Mesoporous SrTiO3 nanowires from a template-free hydrothermal process†
Tian-Yi
Ma
a,
Hui
Li
b,
Tie-Zhen
Ren
*c and
Zhong-Yong
Yuan
*a
aInstitute of New Catalytic Materials Science, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin, 300071, China. E-mail: zyyuan@nankai.edu.cn; Fax: +86 22 23509610; Tel: +86 22 23509610.
bState Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071, China
cSchool of Chemical Engineering & Technology, Hebei University of Technology, Tianjin, 300130, China. E-mail: rtz@hebut.edu.cn
First published on 8th February 2012
Abstract
High-yield SrTiO3 nanowires (NWs) with accessible mesoporosity were synthesized through a template-free hydrothermal process. The highest surface area of 145 m2 g−1 with the pore volume of 0.43 cm3 g−1 was confirmed when the Ti/Sr molar ratio was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 in the precursors. Unlike the previously reported preparation of mesoporous one-dimensional materials, the present method required no substrates, organic templates or additives, making it low cost and environmentally friendly. The formation mechanism of the mesoporous NWs was investigated by sampling and analyzing the reaction mixtures after different autoclaving time. It was suggested that the crystal growth and morphology evolution was governed by the Ostwald ripening process and the Kirkendall effect. The same strategy was also applied for successful preparation of mesoporous BaTiO3 NWs and mesoporous SrTiO3 nanorods and nanocubes. The obtained mesoporous SrTiO3 NWs were used for photodegradation of organic dyes and proved to be useful photocatalysts with excellent reusability.
1.05 in the precursors. Unlike the previously reported preparation of mesoporous one-dimensional materials, the present method required no substrates, organic templates or additives, making it low cost and environmentally friendly. The formation mechanism of the mesoporous NWs was investigated by sampling and analyzing the reaction mixtures after different autoclaving time. It was suggested that the crystal growth and morphology evolution was governed by the Ostwald ripening process and the Kirkendall effect. The same strategy was also applied for successful preparation of mesoporous BaTiO3 NWs and mesoporous SrTiO3 nanorods and nanocubes. The obtained mesoporous SrTiO3 NWs were used for photodegradation of organic dyes and proved to be useful photocatalysts with excellent reusability.
Introduction
One-dimensional (1D) structures, such as nanowires (NWs), nanobelts and nanotubes, have attracted tremendous attention within the last decade. Among the huge variety of 1D nanostructures, semiconducting NWs are particularly interesting not only for fundamental research due to the unique structural and physical properties relative to their bulk counterparts, but also for their fascinating potential in optoelectronic and electronic devices.1 The synthesis of NWs of common semiconductor materials such as Si, GaAs, InP, and ZnO has been accomplished through many strategies such as vapor–liquid–solid processes, vapor–solid processes, electrochemical deposition and solution growth.2,3 A further step beyond preparation of NWs is the incorporation of mesoporosity into this 1D architecture, which could lead to enhanced performances due to the high surface area, large pore size and pore volume of mesoporous materials. For example, mesoporous MnO24 and Pt5 NWs were synthesized with the assistance of anodic aluminium oxide templates, which exhibited improved capacitive performance and electrocatalytic activity, respectively. Mesoporous SnO2 NWs were prepared by porous SiO2 templates and used as efficient lithium battery anode materials.6 Mesoporous Co3O4 NW arrays were synthesized by an ammonia-evaporation-induced method, growing on Ti foils,7 which were also proved to be useful as the anode for lithium ion batteries. However, simpler systems, avoiding the use of additional templates or substrates, are still needed for the mass production of pure mesoporous NWs, which can offer the advantages of low impurities in the final products, easy collection, facile synthesis procedure and low cost.Due to the widespread applications in electronics, nonlinear optics, photoelectricity and catalysis, SrTiO3 with a perovskite structure has been prepared through solid-state reaction techniques, sol–gel methods, molten salt synthesis, hydrothermal techniques, etc.8 Different morphologies of 1D structures were reported such as fibrous SrTiO3 synthesized by using titanate nanofibers as the precursor,9 and single-crystalline SrTiO3 NWs prepared through a template-free hydrothermal process following an Ostwald ripening mechanism.10 For the mesoporosity design, titania spheres were employed in the synthesis of spherical mesoporous SrTiO3 without any templates, but resulted in poor mesoporosity with very low surface areas.11 Either organic additives or expensive polymer templates were found to be necessary for the fabrication of SrTiO3 micrometre spheres with wormhole-like mesopores12 and mesoporous SrTiO3 films.13 Indeed, the introduction of mesopores into 1D SrTiO3 nanostructure-like NWs is scarcely reported to the best of our knowledge. We report herein a template-free hydrothermal process to prepare high-yield mesoporous SrTiO3 NWs. This one-step method required no substrates, organic templates or additives; and the formation mechanism of the well-structured mesoporous NWs with high surface area was proposed according to the Ostwald ripening process combined with the Kirkendall effect, which can be extended to the preparation of other multimetallic oxides such as BaTiO3, and other 1D or even 0D mesoporous nanostructures. The obtained SrTiO3 NWs were used as efficient photocatalysts for organic dye photodegradation under UV light irradiation. The fact that the NWs can be readily recovered by sedimentation is of great significance for their potential application as photocatalysts for the elimination of organic pollutants from water on an industrial scale.14
Experimental
Materials
Tetrabutyl titanate, strontium nitrate (Sr(NO3)2), barium nitrate (Ba(NO3)2), propanol, rhodamine B (RhB), methylene orange (MO) and reactive brilliant blue KN-R were obtained from Tianjin Kermel Chemical Co. Commercial photocatalyst P-25 (surface area: 50 ± 15 m2 g−1, crystalline size: 21 nm, λg: 392 nm, Eg: 3.16 eV, ∼80% anatase and ∼20% rutile) and commercial SrTiO3 powders (surface area: 28 ± 10 m2 g−1, crystalline size: 33 nm, λg: 385 nm, Eg: 3.22 eV, perovskite) were purchased from Beijing J&K Scientific Ltd. All chemicals were used as received without further purification.Synthesis of mesoporous NWs
In a typical synthesis procedure, tetrabutyl titanate was added into 10 mL of propanol to obtain a clear solution [Solution A]. Sr(NO3)2 was dissolved in 30 mL of deionized water [Solution B]. Solution A was added dropwise into Solution B under gentle stirring with a Ti/Sr molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.02, 1
1.02, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05, 1
1.05, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.08 and 1
1.08 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.11, respectively, for four parallel experiments. The pH value was adjusted to 13 by NaOH, followed by sealing in one Teflon-lined autoclave and aging statically at 160 °C for 48 h. The resultant mixture was filtered, dried, and calcined at 500 °C for 6 h to obtain the final mesoporous SrTiO3 NWs. Mesoporous BaTiO3 NWs were prepared by the same method using Ba(NO3)2 as the precursor instead of Sr(NO3)2. To investigate the reaction mechanism, the same reaction was carried out, and aliquots of the reaction mixture were taken after autoclaving for 4, 24 and 48 h, filtered, dried and used for TEM and EDS measurements. Mesoporous SrTiO3 nanorods were synthesized by a similar method to that of SrTiO3 NWs but in a mixed solution of 23 mL of propanol and 30 mL of water, while mesoporous SrTiO3 nanocubes were synthesized in a mixed solution of 20 mL propanol, 5 mL of cyclohexanol and 30 mL of water.
1.11, respectively, for four parallel experiments. The pH value was adjusted to 13 by NaOH, followed by sealing in one Teflon-lined autoclave and aging statically at 160 °C for 48 h. The resultant mixture was filtered, dried, and calcined at 500 °C for 6 h to obtain the final mesoporous SrTiO3 NWs. Mesoporous BaTiO3 NWs were prepared by the same method using Ba(NO3)2 as the precursor instead of Sr(NO3)2. To investigate the reaction mechanism, the same reaction was carried out, and aliquots of the reaction mixture were taken after autoclaving for 4, 24 and 48 h, filtered, dried and used for TEM and EDS measurements. Mesoporous SrTiO3 nanorods were synthesized by a similar method to that of SrTiO3 NWs but in a mixed solution of 23 mL of propanol and 30 mL of water, while mesoporous SrTiO3 nanocubes were synthesized in a mixed solution of 20 mL propanol, 5 mL of cyclohexanol and 30 mL of water.
Characterization
Scanning electron microscopy (SEM) was carried out on a Shimadzu SS-550 microscope at 15 keV. Transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDS) and selected area electron diffraction (SAED) were carried out on a Philips Tecnai G20 at 200 kV. X-Ray diffraction (XRD) patterns of the as-synthesized products were recorded on a Bruker D8 diffractometer with Cu-Kα radiation (λ = 1.5418 Å). Diffuse reflectance UV-vis absorption spectroscopy was employed on a TU-1901 spectrophotometer using BaSO4 as a reference. Thermogravimetry (TG) was performed using a TA SDT Q600 instrument at a heating rate of 5° min−1 using α-Al2O3 as the reference. N2 adsorption–desorption isotherms were recorded on a Quantachrome NOVA 2000e sorption analyzer at liquid nitrogen temperature (77 K). The samples were degassed at 200 °C overnight prior to the measurement. The surface areas were calculated by the multi-point Brunauer–Emmett–Teller (BET) method, and the pore size distribution was calculated from the adsorption branch of the isotherms by non-local density functional theory (NLDFT).Photocatalytic activity testing
The photocatalytic activity experiments on the obtained mesoporous SrTiO3 NWs were performed by the degradation of RhB, MO and KN-R dyes under UV-light irradiation. 5.5 mg of the SrTiO3 sample was placed into a tubular quartz reactor of 100 mL of dye aqueous solution (10 μmol L−1). A 125 W UV-lamp with maximum emission at 365 nm was located 10 cm higher than the solution surrounded by a circulating water tube. The suspensions were magnetically stirred in the dark for 30 min to ensure the establishment of an adsorption/desorption equilibrium, and then exposed to the UV-light irradiation at room temperature. The mixture sampled at different times was centrifuged for 5 min to discard any sediment. The absorbance of reaction solutions was measured by a SP-722 spectrometer at λmax = 554, 464 and 596 nm for RhB, MO and KN-R, respectively. The activity of commercial P-25 and SrTiO3 powders was also investigated under identical conditions. The photocatalytic degradation reaction can be assumed to follow a pseudo-first-order expression: ln(C0/C) = kt, where C0/C is the normalized organic compounds concentration and k is the apparent reaction rate (min−1). The photocatalytic activity has been defined as the overall degradation rate constant of the catalysts. By plotting ln(C0/C) as a function of irradiation time through regression, the k constants from the slopes of the simulated straight lines can be obtained.Results and discussion
Material synthesis and characterization
Sr(NO3)2 and tetrabutyl titanate with different molar ratios were chosen as the precursors herein and the synthesis was performed under alkaline conditions in a mixed solution of propanol and water. The final products were obtained after calcination at 500 °C for 6 h to eliminate any organic species. The wide-angle XRD patterns of SrTiO3 NWs in Fig. 1a show that all of the NWs prepared with different Ti/Sr molar ratios were composed of highly crystalline, cubic perovskite structures of SrTiO3. The unit-cell parameter for the obtained SrTiO3 was determined to 0.39 nm, in agreement with those for the corresponding bulk cubic materials. One single and broad diffraction peak in the range of 1–2.5° (2θ) was present in the low-angle region (Fig. 1b), suggesting the presence of the mesovoids without long-range order.15,16 Noticeably, the sample synthesized at Ti/Sr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 exhibited higher intensity for this broad diffraction peak than others. The obtained mesoporous SrTiO3 NWs were thermally stable up to 900 °C, confirmed by the TG analysis (Fig. S1, ESI†).
1.05 exhibited higher intensity for this broad diffraction peak than others. The obtained mesoporous SrTiO3 NWs were thermally stable up to 900 °C, confirmed by the TG analysis (Fig. S1, ESI†).
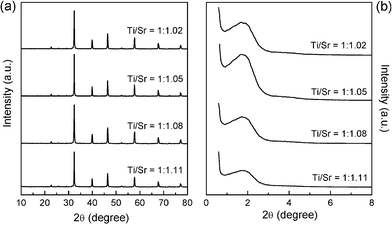 | ||
| Fig. 1 Wide-angle (a) and low-angle XRD (b) patterns of the mesoporous SrTiO3 NWs synthesized with different Ti/Sr molar ratios. | ||
A weight loss of 0.2% at near 100 °C was attributed to the evaporation of water, while another slight weight loss of 0.32% near 400 °C is due to some chemical absorption oxygen being desorbed from the powders.17
N2 sorption analysis was employed to confirm the mesoporosity of the synthesized materials. The N2 sorption isotherms of mesoporous SrTiO3 NWs were of type IV, characteristic of mesoporous materials (Fig. 2a), according to the IUPAC classification.18 The hysteresis loop has a triangular shape and a steep desorption branch, which belongs to the type H2 hysteresis. The specific surface areas were calculated to be 130, 145, 128 and 121 m2 g−1 for the samples synthesized with Ti/Sr molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.02, 1
1.02, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05, 1
1.05, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.08 and 1
1.08 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.11, respectively. And the largest pore volume of 0.43 cm3 g−1 was also confirmed at Ti/Sr = 1
1.11, respectively. And the largest pore volume of 0.43 cm3 g−1 was also confirmed at Ti/Sr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05, which was coincident with the low-angle XRD observation. The pore size distribution, obtained by the NLDFT method (Fig. 2b), presented one peak at 3.4–6.0 nm. Importantly, this well-structured mesoporosity of SrTiO3 with high surface area and pore volume was obtained by a template-free method, which was comparable to the mesoporous SrTiO3 materials synthesized with organic additives or templates;12,13 and the surface area was much higher than some SrTiO3 structures by the template-free method (<20 m2 g−1).9,11 Moreover, the Ti/Sr molar ratio of 1
1.05, which was coincident with the low-angle XRD observation. The pore size distribution, obtained by the NLDFT method (Fig. 2b), presented one peak at 3.4–6.0 nm. Importantly, this well-structured mesoporosity of SrTiO3 with high surface area and pore volume was obtained by a template-free method, which was comparable to the mesoporous SrTiO3 materials synthesized with organic additives or templates;12,13 and the surface area was much higher than some SrTiO3 structures by the template-free method (<20 m2 g−1).9,11 Moreover, the Ti/Sr molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 was proved to be the optimal condition to prepare SrTiO3 NWs with the highest surface area and pore volume, and this sample was used for the following characterizations as illustration.
1.05 was proved to be the optimal condition to prepare SrTiO3 NWs with the highest surface area and pore volume, and this sample was used for the following characterizations as illustration.
 | ||
Fig. 2 N2 sorption isotherms (a) and corresponding pore size distribution curves calculated by the NLDFT method (b) of the mesoporous SrTiO3 NWs synthesized with different Ti/Sr molar ratios. The volume adsorbed was shifted by 300, 200, 100, and dV/dD values were shifted by 0.6, 0.4, 0.2 for the samples with Ti/Sr molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.02, 1 1.02, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05, 1 1.05, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.08, respectively. 1.08, respectively. | ||
The morphology of the synthesized mesoporous SrTiO3 NWs were characterized by SEM and TEM observations. As shown in Fig. 3a and b, high-yield SrTiO3 NWs could be obtained, while other morphologies could be hardly observed. The NWs had a uniform cylindrical structure with a length of several micrometres. In the TEM images (Fig. 3c and d), it could be seen that the surfaces of NWs were smooth, very regular, and without any sheath of amorphous phase; and the average diameter of these NWs was 20–50 nm. According to a generally accepted definition,19,20 nanorods are structures with a width of 1–100 nm and an aspect ratio (major axis/minor axis) less than 20, while nanowires are analogous structures with an aspect ratio greater than 20. Thus the present SrTiO3 materials with an aspect ratio in the range of around 60–200 could be called “nanowires”.
In the magnified TEM images (Fig. 3d and e), accessible mesopores with wormhole-like shapes were observed throughout the NWs, which was consistent with N2 sorption and low-angle XRD analysis. The mesoporosity has scarcely been observed in the previously reported 1D perovskite structures,10,21 which is expected to contribute to the enhanced performances of the synthesized SrTiO3 NWs. By further magnifying the mesopores (Fig. 3einset), lattice fringes with a spacing of around 0.28 nm were clearly observed, indicating the crystalline nature of the mesopore wall. The wormhole-like mesopores with crystalline walls were also confirmed by the high-resolution TEM image in Fig. 4, in which one could see clear lattice fringes and irregular mesopores without long-range order. As the accessible mesopores were formed by the inter-aggregated SrTiO3 nanocrystals without consistent orientations, the polycrystalline diffraction rings in the SAED pattern were observed (Fig. 4inset).
 | ||
| Fig. 4 HRTEM image and SAED pattern (inset) of the mesoporous SrTiO3 NWs. | ||
Because no additional catalysts or templates were introduced into the reaction system, the growth of the NWs is obviously not catalyst-assisted or template-directed. The mechanism of the NW growth should be different from that of previous methods that depend on self-assembled supramolecular forms21 or structure-directing templates in structured solids.22 To investigate the formation mechanism of this novel mesoporous NW structure, the same reaction was carried out, and aliquots of the reaction mixture were sampled for observations after autoclaving for different times. As shown in Fig. 5a after autoclaving for 4 h, the fibrous structure began to appear. EDS spectra taken from the different areas of the body of these fibers show that they were mainly made up of Ti and O, and a very low content of Sr, while the Cu peaks were due to TEM grids (Fig. 5d). It is supposed that the structure here was related to the fibrous titanate species, which were commonly obtained in the strong alkaline system.9,14 Due to the absence of a stabilizing agent and the high free energy, the Sr species could hardly aggregate until the fibrous titanate formed, which acted as a seed to deposit the Sr species. Thus after autoclaving for 24 h, some irregular particles were present along the outside surface of the fibrous structure (Fig. 5b), which were confirmed to be mainly composed of Sr and O by EDS analysis (Fig. 5e). It is attributed to the Ostwald ripening that the small Sr species deposited on the fibrous seeds grew into larger particles under a longer time of autoclaving,23 and thus the titanate fibers were covered with a layer of Sr species (Fig. 5g). The main compositions of the body of these fibrous structures were still Ti and O after 24 h autoclaving (the white square in Fig. 5b). However, the inter-penetration and reaction still happens at the interface of Ti and Sr domains, and thus weak Ti peaks were also observed in the outside particles (Fig. 5e).
 | ||
| Fig. 5 TEM (a, b, c) and EDS (d, e, f) images for aliquots of the mixture after autoclaving for different time. Regions where EDS spectra were taken are circled in the TEM images. A proposed mechanism for the mesoporous NWs (g). | ||
After 48 h of autoclaving, the smooth NWs were obtained with accessible mesopores, which could be explained by the Kirkendall effect. The Kirkendall effect was the first experimental proof that atomic diffusion occurs through vacancy exchange and not by the direct interchange of atoms.24 The net directional flow of matter is balanced by an opposite flow of vacancies, which can commonly lead to the formation of porosity.11,25 In the present system, H2O and highly concentrated OH− would access the inner fibrous titanate to generate titanium hydroxyl species (probably HTiO3−),11,26 which could subsequently react with Sr2+ ions in the outside layer of irregular particles to form SrTiO3. Thus an outward flow of HTiO3− was formed in order to supply enough reagents for the interaction between HTiO3− in the Ti domain and Sr2+ ions in the Sr domain at the interface of these two moieties; according to the Kirkendall effect, this outward flow of ions should be balanced by the inward flow of vacancies into the fibrous structure. As the reaction continues, the outside layer of irregular Sr species was continuously consumed in the reaction and incorporated into the fibrous structure, and the surface became smooth and clean, while the mesopores were formed inside the fibers from the condensation of supersaturated vacancies. The 1D structure grew at the cost of the small particles into longer NWs, with reduction in surface energy as the primary driving force for the morphology evolution. And finally, mesoporous NWs could be obtained (Fig. 5c and g).27 The resultant solids after 48 h of autoclaving were composed homogeneously of Sr, Ti and O (Fig. 5f), which would turn into pure SrTiO3 after calcination. Thus based on TEM and EDS analysis, we proposed that the crystal growth and morphology evolution was governed by the Ostwald ripening process and the Kirkendall effect. Noticeably, Sr(NO3)2 used in the precursors was superfluous (Ti/Sr molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05) due to some dissociative Sr2+ ions in the solvent not crystallizing over fibrous titanate. We also found that propanol was necessary in the preparation, probably because that the microemulsion system containing propanol, butanol (from tetrabutyl titanate hydrolysis) and water benefited the formation of 1D structure.28
1.05) due to some dissociative Sr2+ ions in the solvent not crystallizing over fibrous titanate. We also found that propanol was necessary in the preparation, probably because that the microemulsion system containing propanol, butanol (from tetrabutyl titanate hydrolysis) and water benefited the formation of 1D structure.28
The present method was also applied to synthesize mesoporous BaTiO3 NWs with a perovskite structure. As shown in Fig. 6a, high-yield NWs were obtained with a very large aspect ratio. By magnifying one single NW, wormhole-like mesopores could be clearly seen, similar to that of SrTiO3 NWs. The NWs were composed of cubic perovskite structures of BaTiO3 (Fig. 6b), and the surface area was confirmed to be 109 m2 g−1. We expect that the formation process combining Ostwald ripening and the Kirkendall effect could be extended to other ternary oxides like MgTa2O6 and CoxTi1−xO2−x, or even some quaternary oxides like PbZrTiO3 and BaxSr1−xTiO3.
 | ||
| Fig. 6 TEM image (a), XRD pattern (b) and N2 sorption isotherm (b inset) of the synthesized mesoporous BaTiO3 NWs. | ||
Due to the various and controllable morphologies of metal oxides such as TiO2, the micro-structure of the synthesized mesoporous multimetallic oxides could also be altered. Because the composition of the solvents has great influence on the 1D structure as mentioned above, by varying the alcohol/water ratio and solvent compositions we could get other 1D structures such as SrTiO3 nanorods with much lower aspect ratios (Fig. 7a and b). With the further shortening of the major axis, mesoporous SrTiO3 nanocubes (Fig. 7c) could also be prepared. The surface areas were confirmed to be 71 and 96 m2 g−1 for nanorods and nanocubes, respectively (Fig. 7d). Thus this method might be useful in the synthesis of other 1D or even 0D porous materials.
 | ||
| Fig. 7 TEM images of mesoporous SrTiO3 nanorods (a, b) and nanocubes (c). N2 sorption isotherms of the synthesized nanorods and nanocubes (d). The volume adsorbed for nanocubes was shifted by 20. | ||
Photocatalytic activity
The optical response of the synthesized materials was investigated, and their UV-vis diffuse reflectance spectra were recorded and are shown in Fig. 8a with corresponding physicochemical parameters listed in Table 1. The same tendency in the spectra with a strong absorption in the UV-light range (220–380 nm) was observed for all three samples. The band-gap values of the samples were calculated by the formula Eg (eV) = 1240/λg (nm), where λg stands for the wavelength value corresponding to the intersection point of the vertical and horizontal parts of the spectra.29 The Eg of mesoporous SrTiO3 NWs (3.14 eV) and commercial P-25 (3.16 eV) were similar, which were a little lower than that of commercial SrTiO3 powder (3.22 eV). The photocatalytic activities were evaluated by photodegradation of RhB under UV-light irradiation (Fig. 8b). Catalytic degradation in the dark with mesoporous SrTiO3 NWs and self-degradation under UV-light irradiation without catalysts show no decrease of the dye concentration, which indicated that the loss of dye by adsorption processes could be ignored in this experiment, and the organic dyes could hardly be self-photosensitized and self-decomposed without photocatalysts. The photocatalytic degradation reaction can be assumed to follow a pseudo-first-order expression: ln(C0/C) = kt, where C0/C is the normalized organic compounds concentration and k is the apparent reaction rate (min−1). The k values after the first use of these catalysts follow the sequence of mesoporous SrTiO3 NWs > commercial P-25 > commercial SrTiO3 (Table 1). The photodegradation of other dyes such as MO and KN-R were tested, in which the mesoporous SrTiO3 NWs also show higher activity than commercial P-25 and SrTiO3 nanoparticles (Table 1). The superior photocatalytic activity of mesoporous SrTiO3 NWs could be mainly attributed to the well-structured mesoporosity with a higher specific surface area, which could increase the efficiency of photoabsorption and improve mass transfer.16,30 | ||
| Fig. 8 UV-vis diffuse reflectance spectra (a), and the photocatalytic activity for RhB degradation under UV-light irradiation (b) of the mesoporous SrTiO3 NWs, compared with commercial powders. The green lines in the figure show the fitting results using pseudo-first-order reaction kinetics. | ||
| Sample | Morphology | S BET a (m2 g−1) | λ g b (nm) | E g c (eV) | K d (min−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RhB | MO | KN-R | ||||||||
| First use | Tenth use | First use | Tenth use | First use | Tenth use | |||||
| a BET surface area calculated from the linear part of the 10-point BET plot. b The wavelength value corresponding to the intersection point of the vertical and horizontal parts of the UV-vis diffuse reflectance spectra. c Band-gap value calculated by the formula Eg (eV) = 1240/λg (nm). d Photodegradation rate constant following pseudo-first order reaction kinetics. e Crystalline size: 33 nm. f Crystalline size: 21 nm. | ||||||||||
| Mesoporous SrTiO3 NWs | Mesoporous nanowires | 145 | 394 | 3.14 | 0.0228 | 0.0227 | 0.0351 | 0.0349 | 0.0127 | 0.0126 |
| Commercial SrTiO3 | Irregular particlese | 28 ± 10 | 385 | 3.22 | 0.0187 | 0.0162 | 0.0298 | 0.0254 | 0.0076 | 0.0063 |
| Commercial P-25 | Irregular particlesf | 50 ± 15 | 392 | 3.16 | 0.0207 | 0.0185 | 0.0322 | 0.0282 | 0.0098 | 0.0081 |
Easy recovery and good reusability are very important for a practical catalyst. Taking the photodegradation of RhB as an example, as shown in Fig. 9A, the mesoporous SrTiO3 NWs after the first and the tenth use could sedimentate from an aqueous suspension in less than 1 h, while the aqueous suspension of commercial P-25 and commercial SrTiO3 powders were still turbid. 1D structured photocatalysts with large aspect ratios have an advantage over spherical powder catalysts when separating the catalyst from solution by filtration or sedimentation.14,31 In fact, the high cost of separating the catalyst nanocrystals has seriously impeded their applications on an industrial scale.14 Although ultra-fine catalyst powders with a particle size of several nanometres exhibited a superior activity because of the large surface-to-volume ratios, these powders were very difficult to recover from water after their use in aqueous systems.32 The reaction rate of SrTiO3 NWs shows no obvious decrease after 10-times cycling for RhB photodegradation, while the rates of commercial P-25 and SrTiO3 show decreases of 10.6% and 13.3%, respectively (Fig. 9B). The same phenomenon was observed for these photocatalysts in the degradation of MO and KN-R (Table 1). It is attributed to that the catalysts made up of spherical particles have the tendency to agglomerate into larger particles, which will result in a reduction of the photocatalytic activity during the cycling use.14,32 The fact that the mesoporous SrTiO3 NWs can be readily recovered by sedimentation and maintain their photocatalytic activity after 10-times cycling uses is of great significance for their potential for the elimination of organic pollutants at an industrial scale. Thus two advantages mainly contributed to superior activity of the synthesized photocatalyst, namely, the accessible mesoporosity for improved photoabsorption and mass transfer and 1D nanowire architecture for easy recovery.
 | ||
| Fig. 9 (A) Sedimentation for 1 h in aqueous suspensions of commercial photocatalyst P-25 (a), commercial SrTiO3 powder (b), mesoporous SrTiO3 NWs after the first (c) and the tenth use (d) for RhB photodegradation; (B) overall degradation rate constant k vs. times of cycling uses for RhB degradation of the synthesized photocatalyst compared with commercial powders. | ||
Conclusions
Mesoporous SrTiO3 NWs were prepared through a facile hydrothermal method without employing any template molecules. Accessible mesoporosity with large specific surface areas and high thermal stability were obtained for the synthesized materials. The formation of mesoporous NWs was proposed to be governed by the Ostwald ripening process and the Kirkendall effect, and this mechanism presented here can be extended to synthesize other multimetallic oxide mesoporous 1D structures. Because of the low-cost raw materials without using organic templates and the simple one-pot hydrothermal procedure, the large-scale production of these materials should be very easy. The mesoporous SrTiO3 NWs show a higher photocatalytic activity than commercial P-25 and SrTiO3 powders. Furthermore, these NW photocatalysts can be easily recycled by sedimentation without decrease of the photocatalytic activity. Combining their unique features of low-cost, mesoporous NW structure, high photocatalytic activity with good reusability, the mesoporous SrTiO3 NWs will provide possibilities for future industrial applications in environmental pollutants clean up.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 20973096 and 21073099), the National Basic Research Program of China (No. 2009CB623502), the Key Project of Chinese Ministry of Education (No. 2100016), and the Program for Innovative Research Team in University (IRT0927).References
- S. Barth, F. Hernandez-Ramirez, J. D. Holmes and A. Romano-Rodriguez, Prog. Mater. Sci., 2010, 55, 563 CrossRef CAS.
- H. J. Fan, P. Werner and M. Zacharias, Small, 2006, 2, 700 CrossRef CAS.
- K. Shankar, J. I. Basham, N. K. Allam, O. K. Varghese, G. K. Mor, X. Feng, M. Paulose, J. A. Seabold, K. S. Choi and C. A. Grimes, J. Phys. Chem. C, 2009, 113, 6327 CAS.
- C. Xu, Y. Zhao, G. Yang, F. Li and H. Li, Chem. Commun., 2009, 7575 RSC.
- Y. Zhong, C. L. Xu, L. B. Kong and H. L. Li, Appl. Surf. Sci., 2008, 255, 3388 CrossRef CAS.
- H. Kim and J. Cho, J. Mater. Chem., 2008, 18, 771 RSC.
- Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265 CrossRef CAS.
- S. Tao and J. T. S. Irvine, Nat. Mater., 2003, 2, 320 CrossRef CAS.
- Y. G. Li, X. P. Gao, G. R. Li, G. L. Pan, T. Y. Yan and H. Y. Zhu, J. Phys. Chem. C, 2009, 113, 4386 CAS.
- U. A. Joshi and J. S. Lee, Small, 2005, 1, 1172 CrossRef CAS.
- Y. W. Wang, H. Xu, X. B. Wang, X. Zhang, H. M. Jia, L. Z. Zhang and J. R. Qiu, J. Phys. Chem. B, 2006, 110, 13835 CrossRef CAS.
- X. Wei, G. Xu, Z. Ren, C. Xu, W. Weng, G. Shen and G. Han, J. Am. Ceram. Soc., 2010, 93, 1297 CrossRef CAS.
- D. Grosso, C. Boissiere, B. Smarsly, T. Brezesinski, N. Pinna, P. A. Albouy, H. Amenitsch, M. Antonietti and C. Sanchez, Nat. Mater., 2004, 3, 787 CrossRef CAS.
- H. Y. Zhu, Y. Lan, X. P. Gao, S. P. Ringer, Z. F. Zheng, D. Y. Song and J. C. Zhao, J. Am. Chem. Soc., 2005, 127, 6730 CrossRef CAS.
- A. Vantomme, Z. Y. Yuan and B. L. Su, New J. Chem., 2004, 28, 1083 RSC.
- X. Wang, J. C. Yu, C. Ho, Y. Hou and X. Fu, Langmuir, 2005, 21, 2552 CrossRef CAS.
- Y. L. Xu, X. H. Zhou and O. T. Sorensen, Sens. Actuators, B, 2000, 65, 2 CrossRef.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169 CrossRef CAS.
- C. J. Murphy and N. R. Jana, Adv. Mater., 2002, 14, 80 CrossRef CAS.
- M. H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, Science, 2001, 292, 1897 CrossRef CAS.
- J. J. Urban, W. S. Yun, Q. Gu and H. Park, J. Am. Chem. Soc., 2002, 124, 1186 CrossRef CAS.
- Y. Mao, S. Banerjee and S. S. Wong, J. Am. Chem. Soc., 2003, 125, 15718 CrossRef CAS.
- W. Wang, C. Xu, G. Wang, Y. Liu and C. Zheng, Adv. Mater., 2002, 14, 837 CrossRef CAS.
- A. D. Smigelskas and E. O. Kirkendall, Trans. Aime., 1947, 171, 130 Search PubMed.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2004, 126, 16744 CrossRef CAS.
- M. Pourbaix, in Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd edn, National Association of Corrosion/Engineers, Houston, Texas, 1974, p. 251 Search PubMed.
- Y. Liu, D. Ma, R. A. Blackley, W. Zhou, X. Han and X. Bao, J. Phys. Chem. C, 2008, 112, 4124 CAS.
- X. J. Zhang, T. Y. Ma and Z. Y. Yuan, Eur. J. Inorg. Chem., 2008, 2721 CrossRef CAS.
- L. Korosi and I. Dekany, Colloids Surf., A, 2006, 280, 146 CrossRef.
- J. C. Yu, L. Z. Zhang, Z. Zheng and J. C. Zhao, Chem. Mater., 2003, 15, 2280 CrossRef CAS.
- N. Z. Bao, X. Feng, Z. H. Yang, L. M. Shen and X. H. Lu, Environ. Sci. Technol., 2004, 38, 2729 CrossRef CAS.
- Y. Yu and D. Xu, Appl. Catal., B, 2007, 73, 166 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TG curve and EDS image of the synthesized mesoporous SrTiO3 NWs. See DOI: 10.1039/c2ra00823h |
| This journal is © The Royal Society of Chemistry 2012 |

