Self-assembly of α-Fe2O3 mesocrystals with high coercivity
Ruimin
Yao
and
Chuanbao
Cao
*
Research Center of Materials Science, Beijing Institute of Technology, Beijing, 100081, P. R. China. E-mail: cbcao@bit.edu.cn; Fax: 86 10 6891 2001; Tel: 86 6891 3972
First published on 9th January 2012
Abstract
This paper reports on the non-traditional corrosion method for the synthesis of hematite mesocrystals in the size range of 30 μm. The results clearly show that the self-assembly of hematite mesocrystals were consisted of many small building units which were densely packed. The formation mechanism for hematite mesocrystals is proposed, which belongs to nonclassical crystallization. Ammonium chloride (NH4Cl), as a reagent in the reaction, has played a key role in the formation of hematite mesocrystals. The high coercivity of hematite mesocrystals of 4437.80 Oe was recorded. The origin of the large coercivity is also discussed. The size of the building units is the main reason, the anisotropies of the mesocrystals, defects, strain and exchange coupling are attributed to the enhancement of the large coercivity. All of the reasons for the explanation of the large coercivity can be attributed to the presence of N. The synthesis route is economical and environmentally friendly and is a promising way to fabricate other kinds of mesocrystals.
Introduction
Nanomaterials have attracted great attention in recent decades because of their fascinating properties.1 They have great potential applications in many fields, such as photonics, electronics, magnetics and mechanics, etc. However, their superficial aggregation due to high surface energy results in impairment or even the disappearance of their attractive properties. In addition, the small size of individual nanoparticles makes it very difficult to single them out for practical applications. Therefore, it is important to design, tailor, and create assembly systems of nanomaterials that maintain (or even improve) their unique properties and also perform well when manipulated based on the specific requirements of scientists.2 Thus far, some successful assembly systems have been fabricated, such as nanoarrays,3 mesopore materials,4 thin-films,5 and biomineralization systems,6etc.Recently, the new concept of a ‘mesocrystal’ has been proposed as a self-assembly system of nanomaterials. A mesocrystal is described as a superstructure composed of three-dimensional nanoparticles that is classified as an intermediate between a traditional polycrystal and an ideal single crystal.7 This system has drawn much attention because it is of importance for many fascinating phenomena, such as fabricating nanostructured devices. As intermediates, mesocrystals can be good supplements to polycrystals and single crystals because there is a strong interaction among their ordered units and the size can be tuned by controlling the bottom-up growth conditions. Mesocrystals not only maintain the properties of nanoparticles in microscale, but can even exhibit new effects or improved properties. The structures and properties of mesocrystals are inseparably related to their sizes and the way the assembly units are attached. This suggests that it is possible to achieve various and advanced properties by controlling the structures of mesocrystals.
Diverse mesocrystals, including both inorganic8–11 and organic12 materials, can be fabricated in many media, including non-aqueous,8 aqueous,9,10 gelatine11 and solid,13,14etc. Most mesocrystals require special organic additives to help form superstructures. However, these additives actually act as impurities and are expensive. Because of the disadvantages, it is necessary to develop different routes for the fabrication of mesocrystals without adding organic additives; for example, using ion additives15 or no additives at all16. Furthermore, the products synthesized in liquid phase usually have pores or interspaces. In contrast, a heat treatment process can result in more densely packed mesocrystals,13,14 which is useful for ceramic materials, where density is very important.
Here, we report on the formation of α-Fe2O3 (hematite) mesocrystals by a corrosive route. The obtained mesocrystals, with well crystalline morphologies and three-dimensional superstructures consist of small hematite nanoparticles. The results suggest that this corrosion route could make densely packed hematite mesocrystals with a high coercivity value of 4,437.80 Oe. In addition, the route also holds appeal due to its economical and environmentally friendly reactions.
Experimental section
Preparation of hematite mesocrystals
The fabrication of hematite mesocrystals has two steps. In a typical synthesis: Firstly, an aqueous solution containing FeCl3·6H2O(5.4 g, 20 mmol) and distilled water (75 ml) was mixed with an another solution of ammonia (28 wt%, 25 ml) and distilled water (25 ml) at 80 °C to form reddish brown precipitate of hydrous hematite. The precipitate was washed and filtered with distilled water and absolute ethanol several times separately. Then the product was dried at 60 °C for 6 h. Secondly, grinding a mixture of dry precipitate and ammonium chloride (NH4Cl(s)) according to a proportion (Fe2O3·nH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4Cl = 1
NH4Cl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, wt/wt) for 20 min by hand. At last, the mixture was placed in a muffle furnace and heated at 500 °C for 2 h.
3, wt/wt) for 20 min by hand. At last, the mixture was placed in a muffle furnace and heated at 500 °C for 2 h.
Characterization
The samples were characterized using X-ray powder diffraction (XRD) (Netherlands PANalytical X′ Pert PRO MPD X-ray diffractometer, Cu-Kα radiation, λ = 1.54178 A°), transmission electron microscopy (TEM) (HITACHI-800) and fourier transform infrared (FTIR) spectroscopy (Nicolet 6700 FTIR spectrometer). The morphology and microstructure were observed using scanning electron microscopes (SEM) (HITACHI TM-1000 and S-4800) and energy dispersive spectrometer (EDS) data was performed by S4800. The BET surface areas were determined by Micromeritics ASAP-2400 analyzer (USA). X-ray photoelectron spectroscopy (XPS) data were collected on PHI-5300 ESCA (Pekin–Elmer, USA). Magnetic measurements were performed on a vibrating sample magnetometer (VSM, LakeShore 7407, USA).Results and discussion
Fig. 1 shows the XRD patterns of the products. The precursor of hydrous hematite is nearly amorphous, there are no obvious diffraction peaks. Whereas both the nanoparticles and the mesocrystals are a single phase of well crystallized α-Fe2O3 with the same rhombohedral structures (JCPDS No.89-0596). These results show that the addition of NH4Cl has no effect on the crystal phase of the products.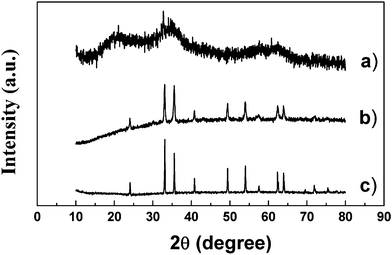 | ||
| Fig. 1 X-ray powder diffraction patterns: (a) hydrous hematite; (b) hematite nanoparticles obtained from simple heat treatment of hydrous hematite; (c) hematite mesocrystals. | ||
The morphology of these samples was investigated by SEM. Fig. 2a and 2b are SEM images of typical hematite mesocrystals with side lengths of about 30 μm. They have the regular morphologies of octahedral or cuboid-like superstructures. Under low magnification, these mesocrystals appeared to have smooth crystalline surfaces, but high-magnification SEM micrographs of the products (Fig. 2c: the surfaces and Fig. 2d: the broken parts) show that they were constructed by the self-assembly of many small nanoparticles. It is evident that these tens of micrometre particles are formed by a great number of small nanoparticles that are aligned very well and in close contact with one another. Moreover, from Fig. 2c and Fig. 2d one can see that a number of the subparticles of the hematite mesocrystals are smaller than 50 nm. When carefully examining the morphologies of the subparticles, one can find out that the subparticles are not spherical but have edges and corners. The shape of these edges and corners of the subparticles were similar to that of the mesocrystals. There is considerable distinction between the hematite nanoparticles and the mesocrystals in morphology and size. The hematite nanoparticles are about 50 nm, on average, with a nearly spherical shape (Fig. 2e), which is very different from the microsize of the mesocrystals. In addition, amorphous hydrous hematite nanoparticles with a mean size of nearly 10 nm are shown in Fig. 2f, they are nearly spherical as well. The different sizes and morphologies of these materials suggest that the crystal growth happened during heat treatment, moreover, there were different growth rates and mechanisms in the formation of hematite nanoparticles and mesocrystals.
 | ||
| Fig. 2 SEM images: (a) and (b) typical morphology structures of hematite mesocrystals; (c) and (d) detailed morphologies of different parts of hematite mesocrystals, pointed out by arrows in image (b), (c) surface morphology; (d) morphology of broken part; (e) hematite nanoparticles obtained after heat treatment of hydrous hematite; (d) hydrous hematite. | ||
TEM images and selected area electron diffraction patterns (SAED) were shown in Fig. 3. Owing to the large sizes, the edges of the hematite mesocrystals are present in the TEM images. The mesocrystals with rough surfaces made of small building blocks were still perfect single crystals, as confirmed in Fig. 3a, which means that all small nanoparticles may be attached along one direction. As expected, the hematite nanoparticles are polycrystalline, Fig. 3b. The nanoparticles average sizes of about 50 nm is agreed with the SEM result.
 | ||
| Fig. 3 TEM images: (a) hematite mesocrystals and (b) hematite nanoparticles. The insets are their corresponding selected area electron diffraction patterns (SAED). | ||
Fig. 4 shows the N2 adsorption-desorption isotherm of hematite nanoparticles and mesocrystals. A significant distinction in the volume adsorbed between the two hematite materials can be clearly seen. The Brunauer–Emmett–Teller (BET) gas sorptometry measurement revealed that the specific surface area of hematite nanoparticles and mesocrystals were 24.96 and 8.27 m2 g−1, respectively. The Barett–Joyner–Halenda (BJH) desorption average pore size of hematite nanoparticles is 39.9 nm, and the single-point adsorption total volume at P/P0 = 0.976 is 0.106 cm3 g−1. The BJH desorption average pore size of hematite mesocrystals is 6.1 nm which may come from the slits among those building blocks, and the single-point adsorption total volume at P/P0 = 0.982 is 0.582 × 10−4 cm3 g−1. Clearly, the latter has a small surface area and a very low value of pore volume, indicating that hematite mesocrystals were indeed densely packed.
 | ||
| Fig. 4 N2 adsorption–desorption isotherm of (a) hematite nanoparticles and (b) hematite mesocrystals. | ||
During the heat treatment of preparing hematite mesocrystals, a visible yellow-green vapor could be observed. This vapor could be collected in a glass jar and solidified after cooling. The solid was composed of two different components: a white crystal form and the other a yellow color. According to the reaction conditions, the white crystal was NH4Cl(s), which sublimes back to a solid upon cooling. After dissolving the solid in water, one droplet of the solution turned the colorless saturated KSCN(aq) solution a blood-red color. This phenomenon showed that the yellow component of the collected solid contained Fe3+ ions because this color change reaction is characteristic for Fe3+ ions. But as to the preparation of hematite nanoparticles, no such vapor occurred. This suggests that the hydrous hematite precursor heated with NH4Cl could produce Fe3+ ions.
In order to understand what chemical reaction occurs, two comparison experiments were carried out. In experiment A, ammonium carbonate ((NH4)2CO3(s)) was used to replace NH4Cl(s). In experiment B, hematite nanoparticles that were obtained from a heat treatment precursor of hydrous hematite (the new starting material, all other experimental conditions were the same) were used. Neither of the results of these two experiments showed formation of the mesocrystals. On one hand, (NH4)2CO3(s) begins to decompose into gases under certain temperatures (58 °C, in theory). The results of experiment A indicated that the product of NH3(g), H2O(g), and CO2(g) decomposed from (NH4)2CO3(s) had nothing to do with mesocrystal formation. On the other hand, experiment B only lacked water of hydration from the hydrous hematite precursor. Compared to the conditions for mesocrystal formation, these results suggest that H2O(g) did participate in the formation process and was not responsible for the formation of hematite mesocrystals alone.
In addition, the result of experiment B indicated that just NH3(g) or HCl(g) alone would not encourage mesocrystal formation. However, when H2O(g) and HCl(g) were involved, the hematite mesocrystals could be obtained. This is because HCl(g) has a corrosive effect in the presence of moisture under high temperature. Due to the corrosive effect of HCl(g), Cl− would react with new hematite nanoparticles (Fe2O3) which crystallized from hydrous hematite (because the temperature of losing crystalline water in amorphous hydrous hematite starting around 100 °C is lower than that of NH4Cl(s) decomposition, 338 °C, and hematite crystallizes at 275 °C)11 to form yellow-green FeCl3(g). Then, FeCl3(g) solidified with sublimed NH4Cl(s) after cooling. During this reaction, the surfaces of the hematite nanoparticles were etched and exposed with some defects as nucleation sites. Hematite nanoparticles can assemble together and re-bond by utilizing these defects.
Although the effect of HCl(g) is discussed above, the role of NH3(g), as another decomposition product of NH4Cl(s), is still not clear. To figure it out, the Fourier transform infrared (FT-IR) spectra measurements for the hydrous hematite precursor, hematite nanoparticles, and their mesocrystals were carried out. The results are shown in Fig. 5. In hydrous hematite (curve a), a wide band from 3,000 cm−1 to 3,500 cm−1 corresponds to O–H stretching, which indicates that the precursor contains crystal water. This hydrous hematite does not have the Fe–O vibration mode of hematite at low wavenumber. However, there is no such O–H stretching band with respect to hematite nanoparticles (curve b) and mesocrystals (curve c), indicating that they have no H2O molecules. The bands at 434 and 515 cm−1 for hematite nanoparticles and 424 and 511 cm−1 for the mesocrystals are Fe–O vibration for hematite. These bands in the mesocrystals are weaker than those in the nanoparticles, and are shifted to lower wavenumbers than the nanoparticles. The weaker vibration and shift indicates that the size of the building units of hematite mesocrystals is smaller than the 50 nm nanoparticles.17
 | ||
| Fig. 5 FT–IR spectra: (a) precursor of hydrous hematite; (b). nanoparticles obtained from the simple heat treatment of hydrous hematite; (c) hematite mesocrystals. | ||
There were yet other small bands at low wavenumbers beyond the Fe–O vibration bands in the hematite mesocrystals (curve c, 403, 411, 454, and 470 cm−1). These bands do not belong to magnetite whose characteristic band is around 600 cm−1.18 The band for magnetite was not detected in the hematite mesocrystals, although it was present in the hematite nanoparticles at 594 cm−1 (curve b). Although NH3(g) has a reducing effect under the proper circumstances, it reacts with oxides to reduce high valence metal ions or form nitrides instead of reducing them to metal.19 According to the theoretical calculations for infrared vibration frequencies for Fe–N20 and Fe–N–O species,21 these small bands are the vibrations of the Fe–N–Fe or Fe–N–O bonds. From this point of view, the decomposition product of NH3(g) can react and bond with the surfaces of the hematite nanoparticles. These chemical bonds can play a role similar as that of surfactants or ions in the formation of various morphologies of nanomaterials by changing the Gibbs free energy, as they have different bonding energies with Fe–O–Fe. Therefore, the building units of hematite mesocrystals showed edges and corners instead of the spherical shapes of the hematite nanoparticles. It is a possible incentive that the bonds can connect different particles, making them assemble. It is also because the edges and corners, there are triple lines and quadruple points among the building units that can limit grain growth.22 Consequently, the size of building units is smaller than that of the nanoparticles of 50 nm even though they had the same heat treatment conditions. However, the Fe–N–Fe and Fe–N–O bonds are a kind of defect in the hematite mesocrystals as well.
To further investigate the existence of nitrogen (N) in the final products, XPS spectra were measured. The spectra (Fig. 6) show the presence of N, O and Fe. There are split peaks in all of the three elements which are distinguishable, and can be attributed to the different chemical atmospheres on the surface of hematite mesocrystals. According to the discussion of FT–IR spectra, the split peaks are arising from the different bonds of Fe–N–Fe or Fe–N–O. When N is involved in these bonds, it is prone to losing electrons so that the binding energy increases, showing a high binding energy peak in the spectra. The O or Fe, affected by N would be prone to gain electrons so that the binding energy decreases which presents low energy peaks. Moreover, the amount of N calculated from the XPS data is about 0.84 at%, which is in agreement with that from the EDS data of 0.80 at%. These results show a trace of N in the hematite mesocrystals, but the XRD pattern (Fig. 1c) still presents as pure hematite.
 | ||
| Fig. 6 The fitted X-ray photoelectron spectra of hematite mesocrystals: (a)N1s (b) O1s (c) Fe2p. And (d) EDS data of hematite mesocrystals. | ||
Fig. 7 shows the different stages of hematite mesocrystal growth under direct viewing of the formation. These samples were obtained at specific reaction temperatures without preservation for further heat treatment. When the temperature reached 400 °C (from room temperature), the particles had already gathered as aggregates, but still had no specific shapes or clear planes (Fig. 7a). These aggregates began to form some specific morphologies when a temperature of 440 °C was reached (Fig. 7b). Specific morphologies became more and more clear when the temperature reached 480 °C. Some distinguishable crystalline planes were formed, but only some aggregates could be recognized because the reaction was not yet complete (Fig. 7c). Fig. 8 shows the results of time-dependent observation. When the temperature reached 500 °C many small mesocrystals with sizes of 4–5 μm were formed (Fig. 8a). From this image, it displays that this self-assembly step is very fast and that mesocrystals had already developed without soaking time. Then, these small mesocrystals would once again aggregate as a new, larger aggregation (Fig. 8b). After that, the boundaries among the small mesocrystals started to blur (Fig. 8c). Over time, these boundaries became invisible and bigger mesocrystals formed (Fig. 8d).
 | ||
| Fig. 7 SEM images of the products at different heat treatment temperatures without preservation for further heating. (a) 400 °C. (b) 440 °C. (c) 480 °C. | ||
 | ||
| Fig. 8 SEM images of the mesocrystal products after heat treatment at 500 °C for different times. (a)0 h (b) 0.5 h (c) 1.0 h (d) 1.5 h. | ||
On the basis of the previous discussion, the mechanism for hematite mesocrystal formation can be assumed to be the following, and classified as nonclassical crystallization:7 (i) amorphous hydrous hematite nanoparticles started to dehydrate, crystallize, and release H2O(g) during heat treatment. (ii) Meanwhile, NH4Cl(s) also began to decompose and generated HCl(g) and NH3(g). (iii) The mixture of HCl(g) and H2O(g) would have a strong corrosive effect. The Fe(III) ion on the surfaces of the poorly crystallized hematite nanoparticles, which had higher reactivity, were etched and exposed with point defects. (iv) At the same time, NH3(g) participated in the process to form Fe–N–Fe or Fe–N–O bonds on the surfaces of particles by utilizing those point defects, as if a surfactant had ‘trapped’ them. This may reduce the surface energy and could benefit the assembly process. Moreover, the bonds were making particles with edges and corners which inhibited them from growing up. (v) The nanoparticles assembled together and quickly re-bonded as small aggregates. (vi) As time passed, these small aggregates had specific morphologies and started to present clear crystalline planes due to the self-limitations of their crystal growth. (vii) After the corrosive reagent was exhausted by etching and evaporating, the key formation procedure was completed, and then the aggregates transformed into small mesocrystals. During this formation process, the nanoparticle units may have randomly collided at arbitrary angles at the first stage and then possibly manoeuvred until a lattice mismatch angle < 15° was reached.23 (viii) Small mesocrystals would then probably aggregate to form bigger aggregations as time passed viaOstwald ripening. Finally, the bigger aggregations turned into well-formed mesocrystals.
It can be determined that the synthesis procedures follow the principles of green chemistry. Firstly, the waste of the two reactions can be recycled as new starting materials: for the preparation of the precursors, the waste was diluted ammonia and NH4Cl; for the next heating treatment, the waste was solid NH4Cl and a small amount of iron(III) chloride salts. All of them can act as raw materials. Secondly, the precursor preparation is surfactant-free. Thirdly, the key assembly step is a solvent-free reaction with a fast reaction rate. According to Hill,24 and based on the green chemistry principles the ideal reaction is one performed without solvent. Therefore, this synthesis route is economical and environmentally friendly.
Hematite can present a weak parasitic ferromagnetism because the electron spin magnetic moments are not in a rigid antiparallel arrangement. There is a slight canting angle between the Morin temperature (TM = 260 K) and the Neél temperature (TN = 948 K).25 The room temperature (RT) magnetic hysteresis loops of the hematite related products are shown in Fig. 9. It is quite evident that the hematite nanoparticles (loop (a)) have higher magnetization than the mesocrystals (loop (c)), while hydrous hematite is paramagnetism (loop (b)). The high magnetization of hematite nanoparticles can be explained by the presence of a small amount of magnetite (testified by the result of FT–IR), lattice defects and uncompensated surface spins. The hematite nanoparticles present a coercivity value of 179.14 Oe. Surprisingly, a high coercivity value for hematite mesocrystals, up to 4,437.80 Oe, was recorded under an magnetic field of 1.8 T. The high coercivity of hematite mesocrystals is slightly lower than the highest value of 5.85 KOe, which is that of hematite microparticles with about 20 nm subparticles.26
 | ||
| Fig. 9 Magnetic hysteresis loops of (a) nanoparticles obtained from simply heat treatment of hydrous hematite. (b) precursor of hydrous hematite. (c) hematite mesocrystals. Measured at RT (300 K). | ||
To the best of our knowledge, there has no report yet on hematite with such a large coercivity in this size range of tens of micrometres. The sizes of the hematite mesocrystals are larger by nearly an order of magnitude than other results.16,25–28 All the hematite mesocrystals with large coercivity reported previously26–28 ranged between 0.5 to 1.5 μm. It appears that there is no close relationship between the coercivity value and the particle size of mesocrystals. When their building units are focused, it in fact turns out that the intrinsic coercivity value is mostly related to the subparticle size. For example, mesocrystals of cantaloupe-like hematite27 are about 1.5 μm in length with 100 nm subparticles, and egglike hematite28 with a diameter of 0.5 μm are composed of 30 nm nanoparticles. In addition, pseudocubic hematite26 consisting of 20 nm subparticles is 1.1 μm in size. The corresponding coercivity values for the three examples were 2,279 Oe, 3,800 Oe, and 5.85 KOe, respectively. When the nanoparticles were smaller in size, the coercivity was higher. The highest coercivity for the pseudocubic hematite with 20 nm subparticles agreed with another result for hematite nanoparticles that also show large coercivity—close to 5.85 KOe for those with a mean size of 20 nm.29 Therefore, the fact that the building units are nanoparticles is an important factor in the large coercivity values of the hematite mesocrystals.
To further understand the magnetic behavior of the hematite mesocrystals, temperature dependence of zero-field-cooled (ZFC) and field-cooled (FC) magnetization from 77 K to 300 K under an applied field of 200 Oe were conducted. Fig. 10 shows the ZFC–FC curves for hematite mesocrystals. The magnetic moment has no abrupt variations, which indicates the absence of a Morin transition. The Morin temperature (TM = 260 K) is the phase transition temperature of hematite. Below TM, bulk hematite is antiferromagnetic, whereas at temperatures above TM, it can present a weak ferromagnetic state. The ZFC–FC magnetization curves of bulk hematite can give rise to an abrupt variation of the magnetic moment around TM. Moreover, the TM value strongly depends on size and morphology. It shifts towards lower values for smaller particles. But if the size of the hematite nanoparticles are smaller than 20 nm, the Morin transition would not occur.30 Therefore, the result here confirms that the microstructure of our hematite mesocrystals are different from that of bulk hematite. Moreover, this result also suggests that the hematite mesocrystals are primarily composed of nanoparticles with a mean size of less than 20 nm, although there are some nanoparticles larger than 20 nm present in the SEM results.
 | ||
| Fig. 10 Temperature dependence of zero-field-cooled (ZFC) and field-cooled (FC) magnetization curves of hematite mesocrystals. Measured from 77 K to 300 K under an applied field of 200 Oe. | ||
The size effect at the nanoscale has a great impact on the coercivity for magnetic materials, because one can have the largest coercivity value at a critical size and the value becomes smaller when the particle size either decreases or increases. The results of FT–IR and the absence of Morin transition testified that most of the building units of the hematite mesocrystals are smaller than 20 nm. This was due to the influence of N which bonded with Fe and O on the surfaces of building units, and these bonds have prevented the fine nanoparticles from growing up further. Within this size of building units, hematite mesocrystals can present large coercivity according to other reports.
Generally, there are two ways to enhance the coercivity of magnetic materials. One is to enhance the resistance of the domain rotation, such as increasing the anisotropies, including shape and magnetization, whereas the other is to increase the resistance of the domain wall displacement, such as inducing internal stress and raising the volume content of impurities.31 In this work, the hematite mesocrystals and their building units with well-formed morphologies suggest that they have high energies for shape and magnetization anisotropies which are induced by the ‘trapping’ of N. When an external magnetic field was applied, the nanoparticles began to become magnetized such that the nanoparticles were deformed due to the formation of densely packed structures. Strain could be produced on their interfaces by deformation due to the intimate contact among the nanoparticles because this kind of contact would discourage the development of deformation. The other possible source of strain comes from the lattice mismatch which was produced at the early stage of nanoparticles aggregation. The intimate contact will establish strong exchange coupling among nanoparticles that can enhance the coercivity as well. Moreover, the chemical bonds of Fe–N–Fe and Fe–N–O on the surfaces of hematite mesocrystals act as defects. As a result of those anisotropies which can enhance the resistance of domain rotation32 and the strain or defects which increase the resistance of the domain wall displacement, the coercivity was enhanced. Thus, the origin of the large coercivity of hematite mesocrystals comes from two parts. One is the size effect, in that the building units are smaller than 20 nm which is the main origin. The other is the enhancement by large anisotropies, strain, defects and exchange coupling. It seems that the large coercivity is mainly induced by the presence of N, and indeed the N induced the small particle size, caused the well morphology, acted as defects and reduced the surface energy for aggregation as described in the earlier discussion.
Conclusions
In summary, a corrosive route was developed for the synthesis of hematite mesocrystals that contained no organic additives and exhibited very high coercivity behavior in large sizes of ∼30 μm. It is the first report on hematite which presents a high coercivity with such a large size. This route is actually economical and environmentally friendly and follows the principles of green chemistry, for example, the waste is recyclable and the reaction is fast and surfactant and solvent-free. The formation mechanism and the origin of high coercivity were discussed in detail. There are several stages of crystallization process. Briefly, the corrosive effect provided the hematite nanoparticles some defects on their surfaces as nucleation sites, a trace of N that bonded as Fe–N–Fe or Fe–N–O made nanoparticles have edges and corners for further crystalline self-limited growth and prevented them growing, many small building units aggregated and formed small hematite mesocrystals, then these small mesocrystals became big mesocrystals viaOstwald ripening. This formation is different from the classical ion-mediated crystallization, instead it is a nonclassical crystallization based on particle-mediated crystal-growth. However, because there was no organic additives, the products were in a dense form without any internal pores, only rough surfaces. Although the size of the building units is the main origin of the large coercivity, the anisotropies of the mesocrystals, defects induced by N, and the strain and exchange coupling all contribute to the enhancement of the coercivity, the presence of N is the true reason for the large coercivity. These characteristics open a threshold to enhance the coercivity in hard magnetic materials by fabricating mesostructures in the field of high frequency or microwave applications, like BaFe12O19 or SrFe12O19, etc.Generally, it is the low-ordered structure of polycrystals that leads to random interactions among their small building units and makes it difficult to maintain novel properties of the materials. Mesocrystals usually have a higher crystallinity with ordered arrangement. However, mesocrystals have distinctively different internal structures from normal single crystals and still maintain, or have improved novel properties like their nanoparticles. One mesocrystal can be fabricated from bottom to top with tunable sizes and can be easily handled. This gives them great potential for MEMS (Micro-Electronic-Mechanical-System) utilization, or to act as a single microdevice in the future. Moreover, due to the special microstructures of the mesocrystals which can produce a fast and efficient electron transfer, they are more promising for sensing, photovoltaic and photocatalytic devices.
This corrosive route reported here can be utilized for other mesocrystal synthesis. Not only for other kinds of single phase advanced materials, but also multifunctional mesocrystals like integrating two different materials, similar to nanoparticle superlattices. Even this method could be attempted to synthesise N–doped materials such as N–doped ZnO, which is still a big challenge. It is a good supplement of how to fabricate mesocrystals which are not obtained via a liquid. Furthermore, grain growth is greatly inhibited in the preparation of nanocrystalline ceramics, leading many researchers to use approaches based on high pressure and high temperature technologies. Our results may provide insight into the synthesis of nanocrystalline ceramics by limiting the grain growth.
References
- Y. N. Xia, P. D. Yang, Y.G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and H. Q. Yan, Adv. Mater., 2003, 5, 353 CrossRef.
- A. N. Shipway, E. Katz and I. Willner, ChemPhysChem, 2000, 1, 18 CrossRef CAS.
- D. Y. Lee, D. H. Lee, H. S. Lim, J. T. Han and K. Cho, Langmuir, 2010, 26, 3252 CrossRef CAS.
- Y. Wan, H. F. Yang and D. Y. Zhao, Acc. Chem. Res., 2006, 39, 423 CrossRef CAS.
- A. R. Tao, J. X. Huang and P. D. Yang, Acc. Chem. Res., 2008, 41, 1662 CrossRef CAS.
- C. S. Choi and Y. W. Kim, Biomaterials, 2000, 21, 213 CrossRef CAS.
- R. Q. Song and H. Cölfen, Adv. Mater., 2010, 22, 1301–1330 CrossRef CAS.
- J. P. Ge, Y. X. Hu, M. Biasini, W. P. Beyermann and Y. D. Yin, Angew. Chem., Int. Ed., 2007, 46, 4342 CrossRef CAS.
- J. S. Chen, T. Zhu, C. M. Li and X. W. Lou, Angew. Chem., Int. Ed., 2011, 50, 650 CrossRef CAS.
- S. W. Cao and Y. J. Zhu, Acta Mater., 2009, 57, 2154 CrossRef CAS.
- H. Tlatlik, P. Simon, A. Kawska, D. Zahn and R. Kniep, Angew. Chem., Int. Ed., 2006, 45, 1905 CrossRef CAS.
- Y. R. Ma, H. Cölfen and M. Antonietti, J. Phys. Chem. B, 2006, 110, 10822 CrossRef CAS.
- G. F. Grom, D. J. Lockwood, J. P. McCaffrey, H. J. Labbé, P. M. Fauchet, B. White, Jr, J. Diener, D. Kovalev, F. Koch and L. Tsybeskov, Nature, 2000, 407, 358 CrossRef CAS.
- Y. H. Zhen and J. F. Li, J. Am. Ceram. Soc., 2007, 90, 3496 CrossRef CAS.
- M. Ocaña, M. P. Morales and C. J. Serna, J. Colloid Interface Sci., 1995, 171, 85 CrossRef.
- B. Judat and M. Kind, J. Colloid Interface Sci., 2004, 269, 341 CrossRef CAS.
- Y. M. Zhao, C. W. Dunnill, Y. Q. Zhu, D. H. Gregory, W. Kockenberger, Y. H. Li, W. B. Hu, I. Ahmad and D. G. McCartney, Chem. Mater., 2007, 19, 916 CrossRef CAS.
- Y. Yu and S. Y. Kwak, J. Mater. Chem., 2010, 20, 8320 RSC.
- L. Volpe and M. Boudart, J. Solid State Chem., 1985, 59, 332 CrossRef CAS.
- G. V. Chertihin, L. Andrews and M. Neurock, J. Phys. Chem., 1996, 100, 14609 CrossRef CAS.
- G. L. Gutsev, M. D. Mochena, E. Johnson and C. W. Bauschlicher, Jr., J. Chem. Phys., 2006, 125, 194312 CrossRef CAS.
- P. Streitenberger and D. Zöllner, Acta Mater., 2011, 59, 4235 CrossRef CAS.
- W. T. Read and W. Shockley, Phys. Rev., 1950, 78, 275 CrossRef CAS.
- A. McCluskey, P. J. Robinson, T. Hill, J. L. Scott and J. K. Edwards, Tetrahedron Lett., 2002, 43, 3117 CrossRef CAS.
- F. Bødker, M. F. Hansen, C. B. Koch, K. Lefmann and S. Mørup, Phys. Rev. B: Condens. Matter, 2000, 61, 6826 CrossRef.
- C. Rath, S. D. Kulkarni, S. Anand, S. K. Date, R. P. Das and N. C. Mishra, Appl. Phys. Lett., 1999, 75, 4171 CrossRef CAS.
- L. P. Zhu, H. M. Xiao and S. Y. Fu, Cryst. Growth Des., 2007, 7, 177 CAS.
- D. D. Wang, C. X. Song, Y. H. Zhao and M. L. Yang, J. Phys. Chem. C, 2008, 112, 12710 CAS.
- L. Lu, L. P. Li, X. J. Wang and G. S. Li, J. Phys. Chem. B, 2005, 109, 17151 CrossRef CAS.
- C. Z. Wu, P. Yin, X. Zhu, C. O. Yang and Y. Xie, J. Phys. Chem. B, 2006, 110, 17806 CrossRef CAS.
- Z. M. Li, X. Y. Lai, H. Wang, D. Mao, C. J. Xing and D. Wang, Nanotechnology, 2009, 20, 245603 CrossRef.
- N. K. Chaudhari, H. C. Kim, D. Son and J. S. Yu, CrystEngComm, 2009, 11, 2274 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
