Polarized spectroscopy and polarized UV light-induced molecular orientation of chiral diphenyl Schiff base Ni(II) and Cu(II) complexes and azobenzene in a PMMA film
Atsuo
Yamazaki
and
Takashiro
Akitsu
*
Department of Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: akitsu@rs.kagu.tus.ac.jp; Fax: +81 3 5261 4631; Tel: +81 3 5228 8271
First published on 15th February 2012
Abstract
We have investigated polarized UV light-induced molecular orientation, namely increasing optical anisotropy of chiral diphenyl Schiff base Ni(II) and Cu(II) complexes (diph(SS)Ni and diph(SS)Cu) as well as azobenzene (AZ), as organic/inorganic hybrid materials in polymethylmethacrylate (PMMA) cast films. The driving force and reason for polarized UV light-induced molecular orientation of AZ are the so-called Weigert effect and accompanying molecular alignment of diph(SS)Ni or diph(SS)Cu is attributed to intermolecular interactions between two components dispersed in a flexible matrix environment of PMMA, which is confirmed with CD spectra. Gradual increase of the degree of dichroism was measured with polarized absorption electronic spectra. The anisotropic parameters were evaluated from them and were discussed based on interactions between electric transition dipole moments calculated by TD-DFT.
Introduction
One of the advantages of organic/inorganic hybrid materials may be facility of designing supramolecular multifunctional materials, for example metal complexes incorporating azo-ligands with large dichromic ratios.1 Along recent development of this field, reversible photo-magnetic switching functional organic/inorganic hybrid materials composed of photochromic organic compounds and magnetic inorganic compounds have been reported as one example.2 Furthermore, we have employed various types of metal complexes as inorganic components to use molecular functions of metal complexes such as fluorescence intermolecular interactions,3 chiral recognitions4 and molecular based magnetism.5 In particular, we also focused on photo-tuning of optical anisotropy of AZ, namely polarized UV light-induced alignment caused by selected photoisomerization and molecular orientation of AZ (Weigert effect),6 in contrast to conventional cis-trans photoisomerization of AZ. During a series of the related studies of such organic/inorganic hybrid materials and photo-induced dichroism observed with polarized spectroscopy,7 it may have been possible to discuss the degree of molecular orientation of components in view of electric transition dipole moments for clear interpretation and deep understanding of angular dependence of absorbance of polarized spectra.Herein, we prepared organic/inorganic hybrid materials as PMMA cast films containing chiral Schiff base Ni(II) and Cu(II) complexes (diph(SS)Ni and diph(SS)Cu, respectively) and AZ (Fig. 1). Investigation of anisotropic parameters taken from polarized spectra was carried out for some organic/inorganic hybrid materials (AZ+diph(SS)Ni+PMMA, AZ+diph(SS)Cu+PMMA and AZ+PMMA for comparison). In this study, we attempted to interpret anisotropic parameters based on transition dipole moments evaluated by TD-DFT.
 | ||
| Fig. 1 Structures of chiral Schiff base Ni(II) complex (diph(SS)Ni) and Cu(II) complex (diph(SS)Cu) and the trans form of AZ. | ||
Experimental
Materials
All reagents and solvents were analytically pure and were used as received without further purification.Preparation of [(1S,2S)-N,N'-bis(3,5-dicholosalicylidene)-diphenyl -1,2-diamine-κ4O,N,N',O]nickel(II) (diph(SS)Ni)
To a solution of 3,5-dichlorosalicylaldehyde (0.3820 g, 2 mmol) dissolved in methanol (100 mL), (1S,2S)-(-)-1,2-diphenylethylenediamine (0.2123 g, 1 mmol), and copper(II) acetate hydrate (0.2488 g, 1 mmol) were added and stirred at 313 K for 4 h to yield brown the complex collected by filtration. Yield 0.5602 g (91%). Anal. Calc. for C28H18Cl4NiN2O2: C, 54.69; H, 2.95; N, 4.56. Found: C, 54.58; H, 2.88; N, 4.40%. IR (Nujol mull):401, 406, 412, 422, 428, 440, 447, 455, 460, 465, 470, 481, 486, 509, 516, 522, 527, 543, 563, 570, 596, 617, 624, 631, 636, 645, 667, 693, 714, 721, 749, 763, 794, 845, 857, 866, 881, 918, 951, 956, 1001, 1076, 1103, 1121, 1160, 1175, 1216, 1274, 1322, 1331, 1347, 1360, 1366, 1377, 1412, 1450, 1588, 1600, 1620(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2671, 2728, 2855, 2905, 2911, 2939, 2957 cm−1.
N), 2671, 2728, 2855, 2905, 2911, 2939, 2957 cm−1.
Preparation of [(1S,2S)-N,N'-bis(3,5-dicholosalicylidene)-diphenyl -1,2-diamine-κ4O,N,N',O]copper(II) (diph(SS)Cu)
To a solution of 3,5-dichlorosalicylaldehyde (0.0955 g, 0.5 mmol) dissolved in methanol (50 mL), (1S,2S)-(-)-1,2-diphenylethylenediamine (0.0531 g, 0.25 mmol), and copper(II) acetate hydrate (0.0499 g, 0.25 mmol) were added and stirred at 313 K for 4 h to yield brown the complex collected by filtration. Yield 0.1288 g (83%). Anal. Calc. for C28H18Cl4CuN2O2: C, 52.73; H, 3.16; N, 4.39. Found: C, 52.73; H, 2.92; N, 4.57%. IR (Nujol mull):401, 403, 406, 412, 417, 422, 425, 431, 438, 444, 447, 453, 455, 460, 465, 471, 480, 489, 495, 498, 509, 649, 666, 693, 721, 750, 763, 788, 851, 870, 889, 918, 972, 990, 1075, 1114, 1169, 1213, 1316, 1366, 1462, 1497, 1512, 1562, 1572, 1589, 1628(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), 1652, 2342, 2368, 2670, 2727, 2857, 2936 cm−1.
N), 1652, 2342, 2368, 2670, 2727, 2857, 2936 cm−1.
Preparation of hybrid materials
Acetone solution (0.5 mL) of AZ (0.0018 g in 10 mL acetone), acetone solution (0.5 mL) of diph(SS)Ni or diph(SS)Cu and acetone solution (2 mL) of PMMA (10%) were cast onto a slide glass to give rise to PMMA film of hybrid materials AZ+PMMA+diph(SS)Ni or AZ+PMMA+diph(SS)Cu. Weight percentages are 0.036%, 0.021%, and 99.94% for AZ, diph(SS)Ni or diph(SS)Cu and PMMA, respectively. AZ+PMMA+diph(SS)Ni: CD spectrum in PMMA films (nm (θ/mdeg)): 586 (−1.95), 495 (−0.71), 482 (−1.13), 457 (+0.20), 420 (−3.34), 389 (−1.36), 367 (−2.04), 340 (−0.48), 276 (−4.90), 253 (+0.34), 245 (−0.73).Physical measurements
Elemental analyses were carried out with a Perkin-Elmer 2400II CHNS/O analyzer at Tokyo University of Science. IR spectra were recorded on a JASCO FT-IR 4200 plus spectrophotometer. UV-vis absorption spectra were recorded on a JASCO V-570 spectrophotometer equipped with a polarizer at 298 K. CD spectra were recorded on a JASCO J-820 spectrophotometer at 298 K. Fluorescence spectra were recorded on a JASCO FP-6200 spectrophotometer at 298 K. Photo-irradiation was carried out with D2 light source for 200–350 nm (with a visible cut filter) and Xe light source 350–800 nm (with a UV cut filter) with a polarizer.Computational methods
All calculations were performed using the Gaussian 09W software Revision A.02 (Gaussian, Inc.).8 The gas phase geometry optimizations were carried out using TD-DFT with B3LYP functional. The vertical excitation energy was calculated with the Lanl2dz for Ni and Cu and with the 6 − 31 + G(d) basis set for H, C, N, O, and Cl method based on doublet (for a Cu(II) complex) or singlet (for a Ni(II) complex) ground state geometry.Results and discussion
Electronic and CD spectra of complexes
Fig. 2 shows comparison between simulated and experimental spectra of electronic and CD spectra in order to confirm reliable band assignment. As for metal complexes, d-d bands in simulated spectra appear at 563 nm and 556 nm and transition electric dipole moment is evaluated to be (0.0396, 0, 0.0127) and (−0.1315, 0, −0.0053) with oscillator strength of 0.0001 and 0.0009 for diph(SS)Ni and diph(SS)Cu, respectively. Moreover, n–π* and π–π* bands in simulated spectra appear at 477 nm and 339 nm and transition electric dipole moment is evaluated to be (0, 0, 0) and (0.1445, −2.9027, 0) with oscillator strength of 0.0000 and 0.7558 for the trans-form of AZ. The corresponding n–π* and π–π* bands appear at 484 nm and 307 nm and transition electric dipole moment is evaluated to be (0.5511, −0.5741, 0) and (0.5471, −0.5755, 0) with oscillator strength of 0.0397 and 0.0625 for the cis-form of AZ. According to preceding studies on the related compounds,9 the optimized structures and assignments of transitions for diph(SS)Ni and diph(SS)Cu are reasonable.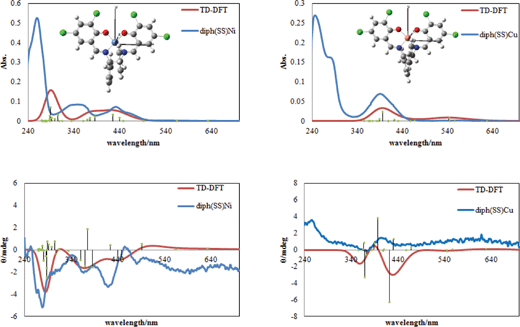 | ||
| Fig. 2 Simulated (red) and experimental (blue) electronic spectra (above) and CD spectra (below) for diph(SS)Ni (left) and diph(SS)Cu (right) with optimized structures. | ||
Polarized light-induced molecular orientation and optical anisotropy
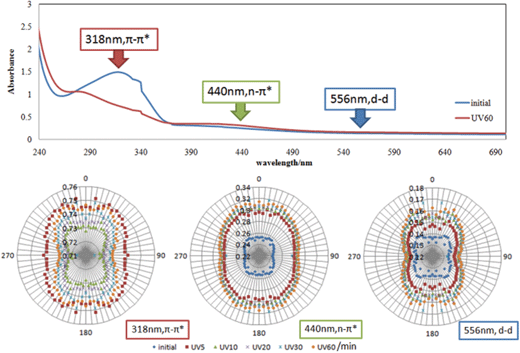
After polarized UV light irradiation, intensity (absorbance as it measured) of some characteristic peaks of polarized absorption spectra gradually were measured. The spectra shown in Fig. 3–5 were obtained with polarizer aligned at 0 degrees before (0 min) and after polarized UV light irradiation for 60 min, and they were also measured for 0–90 degrees at every 5 degree before and after polarized UV light irradiation for 5, 10, 20, 30 and 60 min.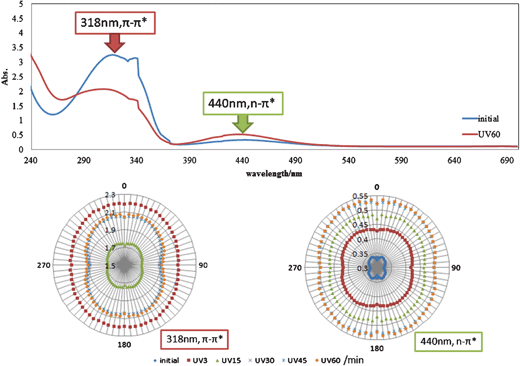 | ||
| Fig. 3 Polarized absorption electronic spectra and angular dependence of a polarizer in absorbance at 318 and 440 nm for AZ after UV light irradiation for 0–60 min. | ||
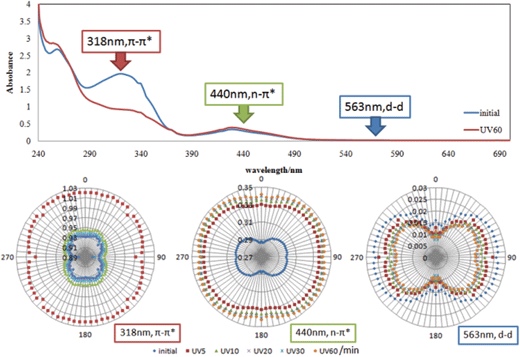 | ||
| Fig. 4 Polarized absorption electronic spectra and angular dependence of a polarizer in absorbance at 318, 440 and 563 nm for AZ+PMMA+diph(SS)Ni after UV light irradiation for 0–60 min. | ||
 | ||
| Fig. 5 Polarized absorption electronic spectra and angular dependence of a polarizer in absorbance at 318, 440 and 556 nm for AZ+PMMA+diph(SS)Cu after UV light irradiation for 0–60 min. | ||
Fig. 3 shows polarized absorption spectra and circular diagrams of angular dependence of absorbance of π–π* and n–π* bands at 318 nm and 440 nm for AZ+PMMA. It should be noted that the large change of π–π* band at initial stage is attributed to photoisomerization from the trans-form of AZ to the cis-form of AZ by UV light irradiation. These circular diagrams about π–π* bands suggest clear optical anisotropy of AZ, which is similar to polymers containing azo-groups.10
Fig. 4 and 5 show polarized absorption spectra and circular diagrams of angular dependence of absorbance of π–π*, n–π*, and d–d bands at 318 nm, 440 nm, 563 and 556 nm for AZ+PMMA+diph(SS)Ni and AZ+PMMA+diph(SS)Cu, respectively. It should be noted that only AZ was influenced by Weigert effect directly among two components. Even weak d–d band at 563 and 556 nm is attributed to a diph(SS)Ni or diph(SS)Cu component, increasing optical anisotropy can be observed, which is obvious proof for supramolecular interactions between AZ and diph(SS)Ni or diph(SS)Cu.
The degree of photoinduced optical anisotropy (spectral dichroism) of polarized absorption electronic spectra can be described commonly by these two parameters:
| S = (Aparrallel − Aperpendicular)/(2Aperpendicular + Aparrallel) |
| R = Aperpendicular/Aparrallel |
Where Aperpendicular and Aparrallel values denote absorbance measured with the measuring polarizers perpendicular or parallel to electric vector of irradiation polarized light. Ideal isotropic systems indicate S = 0 and R = 1 and both S and R parameters are changed as increasing spectral dichroism by molecular alignment.
According to Table 1, we can derive the following information associated with molecular orientations with intermolecular interactions in these organic/inorganic hybrid materials. The degree of increasing optical anisotropy accompanying with Weigert effect of AZ depends on not shapes of (identical) chiral ligands but central Ni(II) or Cu(II) metal ions; in other words, AZ + PMMA > AZ + PMMA + diph(SS)Ni > AZ + PMMA + diph(SS)Cu for π–π* bands, AZ + PMMA + diph(SS)Cu > AZ + PMMA > AZ + PMMA + diph(SS)Cu for n–π* bands, and AZ + PMMA + diph(SS)Ni > AZ + PMMA + diph(SS)Cu for d–d bands. Reducing direct transmission of molecular alignment from AZ by flexibility of metal complexes due to Ni(II) or Cu(II) ions is considered for Schiff base metal complexes,11 discrepancy of tendency about molecular orientation between AZ + PMMA + diph(SS)Ni and AZ + PMMA + diph(SS)Cu cannot be explained reasonably. Therefore, to interpret dichroism of each component properly, dipole–dipole interactions (which could not be estimated from experimental data easily) between AZ and diph(SS)Ni or diph(SS)Cu should be treated appropriately.
| AZ + PMMA | |||
| UV time (min) | π–π* (318 nm) | n–π* (440 nm) | |
| 0 | 1.0235 0.0078 | 1.0074 0.0025 | |
| 3 | 1.0463 0.0152 | 1.0249 0.0082 | |
| 15 | 1.0220 0.0073 | 1.0192 0.0064 | |
| 30 | 1.0838 0.0272 | 1.0534 0.0175 | |
| 45 | 1.0744 0.0242 | 1.0488 0.0160 | |
| 60 | 1.0659 0.0215 | 1.0516 0.0169 | |
| AZ + PMMA + diph(SS)Ni | |||
| UV time (min) | π–π* (318 nm) | n–π* (440 nm) | d–d (563 nm) |
| 0 | 1.0221 0.0073 | 0.9658 −0.0115 | 0.6135 −0.1479 |
| 5 | 1.0290 0.0152 | 0.9825 −0.0059 | 0.5205 −0.1902 |
| 10 | 1.0291 0.0073 | 0.9890 −0.0037 | 0.4840 −0.2077 |
| 20 | 1.0305 0.0272 | 1.0007 0.0002 | 0.6328 −0.1395 |
| 30 | 1.0314 0.0242 | 1.0011 0.0004 | 0.6068 −0.1508 |
| 60 | 1.0320 0.0215 | 1.0030 0.0010 | 0.6504 −0.1319 |
| AZ + PMMA + diph(SS)Cu | |||
| UV time (min) | π–π* (318 nm) | n–π* (440 nm) | d–d (556 nm) |
| 0 | 1.0064 0.0021 | 1.0332 0.0110 | 1.0145 0.0048 |
| 5 | 1.0063 0.0021 | 1.0580 0.0190 | 1.0681 0.0222 |
| 10 | 1.0079 0.0026 | 1.0591 0.0193 | 1.0723 0.0235 |
| 20 | 1.0167 0.0055 | 1.0758 0.0246 | 1.1016 0.0327 |
| 30 | 1.0290 0.0096 | 1.1042 0.0336 | 1.1612 0.0510 |
| 60 | 1.0239 0.0079 | 1.0926 0.0299 | 1.1358 0.0433 |
Comparison between calculated transition dipole moments and experimental parameters of optical anisotropy
Theoretical models and the resulting energy shown in Fig. 6 can be proposed to consider dipole–dipole interactions between metal complexes (AZ + PMMA + diph(SS)Ni and AZ + PMMA + diph(SS)Cu) and AZ. According to eqn (1), when two dipole moments are aligned in a line (θ1 = θ2 = 0) or parallel to each other (θ1 = θ2 = π/2), the energy w takes the maximum value (−2 u1u2/4πε0εrr3) or the minimum (−u1u2/4πε0εrr3) value, respectively. For both cases, the distance between two dipoles’ (r) dependence of energy (w) can be estimated that the w value takes the minimum value at r = 0.01 nm approximately, and the value of w will be convergent to 0 on increasing the r value.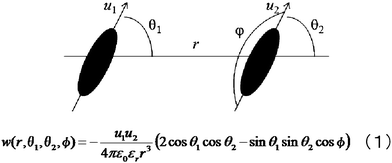 | ||
| Fig. 6 Theoretical model of dipole–dipole interactions and the description of its energy w, eqn (1). | ||
In analogy of Boltzmann distribution, the mechanism of increasing optical anisotropy can be discussed, namely the number of Ni states of the in-line state with Ei = −2 u1u2/4πε0εrr3 and the number of Nj states of the parallel state with Ej = −u1u2/4πε0εrr3. The change of optical anisotropy after polarized UV light irradiation corresponds to the change of the number of parallel dipoles. One of the anisotropy parameters (dichroism ratios, R) can be described by calculated transition electric dipole moments instead of experimental polarized absorbance (A0(Aparrallel) or A90(Aperpendicular)). As shown in Fig. 7, the magnitude of A0 (or A90) can be derived as eqn (2), and the following two cases can be considered about optical anisotropy.
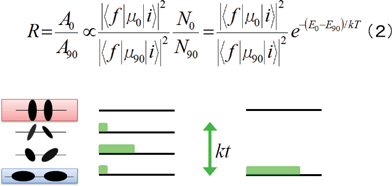 | ||
| Fig. 7 Description of dichroism ratios R with calculated parameters assuming Boltzmann distribution (2) and a model of energy levels dipole–dipole interactions with their energy interval kT. Blue highlight indicated expansion of equation the number of states (N0 or N90 and green bands in pictures) of directed dipoles and their energy by Boltzmann distribution. | ||
[case I] Interval of levels < kT: Because of small differences of energy levels and many levels, small differences between N0 and N90 result in closing to R = 1, namely optical isotropy.
[case II] Interval of levels > kT: Because of large differences of energy levels and a few levels, large differences between N0 and N90 result in closing to R = 0, namely optical anisotropy.
By Weigert effect after polarized UV light irradiation to the hybrid materials, AZ molecules change their molecular orientation (transition electric dipole moments) being perpendicular to electric vector of polarized UV light irradiated. According to the above consideration, discrepancy of tendency of increasing of optical anisotropy among π–π*, n–π*, and d–d bands should be attributed to the magnitude and the direction of transition electric dipole moments for π–π*, n–π*, and d–d transitions. In addition, dipoles can take various angles of orientation between 0 to π/2, which leads to various energy (w) and distribution of energy states.
Conclusions
In summary, we have compared increasing optical anisotropy of components of the hybrid materials of AZ + PMMA + diph(SS)Ni and AZ + PMMA + diph(SS)Cu and AZ + PMMA as a reference due to Weigert effect of AZ by irradiation of polarized UV light. The difference of the degree of optical dichroism between them has been discussed by assuming a qualitative model in analogy of theory of Boltzmann distribution with respect to calculated transition electric dipole moments with TD-DFT. Thus, dipole–dipole interactions can be influenced by molecular electronic structures which results in differences in apparent degree of optical anisotropy based on polarized absorption electronic spectra of both components.References
- (a) O. A. Blackburn, B. J. Coe, J. Fielden, M. Helliwell, J. J. W. McDounall and M. G. Hutchings, Inorg. Chem., 2010, 49, 9136 CrossRef CAS and references therein.; (b) A. A. Khandar and Z. Revani, Polyhedron, 1998, 18, 129 CrossRef.
- (a) Y. Einaga, M. Taguchi, G. Li, T. Akitsu, T. Sugai and O. Sato, Chem. Mater., 2003, 15, 8 CrossRef CAS; (b) R. Mikami, M. Taguchi, K. Yamada, K. Suzuki, O. Sato and Y. Einaga, Angew. Chem., Int. Ed., 2004, 43, 6135 CrossRef CAS; (c) M. Taguchi, K. Yamada, K. Suzuki, O. Sato and Y. Einaga, Chem. Mater., 2003, 17, 4554 CrossRef; (d) T. Yamamoto, Y. Umemura, O. Sato and Y. Einaga, J. Am. Chem. Soc., 2005, 127, 16065 CrossRef CAS; (e) T. Yamamoto, Y. Umemura, O. Sato and Y. Einaga, Chem. Mater., 2004, 16, 1195 CrossRef CAS; (f) Y. Einaga, R. Mikami, T. Akitsu and G. Li, Thin Solid Films, 2005, 493, 230 CrossRef CAS.
- (a) T. Akitsu and A. Yoshida, Current Physical Chemistry, 2011, 1, 76 CrossRef CAS; (b) T. Akitsu and S. Yamamoto, Asian Chem. Lett., 2010, 14, 255 Search PubMed.
- (a) T. Akitsu and Y. Einaga, Polyhedron, 2005, 24, 1869 CrossRef CAS; (b) T. Akitsu and Y. Einaga, Polyhedron, 2005, 24, 2933 CrossRef CAS; (c) T. Akitsu, Polyhedron, 2007, 26, 2527 CrossRef CAS.
- (a) T. Akitsu and Y. Einaga, Inorg. Chem. Commun., 2006, 9, 1108 CrossRef CAS; (b) T. Akitsu and J. Nishijo, J. Magn. Magn. Mater., 2008, 320, 1586 CrossRef CAS; (c) T. Akitsu and J. Nishijo, J. Magn. Magn. Mater., 2007, 315, 95 CrossRef CAS; (d) T. Akitsu, J. Magn. Magn. Mater., 2009, 321, 207 CrossRef CAS.
- (a) F. Weigert, Naturwissenschaften, 1921, 29, 583 CrossRef; (b) K. Ichimura, Chem. Rev., 2000, 100, 1847 CrossRef CAS and references therein..
- (a) T. Akitsu, C. Ishioka and T. Itoh, Cent. Eur. J. Chem., 2009, 7, 690 CrossRef CAS; (b) T. Akitsu and C. Ishioka, Asian Chem. Lett., 2010, 14, 37 CAS; (c) T. Akitsu and T. Itoh, Polyhedron, 2010, 29, 477 CrossRef CAS; (d) T. Akitsu and R. Tanaka, Curr. Phys. Chem., 2011, 1, 82 CrossRef CAS; (e) T. Akitsu and Y. Miura, J. Chem. Chem Eng., 2011, 5, 443 CAS; (f) Y. Aritake, T. Takanashi, A. Yamazaki and T. Akitsu, Polyhedron, 2011, 30, 886 CrossRef CAS.
- M. J. Frisch G.W. Trucks H. B. Schlegel G. E. Scuseria M. A. Robb J. R. Cheeseman G. Scalmani V. Barone B. Mennucci G. A. Petersson H. Nakatsuji M. Caricato X. Li H. P. Hratchian A. F. Izmaylov J. Bloino G. Zheng J. L. Sonnenberg M. Hada M. Ehara K. Toyota R. Fukuda J. Hasegawa M. Ishida T. Nakajima Y. Honda O. Kitao H. Nakai T. Vreven J. A. Montgomery Jr. J. E. Peralta F. Ogliaro M. Bearpark J. J. Heyd E. Brothers K. N. Kudin V. N. Staroverov R. Kobayashi J. Normand K. Raghavachari A. Rendell J. C. Burant S. S. Iyengar J. Tomasi M. Cossi N. Rega J. M. Millam M. Klene J. E. Knox J. B. Cross V. Bakken C. Adamo J. Jaramillo R. Gomperts R. E. Stratmann O. Yazyev A. J. Austin R. Cammi C. Pomelli J. Ochterski R. L. Martin K. Morokuma V.G. Zakrzewski G. A. Voth P. Salvador J. J. Dannenberg S. Dapprich A. D. Daniels O. Farkas J. B. Foresman J. V. Ortiz J. Cioslowski D. J. Fox GAUSSIAN 09 (Revision A.1), Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- (a) S. Yamada, E. Ohno, Y. Kuge, A. Takeuchi, K. Yamanouchi and K. Iwasaki, Coord. Chem. Rev., 1968, 3, 247 CrossRef CAS; (b) S. Yamada, Coord. Chem. Rev., 1967, 2, 83 CrossRef CAS; (c) S. Yamada and A. Takeuchi, Coord. Chem. Rev., 1982, 43, 187 CrossRef CAS; (d) S. Yamada, Coord. Chem. Rev., 1966, 1, 415 CrossRef CAS; (e) S. Yamada, Coord. Chem. Rev., 1999, 190-192, 537 CrossRef CAS; (f) F. Galsbol, P. Steenbol and B. Sondergaard, Acta Chem. Scand., 1972, 26, 3605 CrossRef; (g) R. S. Downing and F. L. Urbach, J. Am. Chem. Soc., 1969, 91, 5977 CrossRef CAS; (h) Y. N. Belokon, J. Fuentes, M. Northa and J. W. Steeda, Tetrahedron, 2004, 60, 3191 CrossRef CAS; (i) T. Storr, P. Verma, R. C. Pratt, E. C. Wasinger, Y. Shimazaki and T. D. P. Stack, J. Am. Chem. Soc., 2008, 130, 15448 CrossRef CAS; (j) F. Zhang, S. Bai, G. P. A. Yap, V. Tarwade and J. M. Fox, J. Am. Chem. Soc., 2005, 127, 10590 CrossRef CAS; (k) Y. Nishida and S. Kida, Bull. Chem. Soc. Jpn., 1970, 43, 3814 CrossRef CAS; (l) S. Routier, J.-L. Bernier, J.-P. Catteau, P. Colson, C. Houssier, C. Rivalle, E. Bisagni and C. Bailly, Bioconjugate Chem., 1997, 8, 789 CrossRef CAS; (m) Y.-M. Shen, W.-L. Duan and M. Shi, J. Org. Chem., 2003, 68, 1559 CrossRef CAS; (n) M. Saito, H. Sato, Y. Mori and Y. Fukuda, Bull. Chem. Soc. Jpn., 2009, 82, 1266 CrossRef CAS; (o) S. Akine, A. Akimoto, T. Shiga, H. Oshio and T. Nabeshima, Inorg. Chem., 2008, 47, 875 CrossRef CAS; (p) A. Trujillo, M. Fuentealba, D. Carrillo, C. Manzur, I. Ledoux-Rak, J.-R. Hamon and J.-Y. Saillard, Inorg. Chem., 2010, 49, 2750 CrossRef CAS; (q) R. Paschke, D. Balkow and E. Sinn, Inorg. Chem., 2002, 41, 1949 CrossRef CAS; (r) M. Hirotsu, N. Kuwamura, I. Kinoshita, M. Kojima, Y. Yoshikawa and K. Ueno, Dalton Trans., 2009, 7678 RSC; (s) L. Rigamonti, F. Demartin, A. Forni, S. Righetto and A. Pasini, Inorg. Chem., 2006, 45, 10976 CrossRef CAS; (t) Y. Shimazaki, T. D. P. Stack and T. Storr, Inorg. Chem., 2009, 48, 8383 CrossRef CAS; (u) Y. Ding, X.-C. Li and D. Ferreira, J. Org. Chem., 2007, 72, 9010 CrossRef CAS; (v) K. Bernardo, S. Leppard, A. Robert, G. Commenges, F. Dahan and B. Meunier, Inorg. Chem., 1996, 35, 387 CrossRef CAS; (w) T. Storr, P. Verma, R. C. Pratt, E. C. Wasinger, Y. Shimazaki and T. D. P. Stack, J. Am. Chem. Soc., 2008, 130, 15448 CrossRef CAS.
- (a) X. Li, X. Lu, Q. Lu and D. Yan, Macromolecules, 2007, 40, 3306 CrossRef CAS; (b) B. Sapich, A. B. E. Vix, J. P. Rabe and J. Stumpe, Macromolecules, 2005, 38, 10480 CrossRef CAS; (c) R. Advincula, M.-K. Park, A. Baba and F. Kaneko, Langmuir, 2003, 19, 654 CrossRef CAS; (d) T. Geue, A. Ziegler and J. Stumpe, Macromolecules, 1997, 30, 5729 CrossRef CAS; (e) N. Bohm, A. Materny, W. Kiefer, H. Steins, M. M. Muller and G. Schottner, Macromolecules, 1996, 29, 2599 CrossRef; (f) N. Bohm, A. Materny, H. Steins, M. M. Muller and G. Schottner, Macromolecules, 1998, 31, 4265 CrossRef; (g) L. De Boni, C. Toro, A. E. Masunov and F. E. Hernandez, J. Phys. Chem. A, 2008, 112, 3886 CrossRef CAS.
- (a) H. Sakiyama, H. Okawa, N. Matsumoto and S. Kida, J. Chem. Soc., Dalton Trans., 1990, 2935 RSC; (b) B. Bosnich, J. Am. Chem. Soc., 1968, 90, 627 CrossRef CAS; (c) H. Okawa, M. Nakamura and S. Kida, Inorg. Chim. Acta, 1986, 120, 185 CrossRef CAS; (d) Y. Nishida and S. Kida, Bull. Chem. Soc. Jpn., 1970, 43, 3814 CrossRef CAS; (e) H. Sakiyama, H. Okawa, N. Matsumoto and S. Kida, Bull. Chem. Soc. Jpn., 1991, 64, 2644 CrossRef CAS; (f) M. Ulusoy, H. Karabiyik, R. Kilincarslan, M. Aygun, B. Cetinkaya and S. Garcia-Granda, Struct. Chem., 2008, 19, 749 CrossRef CAS; (g) T. Akitsu and Y. Einaga, Polyhedron, 2006, 25, 1089 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
