Earth processes cause Zr–Hf and Nb–Ta fractionations, but why and how?
Yaoling
Niu
*ab
aSchool of Earth Sciences, Lanzhou University, Lanzhou 730000, China
bDepartment of Earth Sciences, Durham University, Durham DH1 3LE, UK. E-mail: niuyl@lzu.edu.cn; yaoling.niu@durham.ac.uk; Fax: 441913342311; Tel: 441913342301
First published on 6th February 2012
Abstract
Zr–Hf and Nb–Ta are two elemental twins, each of which has the same valence (i.e. 5+ for Nb and Ta, and 4+ for Zr and Hf) and same ionic size at a given coordination number (e.g. RNb/RTa = 1.000 and RZr/RHf = 1.006 to ∼1.026 for coordination numbers of 6, 7, 8 and 12). As a result, it has been the view that Zr does not fractionate from Hf and Nb does not fractionate from Ta in the formation of minerals and in all Earth processes. Recent studies, however, have shown that this traditional view is in error. Up to 2 orders of magnitude fractionations have been observed in rocks from the world ocean floor and also in the seawater on various scales. I discuss some possible processes that may help explain such large fractionations, but further work is needed to test the validity of these interpretations. Perspectives from the chemistry community are in compelling need to help address this basic problem of scientific significance in understanding the chemical differentiation of our planet.
 Yaoling Niu | Yaoling Niu is Professor of Earth Sciences at Durham University, UK (also honorary Professor of Lanzhou University, China). He obtained a BSc in Geology (Lanzhou University, 1982), an MS in Economic Geology (University of Alabama, USA, 1988) and a PhD in Geology and Geophysics (University of Hawaii, USA, 1992). He was a lecturer/Senior Lecturer at University of Queensland (Australia, 1993–2001), a NERC Senior Research Fellow at Cardiff University (UK, 2001–2003) and an Associate Professor at University of Houston (USA, 2003–2004). He has been in Durham since 2004. His research uses petrology and geochemistry as a means to understand how the Earth works on all scales (see http://www.dur.ac.uk/earth.sciences/staff/?id=2205). |
The theory
Victor Moritz Goldschmidt, Father of Modern Geochemistry,1 recognized that behaviours of chemical elements in the formation of minerals and rocks are largely controlled by their ionic size and valence.2 The typical examples are Zr–Hf and Nb–Ta elemental twins. Each of the two twins has the same valence (e.g., Zr4+ and Hf4+; Nb5+ and Ta5+) and essentially the same ionic radii for a given coordination number (e.g., with CN = 6, Rzr = 0.72, RHf = 0.71 ; RNb = 0.64, RTa = 0.64) in (all or almost all) Earth materials and processes.3 As a result, there has been wide acceptance that Zr/Hf and Nb/Ta ratios should be the same as, or identical to, those of chondrite meteorites (assumed to represent the composition of the bulk Earth and the solar system) in all mantle and mantle derived rocks: Zr/Hf ≈ 36.30 and Nb/Ta ≈ 17.63–6 although it has been noted that the Zr/Hf ratio varies in some carbonatites7 and is superchondritic (variably higher than chondritic ratio) in some alkali basalts8 and that the bulk continental crust has a subchondritic (lower than chondritic ratio) Nb/Ta ratio of 11–12.9Observations
Modern analytical technologies such as inductively coupled plasma mass spectrometry (ICP-MS) has enabled analysis of most elements across the periodic table precisely and accurately in rocks and minerals at all concentration levels, and as low as parts per trillion (10−12). With this method, Niu and co-authors studied mid-ocean ridge basalts (MORB)10–11 – rocks that form the world ocean crust and cover ∼70% of the Earth's surface – solidified from magmas produced by partial melting of mantle rocks along the > 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km long ocean ridge system that encircles the globe. They discovered that these rocks show a factor of 2 correlated variation between Zr/Hf (∼25 to 50) and Nb/Ta (∼9 to 18) (See Fig. 1a).
000 km long ocean ridge system that encircles the globe. They discovered that these rocks show a factor of 2 correlated variation between Zr/Hf (∼25 to 50) and Nb/Ta (∼9 to 18) (See Fig. 1a).
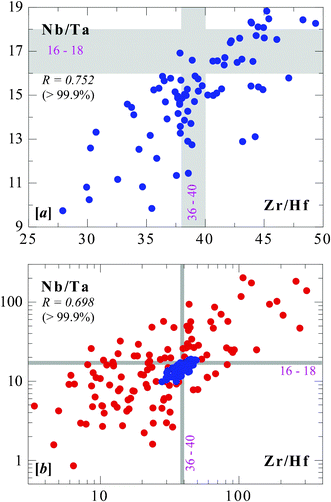 | ||
| Fig. 1 Correlated variations of Zr/Hf and Nb/Ta in seafloor basalts (MORB) (a) and seafloor mantle peridotites (MORP) (b). The grey bands indicate theoretical values of chondrite meteorites that are assumed to represent the values of the solar system and the bulk Earth. These correlations are statistically significant at > 99.9% confidence levels. The blue cluster in (b) is the MORB data in (a). | ||
Furthermore, Niu12 showed that these two ratios in fact show over 2-orders of magnitude correlated variations in mid-ocean ridge peridotites (MORP) (Fig. 1b) that are melting residues complementary to MORB during partial melting of the mantle that produces the ocean crust at ocean ridges. Very recently, Firdaus et al.13 showed very large Zr/Hf and Nb/Ta variations in seawater collected from the surface down to varying depths from a number of stations along two longitudinal sections in the Pacific, although there is no correlation between the two ratios (Fig. 2). More recently, varying Nb/Ta and Zr/Hf ratios have been observed in basalts erupted on oceanic islands,14 along island arcs,15 in some mantle xenoliths,16–17 granitic rocks18 and metamorphic rocks, including the Archean TTG suites.19–22 These are important new observations, but they have rather limited variation ranges without any Nb/Ta–Zr/Hf correlation.
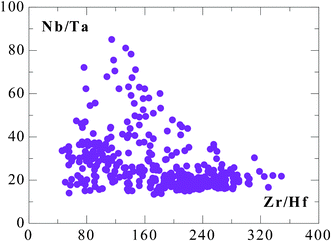 | ||
| Fig. 2 Unexpectedly large Zr–Hf and Nb–Ta fractionations in seawater (data are weight ratios from Firdaus et al.13). | ||
I thus focus here on MORB, MORP and seawater because of their huge fractionations of these two elemental twins and because they represent volumetrically significant Earth materials – mantle rocks (MORP) and mantle-derived rocks (MORB) that cover ∼2/3 of the surface of the solid Earth and the oceans that dominate the Earth's hydrosphere. I hope to inspire testable hypotheses towards revealing the governing principles through a collaborative effort between the chemical and geological community.
All the above observations manifest that the prevailing theory in the geochemistry is invalid, at least in describing processes that have led to the formation of many rocks, especially MORB and MORP, as well as seawater hydro-chemical or hydrodynamic processes. At present, the solid-Earth geochemistry community assumes that the bulk Earth must have a chondritic Nb/Ta = 17.6 (or Nb/Ta = ∼19),6 and, on the basis of this assumption, interprets the subchondritic Nb/Ta ratio (i.e., < 17.6) of bulk continental crust9 and oceanic crust10,23 as indicating a super chondritic Nb/Ta reservoir present deep in the Earth's mantle that has never been sampled by known magmatism.24–25 Wade and Wood26 proposed that Nb is more siderophile (than Ta) and that the missing Nb in crustal rocks may be in the Earth's core.27 All this points to the critical importance in understanding mechanisms that can cause Nb–Ta (and Zr–Hf) fractionation towards an improved understanding on how the Earth works in terms of both chemical and physical processes the planet Earth (maybe the entire solar system) may have experienced over its history. The objective of this short article is to draw the attention of chemists who may offer new perspectives on what may actually cause Zr–Hf and Nb–Hf fractionations, why and how. In the following, I summarize the current hypotheses on this problem.
Discussion
Zr–Hf and Nb–Ta fractionations in MORB and MORP
Elements such as Nb, Ta, Zr and Hf are termed “incompatible” elements in geology because they tend to enter the melt over solid minerals during mantle melting or melt crystallization. This is defined by a parameter called bulk distribution coefficient DSolid/MeltM = CSolidM/CMeltM, which is the concentration ratio of element M in the solid minerals over the melt at equilibrium conditions. If DSolid/MeltM < 1, the element M is termed incompatible. Obviously, if DSolid/MeltA < DSolid/MeltB, then element A is more incompatible than element B.Fig. 3 plots MORB data to manifest that Zr is more incompatible than Hf (i.e., DZr < DHf), and Nb is more incompatible than Ta (i.e., DNb < DTa).10–11 The same is also true in MORP data.12 This is unexpected because of the same valence and similar/identical ionic size. Green et al.28 showed experimentally that in the basaltic system the distribution between clinopyroxene (an effective mineral) and the melt gives DZr/DHf ≈ 0.5 and DNb/DTa ≈ 0.5, which is qualitatively consistent with the observation and could explain the factor of 2 Zr/Hf and Nb/Ta variation in the MORB data (Fig. 1a,3), but cannot explain the over 2 orders of magnitude variation in the MORP data (Fig. 1b). There are more recent experimental data on the relative incompatibility of these elements,29–31 but all these works offer no effective mechanism as to why Zr–Hf and Nb–Ta elemental twins should fractionate from each other.32–33
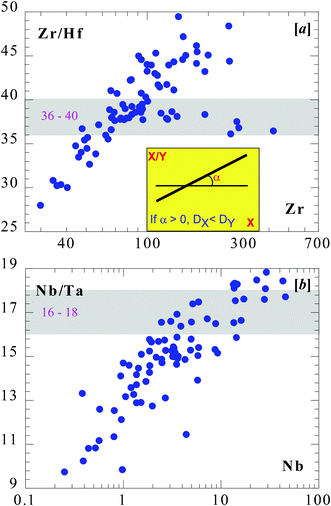 | ||
| Fig. 3 MORB data (as in Fig. 1a) show Zr/Hf vs. Zr (top) and Nb/Ta vs. Nb (bottom). As illustrated in the inset in the top panel, the positive trends on these two plots mean that with increasing X, both X and Y increase, but X increases faster, meaning X is more incompatible than Y.10 | ||
Given the identical valences (5+ for Nb and Ta, and 4+ for Zr and Hf) and essentially the same ionic radii (RNb/RTa = 1.000 and RZr/RHf = 1.006 to ∼1.026 for coordination numbers of 6, 7, 8 and 12) of the two elemental twins, there is a factor of two difference in their respective atomic masses (MZr/MHf = 0.511; MNb/MTa = 0.513), therefore it is logical to reason that mass differences may have effects on the observed fractionations (or the apparent relative incompatibility). For example, for two elements of similar or identical chemical properties, the lighter element (e.g.90–96Zr, 93Nb) is more incompatible than the heavier element (e.g.174–180Hf, 181Ta).10 This allowed Niu & Hekinian11 to suggest mass-dependent fractionation, either differential diffusion rates or differential mass transfer rates. Such mass-dependent fractionation may also explain why DRb < DCs (mass ratio MRb/MCs = 0.643) even though Cs (1+) is about 10% larger in ionic radius than Rb (1+).10–12 The ∼50% mass difference for Zr–Hf and Nb–Ta is significantly greater than the commonly considered isotopic fractionations of light stable elements at relatively low temperatures. Indeed, diffusion or mass transfer coefficients are known to be mass-dependent. For example, KA/KB = (MB/MA)1/2, where KA and KB represent diffusion coefficients of species with mass MA and MB, respectively.34 For a mass ratio of ∼2 (e.g., MTa/MNb and MHf/MZr), the diffusion coefficient ratio would be KNb/KTa (or KZr/KHf) = 1.414, i.e., the lighter element would diffuse, under ideal situations, 41% more efficiently than the heavier element (given all other variables the same: same valence, same ionic radius, same coordination number, etc.). That is, there would be about 41.4% fractionation just from the mass-dependent diffusion coefficients alone.
Such ∼41% (or 410‰) fractionation contrasts with the familiar per-mil level light isotope fractionations. For example, for 16O and 18O fractionation, K18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O/K16
O/K16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O = (16/18)1/2 = 0.943, there would only be a 57‰ (vs. 410‰) fractionation. Is it possible that the apparent DZr/DHf < 1 and DNb/DTa < 1 may be due to mass-dependent fractionation under high temperature magmatic (mantle?) conditions? The ultimate test for the hypothesis of mass-dependent fractionation requires well-designed experimental studies and isotopic analysis of MORP samples (Fig. 1b) at different mass levels (e.g., 46,47,48,49,50Ti, 90,91,92,94,96Zr,174,176,177,178,179,180Hf) using multiple collector ICP-MS as proposed.12 Alternative possibilities have been reviewed by Huang et al.35
O = (16/18)1/2 = 0.943, there would only be a 57‰ (vs. 410‰) fractionation. Is it possible that the apparent DZr/DHf < 1 and DNb/DTa < 1 may be due to mass-dependent fractionation under high temperature magmatic (mantle?) conditions? The ultimate test for the hypothesis of mass-dependent fractionation requires well-designed experimental studies and isotopic analysis of MORP samples (Fig. 1b) at different mass levels (e.g., 46,47,48,49,50Ti, 90,91,92,94,96Zr,174,176,177,178,179,180Hf) using multiple collector ICP-MS as proposed.12 Alternative possibilities have been reviewed by Huang et al.35
While the mass-dependent fractionation hypothesis is attractive and is worth testing, it is also noted that the two elements, Y(3+) and Ho (3+), which are sufficiently similar in chemical properties (RY/RHo = 0.9989 to ∼1.0039 for coordination numbers of 6, 8 and 9),3 yet their mass difference of MY/MHo ≈ 0.539 does not seem to cause significant fractionation between these two elements because Y/Ho = 27.33 ± 0.39 for MORB samples and 27.16 ± 4.67 for MORP samples. A recent study on an Indian Ocean basalt suite shows an obvious Y–Ho fractionation,36 which may be caused by mass-dependent fractionation.
Zr–Hf and Nb–Ta fractionations in seawater
The correlations between total silicate (expressed as Si[OH]4) and Nb, Ta, Zr and Hf abundances in seawater allow Firdaus et al.13 to interpret that these elements in seawater must have derived from terrigenous sources (i.e., sediments derived from the upper continental crust transported through rivers and wind dust). This interpretation seems reasonable, but cannot explain the unexpectedly large Zr/Hf and Nb/Ta variations (Fig. 2) without additional processes because Zr/Hf ≈ 36.4 and Nb/Ta ≈ 13.3 in upper continental crustal rocks.37The positive correlation of Zr/Hf with increasing Zr in Fig. 4a resembles that seen in MORB (Fig. 3a), but the negative correlation of Nb/Ta with Nb in Fig. 4b does not (see Fig. 3b). This observation indicates that there must be different mechanisms at work in causing Zr–Hf and Nb–Ta fractionation. Because the authors recognize that the abundances of these elements in seawater vary as a function of both water depth and geographic locations (latitude and longitude in the Pacific), and because location-dependent variation is more fortuitous, we can examine how these elements and their ratios may vary as a function of water depth so as to gain insights into the mechanism of Zr–Hf and Nb–Ta fractionation. For convenience and to average out effects including analytical uncertainties and location-dependent variations, we can choose to average the data with respect to water depth intervals, as done in Fig. 5, with the averages also plotted in Fig. 4. It is not shown, but significant curvilinear correlations exist between water depth and abundances of Zr, Hf, Nd and Ta, which are best described in terms of a power-law relationship, as given in the caption to Fig. 5. However, the silicate nutrient (Si[OH]4) does not correlate well with the abundances of these elements in having no clear increase with increasing water depth at depths greater than ∼1200 m below the sea surface. On the other hand, Zr/Hf and Nb/Ta ratios only show a change with depth at depths shallower than ∼1000 m, and remain constant at greater depths. (Fig. 5).
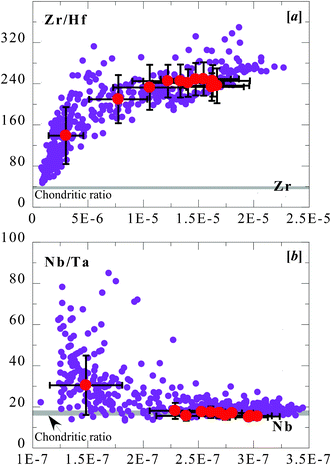 | ||
| Fig. 4 Zr/Hf (weight ratio) vs. Zr (ppm) (a) and Nb/Ta (weight ratio) vs. Nb (ppm) (b) of seawater data from Firdaus et al. (2011). Solid circles in red are ocean depth interval averages with ±1σ from the means explained in Fig. 5. The grey bars are chondrite meteorite ratios plotted for reference. | ||
![Average total silicate (expressed as Si[OH]4 umol l−1), Nb/Ta and Zr/Hf (weight ratios) in seawater plotted as a function of sampling depths from the surface down to the ocean bed. The averages were derived by averaging all the observations from within each of the 500 meter depth intervals (e.g., N = 207 observations in the shallowest interval given on the left panel) regardless of actual locations (latitude and longitude), so as to reveal the first-order variations of these chemical parameters as a function of water depth. It is not shown here, but Zr, Hf, Nb and Ta (in ppm) concentrations exhibit a nonlinear monotonic increase with increasing water depth (D in metres): Zr = 3*10−7D0.4796 (R2 = 0.987), Hf = 4*10−9D0.3317 (R2 = 0.996), Nb = 7*10−8D0.1727 (R2 = 0.986) and Ta = 2*10−9D0.2776 (R2 = 0.959). Data from Firdaus et al.13.](/image/article/2012/RA/c2ra00384h/c2ra00384h-f5.gif) | ||
| Fig. 5 Average total silicate (expressed as Si[OH]4 umol l−1), Nb/Ta and Zr/Hf (weight ratios) in seawater plotted as a function of sampling depths from the surface down to the ocean bed. The averages were derived by averaging all the observations from within each of the 500 meter depth intervals (e.g., N = 207 observations in the shallowest interval given on the left panel) regardless of actual locations (latitude and longitude), so as to reveal the first-order variations of these chemical parameters as a function of water depth. It is not shown here, but Zr, Hf, Nb and Ta (in ppm) concentrations exhibit a nonlinear monotonic increase with increasing water depth (D in metres): Zr = 3*10−7D0.4796 (R2 = 0.987), Hf = 4*10−9D0.3317 (R2 = 0.996), Nb = 7*10−8D0.1727 (R2 = 0.986) and Ta = 2*10−9D0.2776 (R2 = 0.959). Data from Firdaus et al.13. | ||
It is obvious in Fig. 5 that the large Zr–Hf and Nb–Ta variation and fractionation in seawater takes place essentially within the upper ∼1000 m depth interval. If this is indeed the case, then the constant Nb/Ta (18.81 ± 1.01) and Zr/Hf (241 ± 6) ratios at greater depths may be the net result of shallow level fractionation. It is possible that the large variation range of Zr/Hf and Nb/Ta (large ±1σ values in Fig. 5) at shallow water depth may be associated with varying geographic locations. For example, those near the coastlines, such as the Aleutian volcanic arc system to the north and the Tonga–New Zealand system to the south, may receive land-derived sediments relative to locations far from the coastlines. There is also likely to be wind dust (ultimately also from land surface) in shallow water.
However, upper continental crustal rocks have average Nb/Ta ≈ 13.3 and Zr/Hf ≈ 36.4,37 which are significantly lower than Nb/Ta = 13–85 (with a mean of 32.9) and Zr/Hf = 46.5–312 (with a mean of 137), respectively, in the seawater near the surface (Fig. 5). That is, both the extent of Nb/Ta and Zr/Hf fractionation and the large variation range of these two ratios in the near-surface seawater cannot be explained by land-derived materials. Shallow water biological (microorganisms) or biochemical activities are likely the primary cause for such large Nb–Ta and Zr/Hf fractionation, but how they work is unknown and requires chemical and biochemical investigations.
The biochemical effect may be most intense at the surface and declines with depth to ∼1000 m, which would explain the systematic Nb/Ta and Zr/Hf variations with depth to that deep level (Fig. 5). If the microorganisms prefer to intake (in non-chondritic ratios) Ta (vs. Nb) and Zr (vs. Hf), this tendency can explain the decreasing Nb/Ta and increasing Zr/Hf in the dissolved seawater with depth shallower than ∼1000 m (Fig. 5). If the microorganisms demise at depth > 1000 m, the biochemical fractionation would cease. This possibility explains the constant Nb/Ta (18.1 ± 1.01) and Zr/Hf (241 ± 6) in seawater at all depths deeper than ∼1000 m (Fig. 5). Much of these elements may be absorbed on particles, whether biological remains or surface wind dust, and their solubility increase in seawater with depth (pressure effect) explains the their increasing abundances with depth (see caption to Fig. 5).
Conclusions
Contrary to the traditional theory that elemental twins such as Nb–Ta and Zr–Hf should not fractionate from each other in all Earth materials and through all Earth processes, in particular the Earth's mantle and mantle-derived rocks, these elemental twins do fractionate rather significantly as recorded in magmatic rocks such as MORB and mantle rocks like MORP, and as manifested in present-day seawater on a global scale. Mass-dependent fractionation has been proposed to explain the Zr–Hf and Nb–Ta fractionation in Earth processes that form MORB and MORP, but this interpretation requires testing through carefully designed experimental investigations, as well as isotopic analysis of multi-isotope elements of varying mass levels like Ti (masses 46, 47, 48, 49, 50), Zr (90, 91, 92, 94, 96) and Hf (174, 176, 177, 178, 179, 180). A correct understanding of the cause or causes of Nb–Ta and Zr–Hf fractionation in Earth's mantle and mantle-derived rocks is of profound importance in understanding the chemical differentiation of the Earth over its history.More recently, significant Nb–Ta and Zr–Hf fractionations have been observed in rocks from other geological environments (though lesser in extent than in MORP, and without Nb/Ta–Zr/Hf correlation), but the origin of such fractionations remain unknown. Chemical or biochemical processes at near-surface seawater are suspected to be the primary cause of the observed significant Nb–Ta and Zr–Hf fractionations. While this interpretation is logical, the exact chemical or biochemical mechanism is unknown and requires insightful investigations. Firdaus et al.13 proposed that the observed spatial Nb/Ta and Zr/Hf variations in seawater can be used as an effective proxy to monitor ocean circulation patterns. Although the latter is of fundamental scientific value, it remains in doubt whether these two ratios are useful in this regard without understanding the origin of the observed Nb–Ta and Zr–Hf fractionations.
It is well known that to chemically separate Zr from Hf and Nb from Ta is not trivial. For example, one way (without using organic solvents) to separate Zr from Hf is to form Zr–Hf-bearing fluoric complexes (e.g. K2[Zr,Hf]F6) followed by fractional crystallization, because K2ZrF6 is less soluble than K2HfF6 in water. Similarly, one way (without using organic solvents) to separate Nb from Ta also requires the formation of different fluoric complexes (e.g., K2[TaF7] and K2[NbOF5]) and uses their different solubilities in water. In both cases, formation of fluoric complexes is a prerequisite, but except for the rather rare pegmatitic environments (associated with very late stage granitic magma evolution), it is unlikely to form such fluoric complexes through Earth processes because of the difficulties in concentrating fluorine.
Is there any other method to separate Zr from Hf and Nb from Ta without forming fluoric complexes? Perspectives from the chemistry community are in compelling need to help address this basic problem of scientific significance in understanding the chemical differentiation of our planet.
Acknowledgements
The research is supported by Durham University and also the National Natural Science Foundation of China (91014003, 41130314). Two journal reviewers are thanked for their constructive comments.References
- B. Mason, The Geochemical Society Special Publication, 1992, 4, 184 Search PubMed.
- V. M. Goldschmidt, J. Chem. Soc., 1937, 140, 655 RSC.
- R. D. Shannon, Acta Crystallographica, 1976, A 32, 751–767 CrossRef.
- K. P. Jochum, H. M. Seufert and B. Spettel, Geochim. Cosmochim. Acta, 1986, 50, 1173 CrossRef CAS.
- S. S. Sun and W. F. McDonough, Geological Society Special Publications, 1989, 42, 313 CrossRef.
- C. Munker, J.A. Pfander and S. Weyer, Science, 2003, 301, 84 CrossRef.
- F. R. D. Andrade, P. Moller and P. Dulski, Revista Brasileira de Geoscienceas, 2002, 32, 361 Search PubMed.
- C. Dupuy, J. M. Liotard and J. Dostol, Geochim. Cosmochim. Acta, 1992, 56, 2417 CrossRef CAS.
- R. J. Rudnick, Nature, 1995, 378, 571 CrossRef CAS.
- Y. L. Niu and R. Batiza, Earth Planet. Sci. Lett., 1997, 148, 471 CrossRef CAS.
- Y. L. Niu and R. Hekinian, Earth Planet. Sci. Lett., 1997, 146, 243 CrossRef CAS.
- Y. L. Niu, Journal of Petrology, 2004, 45, 2423 CrossRef CAS.
- M. L. Firdaus, T. Minami, K. Norosuye and Y. Sohrin, Nat. Geosci., 2011, 4, 227 CrossRef CAS.
- J. A. Pfander, C. Munker and A. Stracke, Earth Planet. Sci. Lett., 2007, 254, 185 CrossRef.
- S. Konig and S. Schuth, Earth Planet. Sci. Lett., 2011, 301, 265 CrossRef.
- F. Klfoun, D. Iono and C. Merlect, Earth and Planetary Science Letters, 2002, 199, 19 Search PubMed.
- S. Aulbach, S. Y. O'Reilly and N. J. Pearson, Contrib. Mineral. Petrol., 2011, 162, 1047 CrossRef CAS.
- J. Dostal and A. K. Chatterjee, Chem. Geol., 2000, 163, 207 CrossRef CAS.
- A. Schmidt, S. Weyer and T. John, Geochim. Cosmochim. Acta, 2009, 73, 455 CrossRef CAS.
- J. L. Liang, X. Ding and X. M. Sun, Chem. Geol., 2009, 268, 27 CrossRef CAS.
- J. E. Hoffmann, C. Munker and T. Naerass, Geochim. Cosmochim. Acta, 2011, 75, 4157 CrossRef CAS.
- T. John, R. Klemd and S. Klemme, Contrib. Mineral. Petrol., 2011, 161, 35 CrossRef CAS.
- R. L. Rudnick, M. Barth and I. Horn, Science, 2000, 287, 278 CrossRef CAS.
- W. F. McDonough, Philosophical Transaction of the Royal Society of London, 1991, Series A 335, 407 Search PubMed.
- Y. L. Niu and M. J. O'Hara, J. Geophys. Res., 2003, 108, 19, DOI:10.1029/2002JB002048.
- J. Wade and B. J. Wood, Nature, 2001, 409, 75 CrossRef CAS.
- B. S. Kamber, A. Greig and R. Schonberg, Precambrian Res., 2003, 126, 289 CrossRef CAS.
- T. H. Green, J. D. Blundy and J. Adam, Lithos, 2000, 53, 165 CrossRef CAS.
- M. W. Schmidt, A. Dardon, G. Chazot and R. Vannucci, Earth Planet. Sci. Lett., 2004, 226, 415 CrossRef CAS.
- E. C. Fulmer, O. Nebel and W. van Westtrenen, Geochim. Cosmochim. Acta, 2010, 74, 2714 CrossRef.
- X. L. Xiong, H. Keppler and A. Audetat, Geochim. Cosmochim. Acta, 2011, 75, 1673 CrossRef CAS.
- S. Foley, M. Tiepolo and R. Vannucci, Nature, 2002, 417, 837 CrossRef CAS.
- J. Blundy and B. Wood, Earth Planet. Sci. Lett., 2003, 210, 383 CrossRef CAS.
- A. C. Lasaga, Kinetic Theory in the Earth Sciences, 1998, Princeton University Press, 811 pp Search PubMed.
- H. Huang, Y. L. Niu, Z. Zhao, H. Hei and D. Zhu, Journal of Earth Sciences, 22, 52 Search PubMed.
- F. A. Frey, M. Pringle and P. Meleney, Earth Planet. Sci. Lett., 2011, 303, 215 CrossRef CAS.
- R. L. Rudnick and S. Gao, Treatise on Geochemistry, 2003, 3, 1 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
