Synthesis of microporous fluorinated chromia with a sharp pore distribution
Xiao-qing
Jia
a,
Heng-dao
Quan
*b,
Masanori
Tamura
b and
Akira
Sekiya
b
aBeijing Institute of Technology (BIT), Beijing, 100081, P. R. China.
bNational Institute of Advanced Industrial Science and Technology (AIST), Tsukuba Ibaraki, 305-8565, Japan. E-mail: Hengdao-quan@aist.go.jp; Tel: +081-298-4657; Fax: +081-298-4657
First published on 27th June 2012
Abstract
This paper reports a novel micro-porous fluorinated chromia with a sharp pore distribution from 0.5 nm to 1.5 nm. The mentioned fluorinated chromia has a 105.1 m2 g−1 surface area and an excellent chemical stability in the presence of strong corrosive media such as HF, HCl and thermo-stability under 300 °C. A facile process for preparing the target material is carried out by controlling the decomposition rate of urea in a solution of chromium chloride to obtain its precursor, chromia, which has a narrow pore distribution in the range from 0.5 nm to 1.5 nm. Then the chromia is treated with HF via vapor-phase fluorination to obtain porous fluorinated chromia. The prepared material has been used as a fluorination catalyst with a high catalytic activity, and could be a potential membrane material and sorption media.
1 Introduction
The synthesis, structural characterization and properties of porous inorganic solids have been widely investigated.1–4 The reports related to mesoporous materials (with pore diameters from 2 nm to 10 nm) are now quite extensive since the discovery of MCM-41 type mesoporous molecular sieves.5,6 Of these materials, one of the most promising points is to exploit cooperative interactions between an organic template and inorganic precursor molecules to create an ordered porous solid. This general approach offers considerable flexibility, and has led to materials with a wide range of bulk morphologies and controlled pore diameters spanning several orders of magnitude.Micro-porous inorganic materials (with pore diameters of ≤ 2 nm) have been widely used as catalysts, membranes and sorption media due to their similar pore sizes to molecule and the large internal surface area.7,8 The well-known zeolites are more highly crystalline than mesoporous materials. However, most of micro-porous materials, such as zeolites, silicas and molecular sieves, are composed of silicates and metal oxides. They are unstable and result in the collapse of the porous structure in the presence of corrosive media, such as HCl, HF and F2.9,10
Porous metal fluorides are a kind of material inert to strong corrosive media like HF, HCl and widely used as a catalyst in vapor-phase catalytic fluorination, but the existing metal fluorides have a small surface area. The micro-porous metal fluorides with a sharp pore distribution and high surface area have been exploited for their high catalytic activity and potential function as a membrane material. Stacy et al. reported a novel method for converting zeolites to AlF3 with high surface area of 190 m2 g−1via plasma-assisted fluorination with nitrogen trifluoride.11 Kemnitz et al. found a non-aqueous synthesis route to X-ray amorphous metal fluorides with about 206 m2 g−1 surface area by means of the reaction of an organic intermediate with HF.12,13 We reported a facile method for preparing a porous chromium fluoride (PCrF) catalyst with a 187 m2 g−1 surface area and 0.58 cm3 g−1 pore volume. In the process, the large surface area was generated by the produced volatile gas escaping (SiF4), which was formed by the reaction of a siliceous inorganic additive and anhydrous hydrogen fluoride (AHF).10 This kind of porous metal fluoride has an excellent catalytic performance as vapor-phase fluorination catalysts, and has been used widely in vapor-phase catalytic fluorination in industry. Generally speaking, these porous metal fluorides have a wide pore distribution range. Their pore diameter is distributed in the range from 0.5 nm to 40 nm.
In our current research, the goal is to prepare some inert micro-porous metal fluorides, which have a sharp pore distribution and stable chemical performance to corrosive media like HF and HCl. In addition, the prepared materials will be a potential membrane material. Here we will report a novel inert porous material, porous fluorinated chromia, which has a sharp micro pore distribution from 0.5 nm to 1.5 nm and an excellent chemical stability in the presence of strong corrosive media. The synthetic process is described as follows. A suitable amount of urea is added to a solution of chromium chloride, then gradually heating the solution to make the urea decompose into ammonium to obtain a suitably acidic environment. A hydroxyl chromium gel is formed during the decomposing process of urea with the temperature increasing. The formed Cr(OH)3 gel is filtered and calcinated to form chromia, which has a sharp pore distribution. The prepared chromia is then treated by HF via vapor-phase fluorination to obtain a porous fluorinated chromia with a narrow pore distribution. The obtained material has a stable chemical property in the presence of HF and HCl, and can be used as a fluorination catalyst with a high catalytic activity, and will be a potential membrane material and sorption media.
2 Results and discussion
The pores of conventional porous fluorinated chromia are distributed in a wide range from 0.5 nm to 80 nm (see Fig. 1b), which are attributed to the pore distribution of the precursor, chromia (see Fig. 1a). The traditional chromia has a wide pore distribution, which is caused by the in-homogeneous precipitation process. In this process, various sized particles are formed since it is difficult to control the particle size during the formation of the Cr(OH)3 gel. The particles accumulate to form different sized pores, which gives the chromia a wide pore distribution range.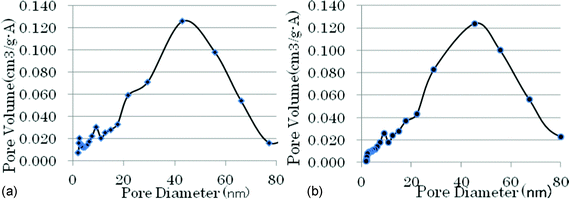 | ||
| Fig. 1 (a) Pore distribution of traditional chromia. (b) Pore distribution of traditional fluorinated chromia. | ||
When the mentioned chromia is treated by AHF in a vapor-phase fluorination process, the AHF reacts with chromia to form fluorinated chromia. The pore size of the chromia becomes larger and larger with the fluorination process, causing the surface area and pore volume to decrease when compared to the original precursor (see Table 1). Also the results in Table 1 indicate that the surface area of the precursor chromia is much larger than that of the fluorinated chromia. However the pore distribution of the material remains in the same state as before. The results are shown in Fig. 1a and 1b clearly.
| Type | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| Cr2O3 | 330.8 | 0.59 | 6.3 |
| F–Cr2O3 | 172.6 | 0.50 | 11.2 |
These porous metal fluorides have been the fundamental pillar for vapor-phase catalytic fluorination processes, in which porous fluorinated chromia has been used as process catalysts and still has an important impact on the fluorine chemical industry today. However due to the wide pore distribution of the traditional fluorinated chromia, its application has been limited to a narrow range. For example, it is difficult to use the fluorinated chromia as an inorganic membrane material for the combination of catalysis and separation processes because this reaction membrane needs one kind of material with a sharp pore distribution.14
According to our design, we would like to prepare a kind of porous metal fluoride with a sharp pore distribution to satisfy the above-mentioned purpose. As we know, urea CO(NH2)2 in solution will gradually decompose to release NH3 with increasing temperature. The released ammonium is rapidly dissolved to change the acidity of the solution. In our experiments, a certain amount of urea is added to a solution of chromium chloride, followed by gradual heating of the solution to make the urea decompose to obtain a suitable acidity, the pH of the solution increases from around 4 to 8. In the above process, the hydroxyl chromium gel with a homogeneous particle size is formed, then filtered, and calcinated under a nitrogen atmosphere to form chromia, which has a sharp pore distribution since the chromia with almost the same particle size is accumulated together (see Fig. 2a). The analytic results of BET indicate that chromia has a 222 m2 g−1 surface area and a sharp pore distribution. Among the pores, more than 90% are distributed in the range from 0.5 nm to 1.5 nm.
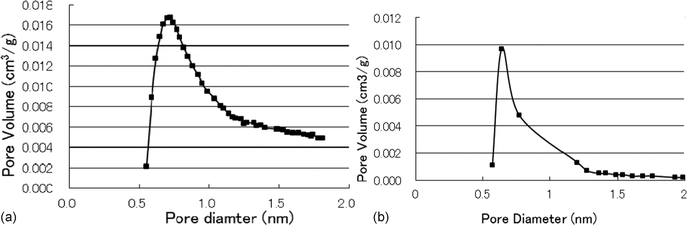 | ||
| Fig. 2 (a) Pore distribution of chromia. (b) Pore distribution of fluorinated chromia. | ||
For preparing porous fluorinated chromia, above prepared chromia is treated by HF via vapor-phase fluorination under nitrogen atmosphere from 200 °C to 400 °C to obtain a porous fluorinated chromia, which has a narrow pore distribution like its precursor (see Fig. 2b). The obtained porous fluorinated chromia has a 105.1 m2 g−1 surface area and a sharp pore distribution, more than 90% of the pores are in the range from 0.5 nm to 1.5 nm.
The nitrogen adsorption isotherm of chromia (see Fig. 3a) and the isotherm of fluorinated chromia (see Fig. 3b) prepared from our novel chromia are quite similar, despite the fact that chromia and fluorinated chromia have different surface areas.
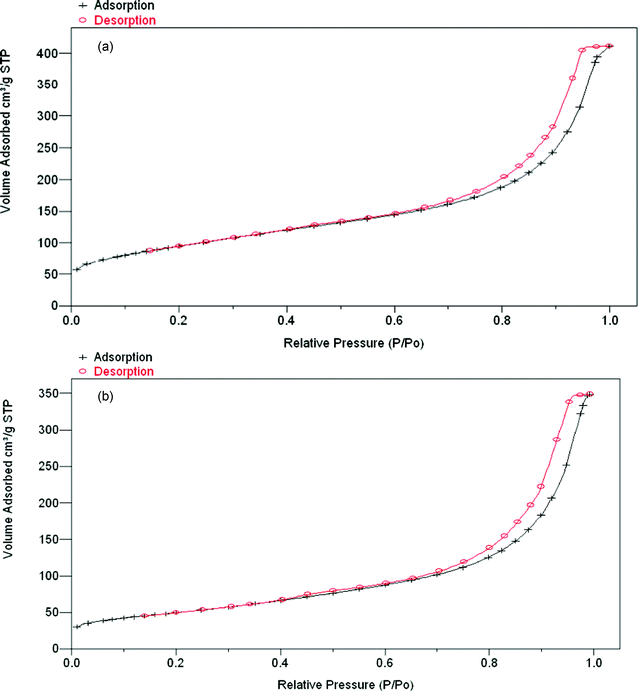 | ||
| Fig. 3 (a) N2 adsorption isotherm plot of chromia (surface area 222 m2 g−1). (b) N2 adsorption isotherm plot of fluorinated chromia obtained from chromia treated with HF (surface area 105.1 m2 g−1). | ||
The pore sizes of chromia and fluorinated chromia in Fig. 2a and 2b are similar to the molecular size, therefore have a high potential as a membrane material and absorption media. Also they have stable chemical properties in the presence of HF and HCl, and fluorinated chromia can been used as a fluorination catalyst with a high catalytic activity.
DSC and TG analysis indicate that the micro-porous metal fluoride has no obvious decomposition until 300 °C. The above-results show that micro-porous fluorinated chromia has an excellent thermal stability.
XRD results indicate that prepared fluorinated chromia keeps amorphous phase when the chromia is treated with AHF under a nitrogen atmosphere from 200 °C to 400 °C for 100 h (Fig. 4). Amorphous fluorinated chromia is advantageous for F/Cl exchange reactions in vapor-phase catalytic fluorination.15
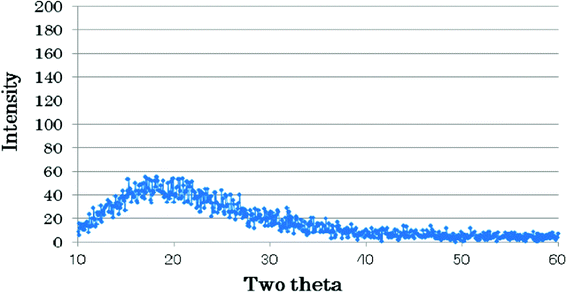 | ||
| Fig. 4 XRD pattern of fluorinated chromia. | ||
Transmission electron microscopy (TEM) shows that most of the micro-porous fluorinated chromia are a regular array of uniform pores (see Fig. 5). The pore diameter is less than 2 nm compared with the solid bar (10 nm). The result is consistent with the results obtained by BET analysis.
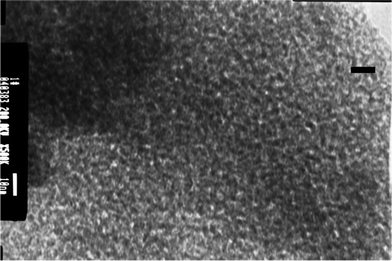 | ||
| Fig. 5 Representative transmission electron micrograph of micro-porous fluorinated chromia. This image was obtained with a transmission electron microscope operated at 200 KV from a thin section prepared by ultrasonic cleaning. | ||
SEM micrographs (see Fig. 6) show that the micro-porous fluorinated chromia is probably agglomerates of much smaller particles. In addition, the surface of its precursor, chromia, appeared to be downy, the particles seem much finer than fluorinated chromia (Fig. 7).
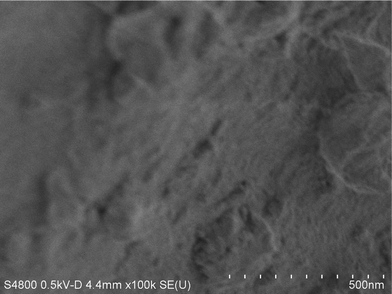 | ||
| Fig. 6 SEM micrographs of micro-porous fluorinated chromia. This micrograph was obtained on an FE-S4800 scanning electron microscope. The sample was prepared by using ultrasonic cleaning. | ||
 | ||
| Fig. 7 SEM micrographs of chromia. This micrograph was obtained on an FE-S4800 scanning electron microscope. The sample was prepared by using ultrasonic cleaning. | ||
A typical F/Cl exchange reaction is the preparation of difluoromethane (HFC-32) from dichloromethane via vapor-phase catalytic fluorination. In this process, the micro-porous fluorinated chromia is used as a process catalyst, which exhibited a higher catalytic activity to effectively run the F/Cl exchange. However, its activity is lower than conventional fluorinated chromia (see Table 2).
| Reaction temp (°C) | Product selectivity (%) | Conv. of CH2Cl2 (%) | Yield (%) | ||
|---|---|---|---|---|---|
| CH2F2 | CH2ClF | CH2F2 | CH2ClF | ||
| 300 | 39.8 | 60.2 | 40.8 | 16.2 | 24.5 |
| 330 | 46.8 | 53.2 | 50.5 | 23.6 | 26.8 |
| 360 | 52.0 | 48.0 | 52.3 | 27.2 | 25.1 |
For the traditional chromia catalyst, the conversion of dichloromethane and selectivity of difluoromethane is 10% higher than our new porous fluorinated chromia. We ascribe the result to the fact that the micropores of fluorinated chromia reach the molecular size of the reactants, for example, CH2Cl2 and CH2F2. These molecules are not so easy to enter inside the fluorinated chromia pores, which made the catalytic efficiency decrease. We speculate the catalytic reaction only occurs on the surface of catalyst. On the other hand, the above fact means that the micro-porous fluorinated chromia has a high potential to be a membrane material, which will be a prime candidate to where the traditional micro materials like molecular sieves cannot be used, especially in corrosive environments.
3 Experimental
The BET surface areas, the pore volumes and BJH adsorption pore-diameter distributions were determined for the samples by means of low-temperature adsorption of argon using a Micromeritics ASAP 2010 instrument. The sample was degassed under vacuum at 300 °C for 3 h before measurement.X-Ray diffraction (XRD) was employed to determine the bulk crystalline phase of the samples. The patterns of samples were recorded with a Mac Science, MPX-18 diffractometer. X-Ray diffraction was completed by using Cu-Kα1 (0.1542 nm) radiation and the X-ray tube was operated at 40 KV and 150 mA.
Differential scanning calorimetry and thermogravimetry patterns of the samples were recorded, respectively, on Rigaku Thermoplus DSC-8230 and Thermoplus 8120 instruments.
Representative scanning electron micrographs and energy-dispersive X-ray (EDX) analysis of samples were obtained on a Hitachi S4800 field-emission scanning electron microscope (SEM). Representative transmission electron micrograph of samples were recorded by a JEOL, JEM 2010 transmission electron microscope (TEM) operated at 200 kV. The sample was dispersed into ethanol by means of ultrasonic cleaning, and then dried under vacuum at room temperature for analysis.
A typical process for preparing micro-pore fluorinated chromia and its precursor is shown as follows;
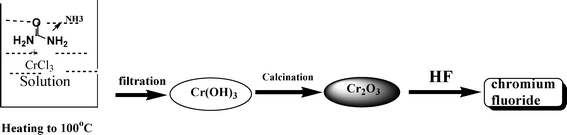 | (1) |
In the process, 6.3 g (NH2)2CO solved into 40 mL de-ionized water was combined with 15.8 g 10% CrCl3 solution. The solution was heated to 100 °C under stirring violently. The gel is formed gradually with the (NH2)2CO decomposition during heating process and the pH of solution increased automatically to around 8 from 4. The resulting gel of chromium hydroxide is filtered, well-washed with de-ionized water, dried at 120 °C for 10 h, ground, and pelletized. The pellets are calcined at 450 °C under a nitrogen atmosphere to obtain the precursor, chromia, with a sharp pore distribution.
Fluorination of chromia is carried out in following step. Apparatus for the fluorination of chromia consists of two mass flow controllers (one is for N2 and the other for AHF) and an electrically heated tubular Inconel reactor, 14 mm in diameter and 300 mm in length, equipped with an inside Inconel tube for inserting type-K thermocouples with a 1 mm diameter. The pelletized chromia is charged into an Inconel reaction tube, treated with an HF steam diluted by N2 (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HF = 200
HF = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) at 200 °C for 4 h, then heated to 400 °C at a rate of 2 °C min−1 and keep at 400 °C for 4 h in a 100% HF steam not diluted by N2. Finally remaining AHF on the precursor is washed by N2. The fluorinated chromia is used in the characterization and fluorination procedure. Element analysis indicated that novel fluorinated chromia (wt. %) is composed of oxygen 6.50, Fluorine 61.40 and Chromium 32.04 (F/O 9.45, F/Cr 1.92).
100) at 200 °C for 4 h, then heated to 400 °C at a rate of 2 °C min−1 and keep at 400 °C for 4 h in a 100% HF steam not diluted by N2. Finally remaining AHF on the precursor is washed by N2. The fluorinated chromia is used in the characterization and fluorination procedure. Element analysis indicated that novel fluorinated chromia (wt. %) is composed of oxygen 6.50, Fluorine 61.40 and Chromium 32.04 (F/O 9.45, F/Cr 1.92).
For the fluorination of dichloromethane, the reactor was charged with 10 mL of the prepared fluorinated chromia, and heated to reaction temperature under N2 atmosphere. Then liquid phase dichloromethane was fed into the reactor via a vaporizer and metering pump. The vapor-phase AHF was supplied to the vaporizer by an HF mass flow-meter. The product stream from the reactor was scrubbed with H2O at 60 °C and then passed first through a dryer packed with CaCl2 and a gas chromatograph (SHIMADZU GC-14 A on-line) with a Poraplot Q capillary column (i.d. 0.32 mm; length 25 m; J & W Scientific Inc.) for calculating the conversion of CH2Cl2 and the yields of CH2FCl and CH2F2. The products were determined by comparing their patterns of NMR with that of authentic samples.16
In order to calculate the conversion of CH2Cl2 and selectivity of CH2FCl and CH2F2, it is necessary to measure their GC relative response factor. The vacuum line is used for the calculation of their absolute amount by measuring the pressure under a certain volume. In vacuum line, each 1 mmol sample was measured, respectively, and filled into a sample tube with around 80 mL. The above gas mixture was carried to GC by carrier gas (He) under a warm atmosphere and their relative GC area was measured. Finally obtaining GC relative response factor is as following. CH2F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2FCl
CH2FCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 1
CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.23
1.23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.67. The data were used to calculate the conversion of CH2Cl2 and selectivity of CH2FCl and CH2F2 in vapor-phase catalytic fluorination.
1.67. The data were used to calculate the conversion of CH2Cl2 and selectivity of CH2FCl and CH2F2 in vapor-phase catalytic fluorination.
4 Conclusion
The present study illustrates for the first time that by controlling the decomposition of urea in a chromium chloride solution can be used to construct micro-porous metal oxides and micro-porous metal fluorides with sharp pore distribution in terms of vapor-phase fluorination. This approach is in contrast to the conventional surfactant templating of mesoporous phases and is distinct from the typical molecular templating of zeolitic structures. Our synthesis enables a pore size below 2 nm with sharp distribution and the prepared metal fluoride has potential as membrane material and catalyst under highly corrosive environments.References
- T. Sun and J. Y. Ying, Angew. Chem., Int. Ed., 1998, 37, 664 CrossRef CAS.
- H. Yang, A. Kuperman, N. Coombs, S. Mamiche-Afara and G. A. Ozin, Nature, 1996, 379, 703 CrossRef CAS.
- Y. Lu, R. Ganguli, C. A. Drewien, M. T. Anderson, C. J. Brinker, W. Gong, Y. Guo, H. Soyez, B. Dunn, M. Huang and J. I. Zink, Nature, 1997, 389, 364 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, M. E. Roth, W. J. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. Chu, D. H. Olsen, W. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- S. Klein, S. Thorimbert and W. F. Maier, J. Catal., 1996, 163, 476 CrossRef CAS.
- B. G. Shpeizer, A. Clearfield and J. M. Heising, Chem. Commun., 2005, 2396 RSC.
- M. E. Davis, Acc. Chem. Res., 1993, 26, 1111 CrossRef.
- H. D. Qaun, H. E. Yang, M. Tamura and A. Sekiya, J. Catal., 2005, 231, 254 CrossRef.
- J. L. Delattre, P. J. Chupas, C. P. Grey and A. M. Stacy, J. Am. Chem. Soc., 2001, 123, 5364 CrossRef CAS.
- E. Kemnitz, U. Groβ, S. Rüdiger and C. S. Shekar, Angew. Chem., Int. Ed., 2003, 42, 4251 CrossRef CAS.
- J. Noack, K. Teinz, C. Schaumberg, C. Fritz, S. Rüdiger and E. Kemnitz, J. Mater. Chem., 2011, 21, 334–338 RSC.
- L. E. Manzer and M. J. Nappa, Appl. Catal., A, 2001, 221, 267 CrossRef CAS.
- S. Akashi, I. Yoshio and K. Satoshi, EP, 1992, 0514932 Search PubMed.
- H. D. Quan, M. Tamura, Y. Matsukawa, J. Mizukado, T. Abe and A. Sekiya, J. Mol. Catal. A: Chem., 2004, 219, 79 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
