhisker-based drug delivery system through their surface chemical modification. Typical drug delivery systems involve natural or synthetic biocompatible polymers, which are coupled to the targeting moiety directly or via a spacer arm. Releasable PEGylation (rPEGylation) is one of the extensively studied and promising drug delivery technologies for amine-containing drugs or biologically active agents, where the drug carrier design constitutes a “trigger” or a “spacer” element attached to the poly(ethylene glycol) (PEG) backbone to provide the specific initiation and rate of release pathway, followed by a “linker” molecule. The linkers comprise a series of customized aliphatic and aromatic molecules known as releasable PEG (rPEG) linkers and they play a key role in achieving controlled delivery.21
The release sequence involves a base or enzyme-cleaved initiation step to separate the PEG followed by a second, faster step through molecular decomposition of the linkers to deliver the unmodified and fully active drug. We examined the use of an aromatic linker, known as a benzyl elimination linker, in designing our nanowhisker delivery system since it relies on a classic and rapid 1,4- or 1,6-benzyl elimination reaction and molecular decomposition to regenerate the amine group of the conjugated drug/biomolecule (Scheme 1). A detailed study of the structure and release mechanism of other linker molecules can be found in the literature.22,23
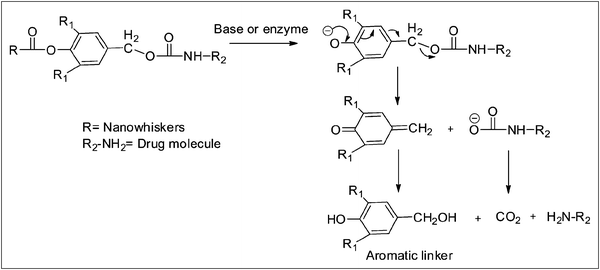 | ||
| Scheme 1 1,6-Benzyl elimination reaction mechanism of the aromatic linker. | ||
In this study, we combined the aromatic linker and cellulose nanowhiskers to synthesize a novel delivery system for amine-containing biologically active compounds and drug molecules. Based on our previous study focused on grafting of small chain primary amines to nanowhiskers employing a sequence of oxidation, condensation and in situ reduction reactions, γ-aminobutyric acid, a spacer molecule, was grafted to the nanowhisker surface. γ-Aminobutyric acid was chosen as a spacer molecule because of its biocompatibility and ease of availability. In the next step, syringyl alcohol was used as an aromatic linker and attached to the carboxylic group of the amino acid. The effective grafting of spacer and linker molecules to the nanowhisker surfaces was investigated by a series of characterization techniques including FT-IR, NMR, XPS and TEM.
Experimental
Materials
A fully bleached commercial softwood kraft pulp was used as a source for cellulose nanowhiskers. All chemicals and solvents were purchased from VWR International and used as received.Preparation of cellulose nanowhiskers
Cellulose nanowhiskers were prepared by sulfuric acid hydrolysis of a bleached softwood pulp based on a literature procedure.24 In brief, 60.00 g (oven-dried weight) of the pulp was mixed with H2SO4 solution (64%, w/w, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g mL−1) with continuous stirring at 45 °C for 45 min. The hydrolysis reaction was stopped by adding an excess (10-fold) of distilled water followed by the removal of acidic solution by successive centrifugation at 12
10 g mL−1) with continuous stirring at 45 °C for 45 min. The hydrolysis reaction was stopped by adding an excess (10-fold) of distilled water followed by the removal of acidic solution by successive centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min until the supernatant became turbid. The sediment was collected and dialyzed (MWCO: 12
000 rpm for 10 min until the supernatant became turbid. The sediment was collected and dialyzed (MWCO: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against tap water until the solution pH became neutral. After dialysis, the content was sonicated for 10 min and centrifuged for 5 min at 10
000) against tap water until the solution pH became neutral. After dialysis, the content was sonicated for 10 min and centrifuged for 5 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The cloudy supernatant, containing nanowhiskers, was collected and the remaining sediment was again mixed with water, sonicated and centrifuged to obtain additional nanowhiskers; this procedure was repeated till the supernatant was clear. Cellulose nanowhiskers were obtained in 20–30% yield.
000 rpm. The cloudy supernatant, containing nanowhiskers, was collected and the remaining sediment was again mixed with water, sonicated and centrifuged to obtain additional nanowhiskers; this procedure was repeated till the supernatant was clear. Cellulose nanowhiskers were obtained in 20–30% yield.
Sodium periodate oxidation of cellulose nanowhiskers
An aqueous mixture of cellulose nanowhiskers (100.00 mL, 2.00 wt%, w/v) and sodium periodate (1.00 g, 4.67 mmol) was stirred for three days in the absence of light at room temperature (Scheme 2). The product was then placed in a sealed dialysis bag (MWCO: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and dialyzed against DI water for 3 days to remove inorganic salts, and then freeze-dried providing a gravimetric yield of 98%. These samples were designated as DAC, where DAC = dialdehyde cellulose nanowhisker.
000) and dialyzed against DI water for 3 days to remove inorganic salts, and then freeze-dried providing a gravimetric yield of 98%. These samples were designated as DAC, where DAC = dialdehyde cellulose nanowhisker.
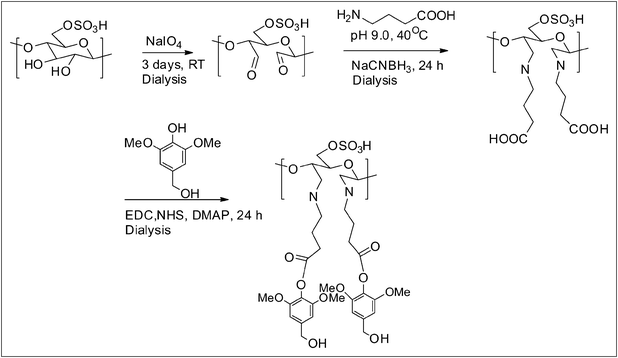 | ||
| Scheme 2 Reaction path followed to modify the cellulose nanowhisker surfaces. | ||
Reaction of dialdehyde cellulose whiskers with γ-aminobutyric acid (GABA)
A dialdehyde cellulose nanowhisker suspension (20.00 mL, 2.00 wt%, w/v) was mixed with acetate buffer (20.00 mL, pH 9.0) and stirred for 15 min. γ-Aminobutyric acid was then slowly added to the suspension (10 equiv. per glucose unit) and the mixture was continuously stirred at 45 °C for 24 h followed by the in situ reduction of the resulting imine intermediates at room temperature employing NaCNBH4 (2.5 equiv. per glucose unit) (Scheme 2). After stirring for 6 h, the product was dialyzed (MWCO: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against DI water until the pH was neutral and, subsequently, the slurry was freeze-dried to obtain the dry product with a yield of 80–90%. The sample was referred to as DAC-GABA.
000) against DI water until the pH was neutral and, subsequently, the slurry was freeze-dried to obtain the dry product with a yield of 80–90%. The sample was referred to as DAC-GABA.
Reaction of DAC-GABA with syringyl alcohol
An aqueous suspension of DAC-GABA (10.00 mL, 2.00 wt%, w/v), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, 1.87 g, 8.00 mmol), sulfo-N-hydroxysuccinimide (NHS, 0.50 mmol), 4-(dimethylamino)pyridine (DMAP, 1.22 g, 1.00 mmol) and syringyl alcohol (1.60 g, 8.00 mmol) was stirred at room temperature for 24 h. Finally, it was dialyzed against DI water for 3–4 days and freeze dried. The product was obtained in 70–80% yield and it was referred to as DAC-GABA-SA (Scheme 2).FT-IR spectroscopy
The starting whisker and oxidized samples were dried at 105 °C for 12 h and then cooled to room temperature for FT-IR analysis. The oven-dried CNWs, periodate oxidized CNWs and freeze-dried DAC-GABA, DAC-GABA-SA samples were pressed into KBr pellets (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). Transmission mode FT-IR spectra were collected with a Nicolet Magna-IR™ 550 spectrometer. Spectra were obtained in 400–4000 cm−1 range and for each sample 64 scans were taken at a resolution of 4 cm−1.
200). Transmission mode FT-IR spectra were collected with a Nicolet Magna-IR™ 550 spectrometer. Spectra were obtained in 400–4000 cm−1 range and for each sample 64 scans were taken at a resolution of 4 cm−1.
NMR spectroscopy
13C NMR spectra were obtained on a Bruker Avance-400 spectrometer operating at a frequency of 100.55 MHz at 45 °C. Freeze-dried DAC-GABA (0.05 g) was dissolved in a mixture of perdeuterated pyridinium ionic liquid and DMSO-d6 (0.60 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with constant stirring at 45 °C for 3 h. DAC-GABA-SA was dissolved in DMSO-d6 (0.60 mL) at 45 °C. An aliquot of this solution (0.50 mL) was transferred to a NMR tube and 30
4) with constant stirring at 45 °C for 3 h. DAC-GABA-SA was dissolved in DMSO-d6 (0.60 mL) at 45 °C. An aliquot of this solution (0.50 mL) was transferred to a NMR tube and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scans were collected for each spectrum.
000 scans were collected for each spectrum.
The DOSY experiments were conducted using the bipolar pulse pair (BPP) pulse sequence. Field gradient calibration was accomplished using the self-diffusion coefficient of pure water at 25 °C (2.299 × 10−9 m2 s−1). The gradients were applied for 3.2 ms and the diffusion time was 80 ms. Homospoil gradients were applied for 600 μs during diffusion and eddy current settling durations. The gradients were incremented 16 times from 1.7 G cm−1 to 63.0 G cm−1. A total of 64 free induction decays containing 8000 complex data points were collected at each gradient with a spectral sweep width of 15.0 ppm. The recycle delay was 5 s.
X-Ray photoelectron spectroscopy (XPS) analysis
X-Ray photoelectron spectra of unmodified and modified whiskers were collected on a Thermo K-alpha XPS spectrometer with a monochromatic aluminium Kα source operated at 12 kV and 6 mA under a pressure of 10−8 mbar. The binding energy scale was shifted to ensure that the main C–C/C–H contribution to the C1s signal occurred at 285.0 kV.TEM experiment
Transmission electron micrographs of cellulose nanowhisker samples were taken in a JEOL 100CX-2 transmission electron microscope at an accelerating voltage of 100 kV. Sample solutions (0.02%, w/v) were deposited on a carbon-coated grid and allowed to dry followed by staining with 2 wt% aqueous uranyl acetate solution.Results and discussion
The purpose of the following chemical modifications is to synthesize a drug delivery system based on cellulose nanowhiskers without the conjugation of the active agent and we expect that this synthetic strategy will open their use as a vehicle for drugs, enzymes and other biomolecules. Cellulose nanowhiskers prepared by the sulfuric acid hydrolysis of the softwood kraft pulp were oxidized in the presence of sodium periodate. Periodate oxidation of nanowhiskers selectively cleaves the C2–C3 bond and introduces carbonyl groups at respective carbon atoms of the glucose ring which was confirmed from the FT-IR spectra due to the appearance of the carbonyl band at 1734 cm−1 (Fig. 1). Oxidized nanowhiskers were further grafted with the spacer molecule, γ-aminobutyric acid following Schiff's base reaction and in situ sodium cyanoborohydride reduction. As a result, the carbonyl band disappeared and an additional band at 1568 cm−1 appeared indicating successful amination-reduction reaction between the carbonyl and amine groups. The final step involved the activation of the carboxylic group of the amino acid by EDC in the presence of NHS followed by esterification with syringyl alcohol. As observed in the FT-IR spectra, the presence of an ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1680 cm−1, in addition to a characteristic C
O at 1680 cm−1, in addition to a characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band of a benzene ring at 1609 cm−1, supports the esterification reaction. The results from FT-IR spectra support the sequence of changes that occurred during the series of reactions.
C band of a benzene ring at 1609 cm−1, supports the esterification reaction. The results from FT-IR spectra support the sequence of changes that occurred during the series of reactions.
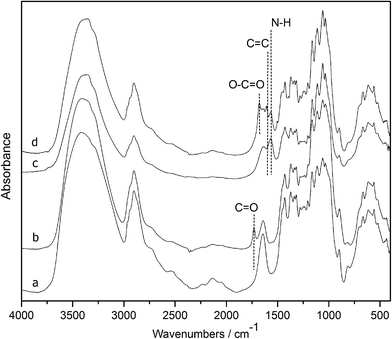 | ||
| Fig. 1 FT-IR spectra of (a) nanowhiskers, (b) DAC, (c) DAC-GABA, and (d) DAC-GABA-SA. | ||
Cellulose nanowhisker derivatives were further characterized by NMR spectroscopy. The DAC-GABA sample was found to be insoluble in DMSO-d6; hence it was dissolved in a mixture of perdeuterated pyridinium ionic liquid and DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to study the structural changes by 13C NMR.25 As shown in Fig. 2, the signals centered at δ 103.5, 73.4, 74.6, 81.0, 76.4, and 60.2 were readily assigned to C1, C2, C3, C4, C5, and C6 of the glucopyranoside ring in the cellulose nanowhiskers, respectively. The peaks corresponding to the carboxylic group and the alkyl chain of γ-aminobutyric acid appeared at 172.4 and 20–35 ppm respectively, suggesting its attachment to nanowhiskers. Signals in the region of 120–150 ppm belong to the ionic liquid. The DAC-GABA-SA sample was found to be soluble in DMSO-d6 possibly, due to the incorporation of non-polar moieties on to the surface of the nanowhiskers. So, the NMR spectra were collected in DMSO to avoid the interference of peaks from ionic liquid with the syringyl group. As shown in Fig. 3, signals at δ 57.3, 63.5, 102.9, 142.6, 152.4, 157.2, and 174.5 correspond to C16, C15, C13, C11, C14, C12, and C10 of syringyl group, respectively, indicating the chemical modification of DAC-GABA by the syringyl alcohol molecule.
4) to study the structural changes by 13C NMR.25 As shown in Fig. 2, the signals centered at δ 103.5, 73.4, 74.6, 81.0, 76.4, and 60.2 were readily assigned to C1, C2, C3, C4, C5, and C6 of the glucopyranoside ring in the cellulose nanowhiskers, respectively. The peaks corresponding to the carboxylic group and the alkyl chain of γ-aminobutyric acid appeared at 172.4 and 20–35 ppm respectively, suggesting its attachment to nanowhiskers. Signals in the region of 120–150 ppm belong to the ionic liquid. The DAC-GABA-SA sample was found to be soluble in DMSO-d6 possibly, due to the incorporation of non-polar moieties on to the surface of the nanowhiskers. So, the NMR spectra were collected in DMSO to avoid the interference of peaks from ionic liquid with the syringyl group. As shown in Fig. 3, signals at δ 57.3, 63.5, 102.9, 142.6, 152.4, 157.2, and 174.5 correspond to C16, C15, C13, C11, C14, C12, and C10 of syringyl group, respectively, indicating the chemical modification of DAC-GABA by the syringyl alcohol molecule.
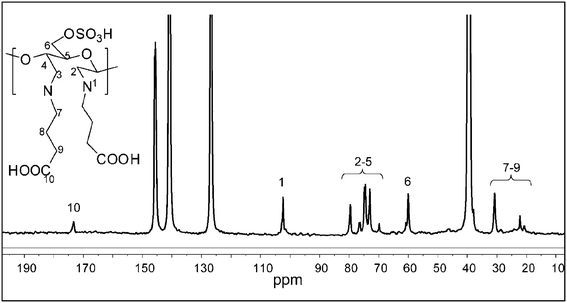 | ||
| Fig. 2 13C NMR spectrum of DAC-GABA in perdeuterated pyridinium ionic liquid-DMSO-d6. | ||
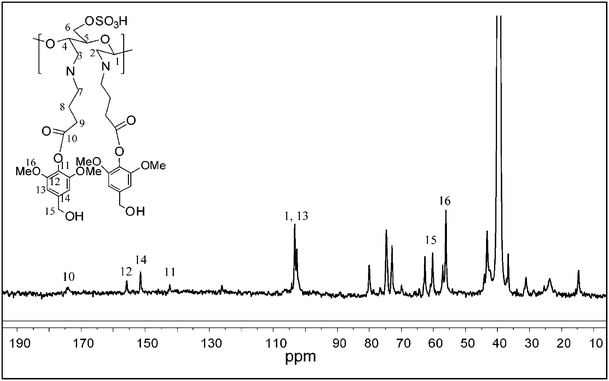 | ||
| Fig. 3 13C NMR spectrum of DAC-GABA-SA in DMSO-d6. | ||
In order to confirm that the 13C NMR spectrum of DAC-GABA-SA is a consequence of the effective chemical attachment of spacer and linker molecule to the nanowhiskers, we further collected its 2D DOSY spectrum. Diffusion ordered spectroscopy (DOSY) is a powerful technique to study the interactions between the components in a solution based on their difference in apparent diffusion coefficients. DOSY employs pulsed field gradient (PFG) to acquire a series of diffusion-attenuated 1D PFG-spin echo experiments. A suitable Laplace transform of the decaying signal results in a 2D spectrum with the chemical shift on one axis and the distribution of diffusion coefficients on the other axis.26,27
Interaction between the molecules will be determined by comparing their diffusion coefficients at their chemical shifts; for instance, if the molecules with different diffusion coefficients are chemically associated with each other then they will diffuse at the same rate, whereas no interaction will give rise to separate diffusion coefficients at their respective chemical shifts. As shown in Fig. 4, signals belonging to the cellulose nanowhiskers, amino acid and syringyl molecule are aligned at the same diffusion coefficients, confirming that the three molecules diffuse together, which suggests they are chemically bonded to each other.
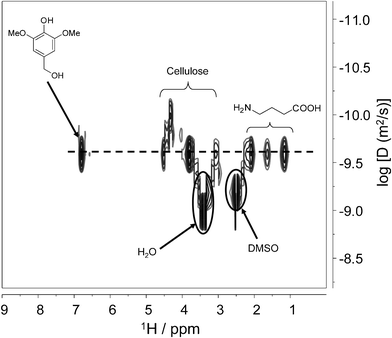 | ||
| Fig. 4 Contour-plot 1H NMR DOSY spectrum of DAC-GABA-SA in DMSO-d6. | ||
The surface elemental composition of pure cellulose nanowhiskers and their derivatives was studied by XPS. As expected, the spectrum of pure nanowhiskers (Fig. 5) exhibits two peaks at 285 and 532.6 eV which correspond to carbon and oxygen respectively. After treating with γ-aminobutyric acid and syringyl alcohol the nanowhisker surface shows the presence of nitrogen at 400 eV in addition to carbon and oxygen. As summarized in Table 1, coupling of cellulose nanowhiskers with the spacer and linker molecule caused an increase in the concentration of carbon and nitrogen which is a clear indication of their attachment to the nanowhisker surface.
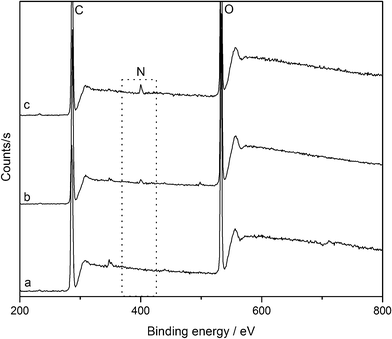 | ||
| Fig. 5 XPS spectra of (a) nanowhiskers, (b) DAC-GABA, and (c) DAC-GABA-SA. | ||
| Sample | Elemental composition (%) | ||
|---|---|---|---|
| O | C | N | |
| Nanowhiskers | 37.61 | 53.25 | 0 |
| DAC-GABA | 36.43 | 55.92 | 1.47 |
| DAC-GABA-SA | 37.22 | 56.83 | 1.80 |
The surface morphology of cellulose nanowhiskers and their derivatives was investigated by TEM as shown in Fig. 6. TEM images show that the nanowhiskers maintained their rod-like characteristic morphology even after the series of reactions. The dimensions of CNWs and their derivatives were found to be in the range of 150–300 nm in length and 4–8 nm in diameter.
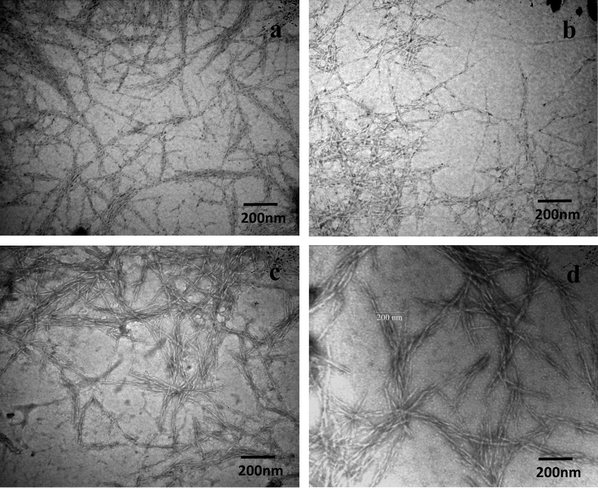 | ||
| Fig. 6 TEM images of 0.02 wt% suspensions of (a) nanowhiskers, (b) DAC, (c) DAC-GABA, and (d) DAC-GABA-SA. | ||
Conclusion
In conclusion, the unique assembly of cellulose nanowhiskers attached to the syringyl alcohol linker through a γ-aminobutyric acid spacer molecule enables the successful synthesis of a novel cellulose nanowhisker-based delivery system by following a series of oxidation, reductive-amination and esterification reactions in aqueous media. The coupling of the spacer and the linker molecule to nanowhiskers was confirmed from the sequential changes in functional groups and structure through FT-IR, 13C NMR and XPS. Further, their effective binding to the cellulose nanowhisker surface was evidenced by 2D-DOSY NMR spectroscopy. TEM observations showed no change in size and shape of the nanowhiskers after the series of reactions, which is substantial for a delivery system. Our future study involves exploring the conjugation of a small model amine drug (phenylpropanolamine)28 to the nanowhisker delivery system. Finally, the concept of carrier molecule from a renewable, biocompatible and biodegradable resource provides a platform that could be widely adapted for the controlled delivery of enzymes, proteins and amine-containing drugs with the selection of desired linker molecules.Acknowledgements
The authors thank DOE (DE-EE0003144) for the support of this study.References
- Z. Liu, Y. Jiao, Y. Wang, C. Zhou and Z. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1650–1662 CrossRef CAS.
- L. Zhang, F. X. Gu, J. M. Chan, A. Z. Wang, R. S. Langer and O. C. Farokhzad, Clin. Pharmacol. Ther., 2008, 83, 761–769 CrossRef CAS.
- C. S. Ha and J. A. Gardella, Chem. Rev., 2005, 105, 4205–4232 CrossRef CAS.
- B. L. Peng, N. Dhar, H. L. Liu and K. C. Tam, Can. J. Chem. Eng., 2011, 9999, 1–16 Search PubMed.
- H. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef.
- G. Siqueira, J. Bras and A. Dufresne, Biomacromolecules, 2009, 10, 425–432 CrossRef CAS.
- S. Beck-Candanedo, M. Roman and D. G. Gray, Biomacromolecules, 2005, 6, 1048–1054 CrossRef CAS ; http://pubs.acs.org/doi/abs/10.1021/bm049300p.
- H. Angellier, S. Molina-Boisseau, N. M. Belgacem and A. Dufresne, Langmuir, 2005, 21, 2425–2433 CrossRef CAS.
- M. A. S. Azizi Samir, F. Alloin and A. Dufresne, Biomacromolecules, 2005, 6, 612–626 CrossRef.
- M. M. de Souza Lima and R. Borsali, Macromol. Rapid Commun., 2004, 25, 771–787 CrossRef CAS.
- S. Dong and M. Roman, J. Am. Chem. Soc., 2007, 129, 13810–13811 CrossRef CAS.
- C. A. Cateto and A. Ragauskas, RSC Adv., 2011, 1, 1695–1697 RSC.
- X. L. Zhang, J. Huang, P. R. Chang, J. L. Li, Y. M. Chen, D. X. Wang, J. H. Yu and J. H. Chen, Polymer, 2010, 51, 4398–4407 CrossRef CAS.
- K. A. Mahmoud, K. B. Male, S. Hrapovic and J. H. T. Luong, ACS Appl. Mater. Interfaces, 2009, 1, 1383–1386 CAS.
- A. P. Mangalam, J. Simonsen and A. S. Benight, Biomacromolecules, 2009, 10, 497–504 CrossRef CAS.
- M. Roman, S. P. Dong, H. Anjali and Y. W. Lee, Polysaccharide Materials: Performance by Design, ACS Symp. Ser., vol. 1017, 2010, ch. 4, pp. 81–91 Search PubMed.
- T. Kovacs, V. Naish, B. O'Connor, C. Blaise, F. Gagne, L. Hall, V. Trudeau and P. Martel, Nanotoxicology, 2010, 4, 255–270 CrossRef CAS.
- J. M. Dugan, J. E. Gough and S. J. Eichhorn, Biomacromolecules, 2010, 11, 2498–2504 CrossRef CAS.
- J. M. Dugan, J. E. Gough and S. J. Eichhorn, Eur. Cells Mater., 2011, 22, 41–42 Search PubMed.
- S. Dumitriu, Polysaccharides as Biomaterials, in Polymeric Biomaterials, CRC, Canada, 2nd edn, 2002, pp. 1–61 Search PubMed.
- R. B. Greenwald, Y. H. Choe, J. McGuire and C. D. Conover, Adv. Drug Delivery Rev., 2003, 55, 217–250 CrossRef CAS.
- D. Filpula and H. Zhao, Adv. Drug Delivery Rev., 2008, 60, 29–49 CrossRef CAS.
- R. B. Greenwald, A. Pendri, C. D. Conover, H. Zhao, Y. H. Choe, A. Martinez, K. Shum and S. Guan, J. Med. Chem., 1999, 42, 3657–3667 CrossRef CAS.
- D. Bondsen, A. Mathew and K. Oksman, Cellulose, 2006, 13, 171–180 CrossRef.
- N. Jiang, Y. Pu, R. Samuel and A. J. Ragauskas, Green Chem., 2009, 11, 1762–1766 RSC.
- C. S. Johnson Jr., Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 203–256 CrossRef.
- A. Jerschow and N. Müller, Macromolecules, 1998, 31, 6573–6578 CrossRef CAS.
- L. Zhu, V. Kumar and G. S. Banker, Int. J. Pharm., 2001, 223, 35–47 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
