A green synthetic route to antimalarial and antibacterial agent CJ-15,801 and its isomer cis-CJ-15,801†
K.
Kashinath
,
Pandrangi Siva
Swaroop
and
D. Srinivasa
Reddy
*
CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune, 411008, India. E-mail: ds.reddy@ncl.res.in; Fax: +91 20 25902629; Tel: +91 20 25902445
First published on 13th March 2012
Abstract
An efficient and rapid synthesis of antiplasmodial and antibacterial agent CJ-15,801 and its isomer cis-CJ-15,801 is disclosed. We have made an attempt towards “Ideal Synthesis” or a step towards “Dial-a-Molecule” by reducing the number of steps, following atom economy, and a green synthetic route.
In 2001, Sugie's group from Pfizer isolated an antibiotic CJ-15,801, 1 (Fig. 1), from the fermentation broth of the fungus Seimatosporium sp. CL28611.1 Compound 1 showed selective antibacterial activity against MDR Staphylococcus aureus strains (MIC = 6.25 to 50 μg ml−1). However, structurally very closely related (R)-pantothenic acid (vitamin B5), 3 (Fig. 1), showed no antibacterial activity.1 Recent findings from the Australian group revealed that compound 1 shows interesting antiplasmodial activity against Plasmodium falciparum and it can be used as a lead compound for the development of drugs to cure malaria.2 Unlike antibacterial activity, compound 1’s effect on P. falciparum can be attributed to its structural similarity with pantothenic acid 3. The promising biological profile coupled with its interesting N-acyl vinylogous carbamic acid bearing structure has already attracted the attention of leading synthetic groups such as those of Porco3 and Nicolaou.4 Very recently, the Marquez group reported the fast and efficient synthesis of compound 1 by taking advantage of the imide olefination methodology developed in his group.5 The first synthesis by Porco et al. utilizes the key coupling of a vinyl-halide and an amide under Cu catalyzed conditions.3 The second synthesis by Nicolaou et al. relies on the Dess–Martin periodinane oxidation of a saturated amide.4 Although, all three syntheses can produce the desired compound 1 following independent routes, the invention of new and green routes to 1 is always rewarding and these can be complementary to the existing ones. Here, we have made an attempt towards “Ideal Synthesis”6 or a step towards the EPSRC's “Dial-a-Molecule”7 of CJ-15,801 and its cis isomer4,8 starting from readily accessible unnatural amino acids and D-(−)-pantolactone.9
 | ||
| Fig. 1 Structures of CJ-15,801 and related compounds. | ||
Retrosynthetically, we have envisioned the target molecules 1 or 2 (Fig. 1) as retro Diels–Alder products of a bicyclic amino acid derivative such as A, which in turn could be prepared by the opening of pantolactone with amino acid ester B (Scheme 1). Our plan was to carry out both the key steps only by heating without using additional reagents.
 | ||
| Scheme 1 Retrosynthesis. | ||
Our synthesis commenced with the preparation of bicyclic amino acid ester 5 through esterification with allyl alcohol from a known bicyclic amino acid 4 (Scheme 2). It was reported in previous syntheses of 1 that final ester hydrolysis was cumbersome as the target compound is sensitive to acid or base hydrolysis. As the hydrolysis step was already optimized with allyl ester, we decided to go with the same. D-(−)-Pantolactone was readily opened with bicyclic amino acid ester 5 by refluxing in toluene to give compound 6 (dr ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in 60% isolated yield. After several attempts, the desired retro Diels–Alder reaction was successfully carried out by heating in diphenyl ether at ∼230 °C for 20 min. The allyl ester of cis-CJ-15,801 (7) was obtained in 77% yield. The spectral data of 7 were compared with the reported data4 and it was found to be identical. We have also carried out the two-step procedure in a one-pot sequence (61% yield) by using diphenyl ether as solvent, initially heating at ∼120 °C for 24 h followed by heating at ∼230 °C for 20 min.10 The allyl deprotection of 7 to produce 2 was already documented by Nicolaou's group in very high yield (87%).
2) in 60% isolated yield. After several attempts, the desired retro Diels–Alder reaction was successfully carried out by heating in diphenyl ether at ∼230 °C for 20 min. The allyl ester of cis-CJ-15,801 (7) was obtained in 77% yield. The spectral data of 7 were compared with the reported data4 and it was found to be identical. We have also carried out the two-step procedure in a one-pot sequence (61% yield) by using diphenyl ether as solvent, initially heating at ∼120 °C for 24 h followed by heating at ∼230 °C for 20 min.10 The allyl deprotection of 7 to produce 2 was already documented by Nicolaou's group in very high yield (87%).
 | ||
| Scheme 2 Synthesis of cis-CJ-15,801. | ||
Having seen the success of our strategy to access cis-CJ-15,801, next we attempted the synthesis of E-isomer, 1. The bicyclic amino acid ester 8 was prepared starting from (E)-allyl 3-nitroacrylate and cyclopentadiene in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio (Scheme 3).11 The inseparable mixture of 8a and 8b was treated with D-(−)-pantolactone to furnish the corresponding adducts 9 as a mixture of four diastereomers in 61% total yield. As the stereoselectivity has no role in our case, we took forward the mixture as such. However, the next step had limited success despite several efforts. The conversion of 9 to 10 was poor and also suffered on purification to obtain clean compound 10. We took forward the inseparable mixture of 9 and 10 to the allyl deprotection step using a catalytic amount of Pd(PPh3)4 and isolated the desired compound 1 in very poor yield (<10%).
1 diastereomeric ratio (Scheme 3).11 The inseparable mixture of 8a and 8b was treated with D-(−)-pantolactone to furnish the corresponding adducts 9 as a mixture of four diastereomers in 61% total yield. As the stereoselectivity has no role in our case, we took forward the mixture as such. However, the next step had limited success despite several efforts. The conversion of 9 to 10 was poor and also suffered on purification to obtain clean compound 10. We took forward the inseparable mixture of 9 and 10 to the allyl deprotection step using a catalytic amount of Pd(PPh3)4 and isolated the desired compound 1 in very poor yield (<10%).
 | ||
| Scheme 3 Initial attempt towards the synthesis of CJ-15,801. | ||
To overcome the problem with the retro Diels–Alder reaction of trans isomer 9, we decided to use cyclopropyl-containing bicyclic amino acid ester 11 which is expected to undergo the retro Diels–Alder reaction at lower temperatures.12 Compound 11 was prepared in a similar manner to compound 8 starting from (E)-allyl 3-nitroacrylate and 1,1-cyclopropylcyclopentadiene (CPCP) (Scheme 4).13 In this case, we attempted the one-pot sequence in a similar fashion to that for the cis compound but at slightly lower temperatures (initially heating at ∼100 °C for 24 h followed by heating at ∼200 °C for 20 min.). To our delight, the desired N-acyl vinylogous carbamic acid ester 10 was obtained in 50–55% yield.11 All the spectral data of 10 were identical with those of the published data.3 As the final allyl ester deprotection was already documented by Porco et al. in high yield using a catalytic method, we did not attempt further optimization. In principle, the liberated dienes (cyclopentadiene or CPCP) during the retro Diels–Alder step can be recycled to generate the starting bicyclic amino acid esters. It is noteworthy to mention that we have minimized the use of reagents and used only “heat” in both the key steps. Thus, the present green route can be used for the preparation of a variety of analogs of CJ-15,801/pantothenic acids and for the scale up to compounds which are essential for the development of antibacterials or antimalarials based on this chemotype (Scheme 5).
 | ||
| Scheme 4 Synthesis of CJ-15,801. | ||
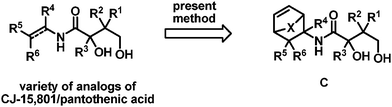 | ||
| Scheme 5 Ready access to analogs of CJ-15,801/pantothenic acid. | ||
In summary, we have described the synthesis of biologically important CJ-15,801 and its isomer cis-CJ-15,801 using a short and green synthetic route. This method will be useful for the generation of compound libraries of medicinal value. Our efforts are on to achieve the ultimate goal of ‘complete ideality’ in CJ-15,801 synthesis by removing even allyl ester protection and final hydrolysis and thereby improving the total efficiency.14
We gratefully acknowledge financial support from NCL, Pune (Start-up grant MLP022126). The authors thank Venkat Panchangula and Ajeet Singh of NCL, Pune, for the HRMS spectra. We appreciate Satish Chandra Philkhana for his help in starting material preparation. The authors thank Mrs S. S. Kunte for her help in HPLC analysis. KK thanks CSIR, New Delhi, for the award of a research fellowship. PSS thanks CSIR, New Delhi, for financial support as part of the NCL-IGIB collaboration program (NWP0013-G).
References
- Y. Sugie, K. A. Dekker, H. Hirai, T. Ichiba, M. Ishiguro, Y. Shiomi, A. Sugiura, L. Brennan, J. Duignan, L. H. Huang, J. Sutcliffe and Y. Kojima, J. Antibiot., 2001, 54, 1060 CAS.
- K. J. Saliba and K. Kirk, Mol. Biochem. Parasitol., 2005, 141, 129 Search PubMed.
- C. Han, R. Shen, S. Su and J. A. Porco, Org. Lett., 2004, 6, 27 CrossRef CAS.
- K. C. Nicolaou and C. J. N. Mathison, Angew. Chem., Int. Ed., 2005, 44, 5992 CrossRef CAS.
- A. L. Sewell, M. V. J. Villa, M. Matheson, W. G. Whittingham and R. Marquez, Org. Lett., 2011, 13, 800 Search PubMed.
- (a) T. Gaich and P. S. Baran, J. Org. Chem., 2010, 75, 4657 CrossRef CAS; (b) J. B. Hendrickson, J. Am. Chem. Soc., 1975, 97, 5784 CrossRef CAS.
- http://dialamolecule.chem.soton.ac.uk/site/ .
- J. M. Lee, D.-S. Ahn, D. Y. Jung, J. Lee, Y. Do, S. K. Kim and S. Chang, J. Am. Chem. Soc., 2006, 128, 12954 CrossRef CAS.
- See previous references from this group where the (−)-pantolactone chiral pool was used in total synthesis: (a) A. Hajare, V. Ravikumar, S. Khaleel, D. Bhuniya and D. S. Reddy, J. Org. Chem., 2011, 76, 963 CrossRef CAS; (b) S. E. Kurhade, A. I. Sanchawala, V. Ravikumar, D. Bhuniya and D. S. Reddy, Org. Lett., 2011, 13, 3690 Search PubMed and refs cited therein.
- Experimental details for the one-pot method: a mixture of 4 (250 mg, 1.3 mmol) and D-(−)-pantolactone (340 mg, 2.6 mmol) in diphenyl ether (15 mL) was heated to ∼120 °C for 24 h and the progress of the reaction was monitored by TLC. After a considerable amount of compound 4 was consumed with no further progress in the reaction, the reaction mixture was further heated to ∼220 °C for 20 min. After the completion of the reaction (TLC monitoring), the crude reaction mixture was cooled to room temperature and purified by silica gel column chromatography eluting with methanol
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM (3
DCM (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97) affording (202 mg, 61%) 6 as a brown colored mass. Compound7: IR: νmax (film): 3408, 2962, 1695, 1634 cm−1; 1H NMR (500 MHz, CDCl3): δ 11.25 (1H, br d, J = 11.6 Hz), 7.46 (1H, dd, J = 11.6, 8.8 Hz), 5.93 (1H, ddt, J = 17.2, 10.4, 5.6 Hz), 5.32 (1H, ddd, J = 17.2, 2.9, 1.3 Hz), 5.24 (1H, overlapped dd, J = 10.4, 1.3 Hz), 5.22 (1H, d, J = 8.8 Hz), 4.63 (2H, m), 4.20 (1H, s), 3.58 (1H, d, J = 10.8 Hz), 3.53 (1H, d, J = 10.8 Hz), 1.03 (3H, s), 0.98 (3H, s); 13C NMR (125 MHz, CDCl3): δ 171.8, 168.2, 136.9, 131.9, 118.4, 97.5, 78.1, 71.4, 64.8, 39.3, 20.9, 20.2; LCMS [M+Na]+: 280. [α]23D = +26.2 (c = 0.8, CHCl3). All the spectral data compared with those of reported data7 and it was found to be identical. Compound10: IR νmax (film): 3344, 2964, 2931, 2877, 1687, 1633 cm−1; 1H NMR (400 MHz, CD3OD, 3.31): δ 7.98 (1H, d, J = 14.3 Hz), 5.98 (1H, ddt, J = 17.3, 10.5, 5.5 Hz), 5.78 (1H, d, J = 14.3 Hz), 5.34 (1H, dd, J = 17.3, 1.5 Hz), 5.24 (1H, dd, J = 10.5, 1.2 Hz), 4.65 (2H, app dt, J = 6.7, 5.5 Hz), 4.05 (1H, s), 3.50 (1H, d, J = 10.8 Hz), 3.39 (1H, d, J = 10.8 Hz), 0.96 (3H, s), 0.95 (3H, s); 13C NMR (50 MHz, CDCl3): δ 171.3, 167.1, 136.8, 132.2, 118.1, 102.3, 77.9, 71.51, 64.9, 39.3, 20.9, 20.1; LCMS [M+Na]+: 280. [α]24D = +73.2° (c = 0.75, CH3CN); Lit.3 [α]25D = +80.0° (c = 0.12, CH3CN). All the spectral data of 10 compared with those of reported data3 and it was found to be identical.
97) affording (202 mg, 61%) 6 as a brown colored mass. Compound7: IR: νmax (film): 3408, 2962, 1695, 1634 cm−1; 1H NMR (500 MHz, CDCl3): δ 11.25 (1H, br d, J = 11.6 Hz), 7.46 (1H, dd, J = 11.6, 8.8 Hz), 5.93 (1H, ddt, J = 17.2, 10.4, 5.6 Hz), 5.32 (1H, ddd, J = 17.2, 2.9, 1.3 Hz), 5.24 (1H, overlapped dd, J = 10.4, 1.3 Hz), 5.22 (1H, d, J = 8.8 Hz), 4.63 (2H, m), 4.20 (1H, s), 3.58 (1H, d, J = 10.8 Hz), 3.53 (1H, d, J = 10.8 Hz), 1.03 (3H, s), 0.98 (3H, s); 13C NMR (125 MHz, CDCl3): δ 171.8, 168.2, 136.9, 131.9, 118.4, 97.5, 78.1, 71.4, 64.8, 39.3, 20.9, 20.2; LCMS [M+Na]+: 280. [α]23D = +26.2 (c = 0.8, CHCl3). All the spectral data compared with those of reported data7 and it was found to be identical. Compound10: IR νmax (film): 3344, 2964, 2931, 2877, 1687, 1633 cm−1; 1H NMR (400 MHz, CD3OD, 3.31): δ 7.98 (1H, d, J = 14.3 Hz), 5.98 (1H, ddt, J = 17.3, 10.5, 5.5 Hz), 5.78 (1H, d, J = 14.3 Hz), 5.34 (1H, dd, J = 17.3, 1.5 Hz), 5.24 (1H, dd, J = 10.5, 1.2 Hz), 4.65 (2H, app dt, J = 6.7, 5.5 Hz), 4.05 (1H, s), 3.50 (1H, d, J = 10.8 Hz), 3.39 (1H, d, J = 10.8 Hz), 0.96 (3H, s), 0.95 (3H, s); 13C NMR (50 MHz, CDCl3): δ 171.3, 167.1, 136.8, 132.2, 118.1, 102.3, 77.9, 71.51, 64.9, 39.3, 20.9, 20.1; LCMS [M+Na]+: 280. [α]24D = +73.2° (c = 0.75, CH3CN); Lit.3 [α]25D = +80.0° (c = 0.12, CH3CN). All the spectral data of 10 compared with those of reported data3 and it was found to be identical. - S. Mukherjee and E. J. Corey, Org. Lett., 2010, 12, 1024 Search PubMed.
- The lower kinetic barrier for the retro Diels–Alder reaction of 11 is expected from the superior stabilization of the transition state resulting from effective orbital interactions of cyclopropane bond orbitals. See: S. Kotha, S. Banerjee, M. P. Patil and R. B. Sunoj, Org. Biomol. Chem., 2006, 4, 1854 Search PubMed.
- J. W. Coe, M. C. Wirtz, C. G. Bashore and J. Candler, Org. Lett., 2004, 6, 1589 Search PubMed.
- Our attempts to use bicyclic amino acids directly in the reactions to synthesize the target compounds were not successful. Nonetheless, we continue our efforts in that direction.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of spectra and spectroscopic data for all the compounds. See DOI: 10.1039/c2ra01051h |
| This journal is © The Royal Society of Chemistry 2012 |
