Electrochemical hydrogen storage properties of a non-equilibrium Ti2Ni alloy†
Xiangyu
Zhao
,
Jiajia
Li
,
Yan
Yao
,
Liqun
Ma
and
Xiaodong
Shen
*
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Materials Science and Engineering, Nanjing University of Technology, 5 Xinmofan Road, Nanjing, 210009, PR China. E-mail: xdshen@njut.edu.cn; Fax: +86 25 83240205; Tel: +86 25 83587234
First published on 19th January 2012
Abstract
A crystalline Ti2Ni alloy was mechanically milled and subsequently annealed to form a non-equilibrium Ti2Ni alloy. This non-equilibrium structure could restrain the formation of irreversible metal hydride during charging. Consequently, we demonstrated that the alloy had a discharge capacity of 336 mAh g−1, which was much higher than those of other Ti2Ni alloys reported in the literature and also higher than that of the commercial LaNi5–based alloy. An electrochemical hydrogen absorption–desorption mechanism of the alloy during cycling is also presented.
Hydrogen storage at room temperature and atmospheric pressure, including electrochemical and gaseous storage, is one of the key points for developing hydrogen applications, such as Ni–MH secondary batteries1–3 and hydrogen supplying systems for fuel cells.4–6 Some metals and their alloys such as AB5-type LaNi5–based alloys and AB3+x-type La–Mg–Ni–based alloys are considered to be good hydrogen storage materials and have been extensively investigated for decades. Moreover, A2B-type Ti2Ni–based alloys have also been attracting much attention because of their high number of interstitial sites for hydrogen storage.7–10 A Ti2Ni alloy could absorb 1.85 wt% hydrogen, corresponding to a high theoretical electrochemical hydrogen storage or charge capacity of 500 mAh g−1.11,12 However, the irreversible formation of metal hydride during hydrogen absorption of the alloy suppressed its hydrogen desorption, meaning in that the alloy had a low maximum discharge capacity, less than 250 mAh g−1.9,13 This severely limited the application of Ti2Ni–based alloys, which were almost removed from the list of hydrogen storage alloys.
We have found that phase structure played an important role in the discharge properties of the Ti2Ni alloy.14 However, the discharge capacities of the non-equilibrium Ti2Ni alloys prepared by milling a sintered crystalline Ti2Ni alloy were very low, although they had a better cycling stability as compared to the crystalline alloy. In this present work, we obtained a Ti2Ni alloy with a high discharge capacity of 336 mAh g−1, which was higher than 310 mAh g−1 of the commercial LaNi5–based alloy. There was no evident formation of an irreversible phase during hydrogen absorption of this alloy. Moreover, the electrochemical reaction mechanism of the alloy was presented.
A crystalline Ti2Ni alloy fabricated by an induction melting method13 was crushed into powders below a 200 mesh, and then mechanically milled in a stainless steel vial containing stainless steel balls (10 and 6 mm) at 1400 rpm for 10 h under an argon atmosphere to form an amorphous Ti2Ni alloy (see ESI, Fig. S1†), which was subsequently heat treated at 693 K under an argon atmosphere with a purity of 99.99%, leading to the formation of a non-equilibrium Ti2Ni alloy containing amorphous and nanocrystalline phases. X-ray diffraction (XRD) with Cu-Kα radiation was carried out in a Thermo ARL X' TRA diffractometer system to determine the structure of the alloy powders. High resolution transmission electron microscopy (HRTEM) was also employed for structure observation on the powders, embedded in copper, using a JEM 2000 EX unit. X-Ray photoelectron spectroscopy (XPS) measurements were performed using a K–Alpha (Thermo Scientific) spectrometer with Al-Kα radiation as the X-ray source. The C 1s line with a binding energy of 284.6 eV was used as a standard. The morphology observation of the sample was analyzed by metallographic microscopy and a JSM-5610LV scanning electron microscope (SEM).
The metal-hydride electrodes were prepared by pressing 0.1 g alloy powders and 0.3 g nickel powders into a pellet 10 mm in diameter, under a pressure of 15 MPa. The charge and discharge testing was conducted in a half-cell consisting of a metal-hydride electrode, a Ni(OH)2/NiOOH counter electrode, and a Hg/HgO reference electrode in a 6 M KOH solution under a BT-2000 testing equipment (Arbin, USA). The electrodes were charged at 60 mA g−1 for several hours, allowed to rest for 10 min, and then discharged at 60 mA g−1 to the cut-off potential of −0.6 V vs. the Hg/HgO reference electrode.
Fig. 1 shows the XRD patterns of the crystalline and non-equilibrium Ti2Ni alloy powders. The crystalline alloy exhibits the diffraction peaks corresponding to the Ti2Ni phase (PDF card 72-0442) with a cubic structure. Mechanical milling and subsequent heat treatment contributed to the formation of amorphous and nanocrystalline phases, as shown in Fig. 1 (b). This can be confirmed by the results of the selected area electron diffraction (SAED) and HRTEM (see ESI, Fig. S2†). The crystalline alloy powders show a gray color, while the non-equilibrium Ti2Ni alloy powders show a blue color, as shown in the pictures embedded in Fig. 1. This may be attributed to the formation of a protective oxide layer during heat treatment.15
 | ||
| Fig. 1 XRD patterns of Ti2Ni alloy powders: (a) crystalline (gray) and (b) non-equilibrium (blue). | ||
Fig. 2 shows the discharge capacities of the crystalline and non-equilibrium Ti2Ni electrodes as a function of cycle number at several temperatures. The charge process was performed for 6 h. The non-equilibrium electrode had a stable discharge capacity of about 215 mAh g−1 after three charge and discharge cycles for activation at 293 K. There was no severe capacity loss during the initial cycles, probably due to the fact that the non-equilibrium phase structure restrained the formation of irreversible metal hydride and the protective layer had good corrosion resistance in the alkaline electrolyte. When the electrolyte temperature was 313 K, the non-equilibrium electrode showed a high discharge capacity of 336 mAh g−1 at the first cycle, which was evidently higher than those of the Ti2Ni alloys reported in the literature7–10,12–15 and 310 mAh g−1 of the commercial LaNi5–based alloys.16,17 This is quite different from the discharge properties of the crystalline Ti2Ni alloy, which showed a significant decrease of discharge capacity when the electrolyte temperature was increased,13 as shown in Fig. 2. Furthermore, the discharge capacity of the non-equilibrium electrode decreased drastically by increasing the charge and discharge cycles. The further increase of the electrolyte temperature showed a slight effect on the discharge properties. Note that the non-equilibrium alloy at 313 and 333 K had a good activation property, which could be attributed to the significant improvement of hydrogen penetration through the alloy surface at a high temperature.
 | ||
| Fig. 2 Discharge capacities of the crystalline (A) and non-equilibrium (B) Ti2Ni electrodes as a function of cycle number at several temperatures. | ||
The charge and discharge curves, phase structure, and surface structure and morphology of the Ti2Ni alloys were studied, in order to understand the electrochemical reaction mechanism of the non-equilibrium alloy. Fig. 3 shows the charge and discharge curves of the crystalline (A) and non-equilibrium (B) Ti2Ni electrodes at a current density of 60 mA g−1. The crystalline electrode charged for a short time (6 h) at 293 K showed an electrode potential of about –0.9 V after charging, which meant that the electrode absorbed many hydrogen atoms and was not fully charged. However, the discharge capacity of this electrode was only 154.6 mAh g−1, corresponding to 42.9% of the hydrogen absorbed by the electrode. A long time charging (8.5 h) was carried out for the crystalline electrode at 293 K, which had a high hydrogen storage capacity of about 450 mAh g−1. Nevertheless, only 55.0% of the hydrogen in the fully charged crystalline electrode was desorbed. A large number of hydrogen atoms were fixed in the bulk alloy in the initial charging. Note that the electrochemical hydrogen storage capacity of Ti2Ni alloy was almost equal to the theoretical capacity at 293 K, while the hydrogen desorption of the alloy was difficult, leading to a low discharge capacity. It could be inferred that most of the irreversible phase was formed during the initial charge process at the first cycle of the crystalline electrode. For the second cycle of the crystalline electrode, no evident new irreversible phase formed according to the charge and discharge curves. The structural change during cycling has been carried out by XRD.13Hydrogen absorption led to a non-elastic lattice expansion of the crystalline alloy. The charge and discharge curves of the non-equilibrium electrode at 313 K were also shown in Fig. 3. It showed a high discharge efficiency that the hydrogen absorbed was almost desorbed at the first and second cycles. The formation of the irreversible phase was significantly restrained. We have found in our previous work14 that both nanocrystalline and amorphous phases were beneficial to improvement of kinetics of hydrogen absorption–desorption. Moreover, the amorphous structure showed better corrosion and pulverization resistance. However, the formation of nanocrystalline and amorphous phases during mechanical milling of a crystalline Ti2Ni alloy led to a decrease of the discharge capacity. The amorphous alloy showed the lowest discharge capacity. The low discharge capacity of nanocrystalline and amorphous phases formed during mechanical milling could be attributed to the fact that some of the hydrogen storage sites of the alloy were destroyed by mechanical milling, and thus a decrease of discharge capacity. However, heat treatment for the milled alloy could recover the hydrogen storage sites destroyed during milling. The nanocrystalline phase was more beneficial to hydrogen storage capacity, while the amorphous phase mainly contributed to kinetics improvement of hydrogen absorption–desorption and also enhancement of corrosion resistance. Therefore, a non-equilibrium Ti2Ni alloy with a high discharge capacity was obtained by mechanical milling and subsequent proper heat treatment.
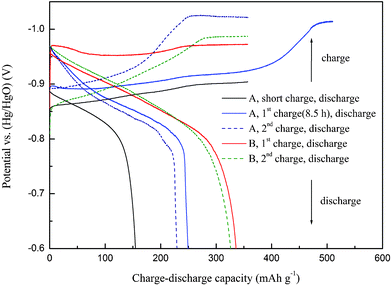 | ||
| Fig. 3 Charge and discharge curves of the crystalline (A) and non-equilibrium (B) Ti2Ni electrodes at a current density of 60 mA g−1 | ||
Fig. 4 shows XPS region spectra of Ti 2p for the non-equilibrium Ti2Ni alloy and the alloy after 10 cycles. Ti 2p1/2 and Ti 2p3/2 peaks corresponding to the oxide of TiO2 are located at 464.0 and 458.4 eV for both alloys, respectively. Ar+ sputtering removed part of the surface layer, inducing the appearance of Ti 2p peaks relating to TiO at 460.3 and 454.7 eV and a drastic decrease in the signal intensity attributed to metallic TiO2 for the as-prepared alloy. While the Ar+ sputtering had almost no effect on the surface structure of the alloy after cycling according to the results of Ti 2p peaks. The TiO2 layer of the alloy after cycling was thicker and more stable than that of the as-prepared alloy, which could decrease the charge efficiency and limit the hydrogen penetration through the alloy surface, leading to the decrease of discharge capacity of the alloy.
 | ||
| Fig. 4 XPS region spectra of Ti 2p of the non-equilibrium Ti2Ni alloy powders: (a) as-prepared and (b) after 10 cycles. Ar+ sputtering was carried out at 1 keV with a medium speed. | ||
Hydrogen absorption–desorption may lead to volume expansion of the alloy, which probably results in the pulverization of the alloy powders.18,19 This will accelerate the corrosion of the alloy if it has a poor corrosion resistance ability in an alkaline electrolyte, and thus a decay of the discharge capacity. Fig. 5 shows the variation of discharge capacity of the non-equilibrium Ti2Ni electrodes not fully charged at 313 K. The alloy charged for 4 h showed a higher maximum discharge capacity as a function of cycle number than that charged for 3 h. However, a drastic decrease of the discharge capacity occurred after five cycles. While the alloy charged for 3 h has a stable capacity of about 170 mAh g−1, as shown in Fig. 5. It could be inferred that the decrease of the discharge capacity of the non-equilibrium alloy during cycling was caused by the corrosion in the alkaline electrolyte instead of by a formation of irreversible phases. The variation of the bulk structure of the alloy charged for 4 h during cycling was also presented, as shown in Fig. 6. The diffraction peaks shifted to lower angles after charging for the second cycle. The XRD pattern of the alloy after discharging on the tenth cycle indicated that this volume expansion by hydrogen absorption disappeared, in spite of the drastic decay of the discharge capacity. The non-equilibrium Ti2Ni alloy, quite different from the crystalline alloy,9,13 showed a reversible change of the bulk structure during cycling.
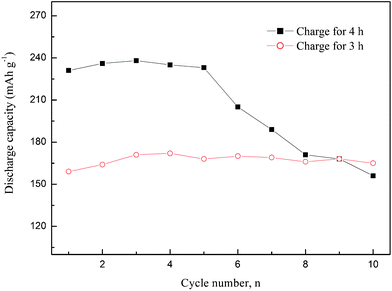 | ||
| Fig. 5 Discharge capacities of the non-equilibrium Ti2Ni electrodes as a function of cycle number at different charge terms. | ||
 | ||
| Fig. 6 XRD patterns of the non-equilibrium Ti2Ni alloy before and after cycling. | ||
Fig. 7 shows the SEM morphology after 10 cycles and a schematic illustration of hydrogen absorption–desorption of the non-equilibrium Ti2Ni alloy during cycling. There was no occurrence of pulverization for the alloy powders, indicating that the non-equilibrium phases had a good strength and toughness. However, the surface layer of the alloy powders was destroyed after cycling, as shown in Fig. 7 (a). Based on the results mentioned above, the schematic illustration of the hydrogen absorption–desorption of the alloy powders during cycling is presented in Fig. 7 (b), in order to provide a further understanding on the decay of the capacity of the electrode. It contains two steps, (1) the hydrogen absorption of the powder with good pulverization resistance resulted in a significant volumetric expansion. However, while the bulk structure was stable, the surface layer was destroyed. Many micro-cracks formed on the surface layer, and consequently, fresh surface was exposed to the alkaline solution leading to the subsequent corrosion of the alloy. (2) The small fresh surface as an anode and the large passive surface as a cathode formed a micro corrosion cell, namely the formation of a galvanic corrosion, accelerating the corrosion of the alloy in the electrolyte. As such the amount of active material for hydrogen absorption–desorption would be reduced. Moreover, the expansion and contraction of the powder and hydrogen penetration from the surface layer could separate part of the protective layer formed during heat treatment from the bulk powder. Finally, repeated formation and destruction of the new oxide layer would bring about a continuous depression of the alloy powders during charge and discharge cycles. The alloy surface was covered by a large amount of TiO2 so the charge efficiency was significantly decreased and thus a decrease of charge capacity, resulting in a low discharge capacity.
 | ||
| Fig. 7 (a) SEM morphology after 10 cycles and (b) schematic illustration of hydrogen absorption–desorption of the non-equilibrium Ti2Ni alloy during cycling. | ||
The hydrogen storage alloys utilized in Ni–MH secondary batteries require not only good hydrogen storage properties, but also excellent corrosion resistance properties. Although we contribute to the suppression of the formation of irreversible phases for the Ti2Ni alloy during cycling and a significant increase of discharge capacity of the alloy, more work is required for improving its overall properties.
Our conclusions are as follows: 1. The crystalline Ti2Ni alloy has an electrochemical hydrogen storage capacity of about 450 mAh g−1. However, only 55% of the hydrogen in the bulk alloy can be desorbed.
2. The non-equilibrium Ti2Ni alloy possesses a high reversible discharge capacity of 336 mAh g−1, which is much higher than those of the Ti2Ni alloy reported in the literature and also higher than that of the commercial LaNi5-based alloy.
3. The capacity loss of the non-equilibrium alloy is attributed to the damage of the surface layer and subsequent corrosion by the alkaline electrolyte during cycling.
Acknowledgements
We would like to acknowledge the financial support from the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions of China. We are also grateful to the support from a Project Funded by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (11KJB430009).References
- B. Drenchev, T. Spassov and D. Radev, J. Appl. Electrochem., 2007, 38, 437 CrossRef.
- J. E. Thomasa, E. B. Castro and A. Visintin, Int. J. Hydrogen Energy, 2010, 35, 5981 CrossRef.
- M. Anik, J. Alloys Compd., 2010, 491, 565 CrossRef CAS.
- K. Akiyama, S. Matsumoto, A. Miyasaka and T. Shodai, J. Power Sources, 2009, 186, 37 CrossRef CAS.
- F. Fang, Y. T. Li, Q. A. Zhang, L. L. Sun, Z. P. Shao and D. L. Sun, J. Power Sources, 2010, 195, 8215 CrossRef CAS.
- Y. Wang, C. X. Guo, X. Wang, C. Guan, H. B. Yang, K. Wang and C. M. Li, Energy Environ. Sci., 2011, 4, 195 CAS.
- E. W. Justi, H. H. Ewe, A. W. Kalberlah, N. M. Saridakis and M. H. Schaefer, Energy Convers., 1970, 10, 183 CrossRef CAS.
- B. Luan, N. Cui, H. K. Liu, H. Zhao and S. X. Dou, J. Power Sources, 1994, 52, 295 CrossRef CAS.
- B. Luan, N. Cui, H. Zhao, H. K. Liu and S. X. Dou, J. Power Sources, 1995, 55, 101 CrossRef CAS.
- B. Luan, S. J. Kennedy, H. K. Liu and S. X. Dou, J. Alloys Compd., 1998, 267, 224 CrossRef CAS.
- H. Buchner, M. A. Gutjahr, K. D. Beccu and H. Saufferer, Zeitschrift Fur Metalkunde, 1972, 63, 497 CAS.
- M. A. Gutjahr, H. Buchner, K. D. Beccu and H. Säufferer, A new type of reversible negative electrode for alkaline storage batteries based on metal alloy hydrides, in Proceedings of the Power Sources Symposium, Brighton, 1972, pp. 1973 Search PubMed.
- X. Y. Zhao, L. Q. Ma, Y. Ding and X. D. Shen, Intermetallics, 2010, 18, 1086 CrossRef CAS.
- X. Y. Zhao, L. Q. Ma, X. X. Qu, Y. Ding and X. D. Shen, Energy Fuels, 2009, 23, 4678 CrossRef CAS.
- X. Y. Zhao, L. Q. Ma, Y. Yao, Y. Ding and X. D. Shen, Energy Environ. Sci., 2010, 3, 1316 CAS.
- S. Corré, M. Bououdina, N. Kuriyama, D. Fruchart and G. Adachia, J. Alloys Compd., 1999, 292, 166 CrossRef.
- K. Hong, J. Alloys Compd., 2001, 321, 307 CrossRef CAS.
- Y. F. Liu, H. G. Pan, M. X. Gao and Q. D. Wang, J. Mater. Chem., 2011, 21, 4743 RSC.
- M. X. Gao, S. C. Zhang, H. Miao, Y. F. Liu and H. G. Pan, Int. J. Hydrogen Energy, 2010, 489, 552 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra00846g |
| This journal is © The Royal Society of Chemistry 2012 |
