Uniform square-like BaFBr:Eu2+ microplates: controlled synthesis and photoluminescence properties†
Qinghua
Liang
ab,
Yao
Shi
a,
Wangjing
Ma
a,
Zhi
Li
*a and
Xinmin
Yang
*a
aTechnical Institute of Physics and Chemistry of the Chinese Academy of Sciences (CAS), 29 Zhongguancun East Road, Beijing, 100190, PR China. E-mail: xmyang@mail.ipc.ac.cn; lizhi@mail.ipc.ac.cn; Fax: +86 10 62554670; Tel: +86 10 82543551
bGraduate University of Chinese Academy of Sciences, Beijing, 10039, PR China
First published on 14th May 2012
Abstract
Uniform square-like BaFBr:Eu2+ microplates were successfully fabricated via a simple water/oil emulsion method. Various characterization techniques such as X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, Scanning electron microscopy (SEM) and Transition electron microscopy (TEM) were employed to examine the phase, size, morphology, and structure of the products. It was found that the morphology and size of products were strongly dependent on the reaction parameters, such as the ratio of reactants, the reaction time and the reaction temperature. The length and thickness of these square-like BaFBr:Eu2+ microplates can be tuned by adjusting the reaction time and temperature. Well-dispersed cuboctahedron and corner cut cuboid-like BaFBr:Eu2+ particles were produced when the reaction temperature was increased above 60 °C. A possible formation mechanism of the products was discussed based on the time and temperature-dependent experimental results. The optical properties were characterized by photoluminescence (PL) spectroscopy as well as kinetic decay. Eu2+-doped dependent luminescent intensity was investigated in detail. The prepared BaFBr:Eu2+ phosphors are considered promising materials for fluorescent applications.
1. Introduction
Design and synthesis of nano- or microcrystals with controlled shape and size have received considerable attention over the past decades due to their dimensionality and morphology-dependent properties as well as their potential applications in many fields.1–8 Rare earth ion-doped materials have been widely applied in various fields including solid-state lighting,9 optical imaging,10 3D volumetric display,11 drug delivery,12etc. BaFX (X = Cl, Br, I) are layered ionic crystals with a tetragonal matlockite structure (space group: P4/nmm; Z = 2).13 They are well known as important host materials in which rare earth ions can occupy the Ba2+ sites of the BaFX lattice. Rare earth ion-doped BaFX compounds have found many applications as pressure sensors,14 pressure celebrants15,16 and photodynamic activators.17 Above all, BaFX:Eu2+ materials are efficient X-ray storage phosphors (XSP) for exhibiting photo-stimulated luminescence (PSL) properties. Their wide uses include commercial imaging plates (IPs) for medical radiography, security screening and non-destructive testing.18 In comparison with conventional film-screen systems, IPs have many advantages, such as better reusability, higher sensitivity, larger dynamic range and better detective quantum efficiency.18 Imaging plates also allow computed radiography (CR) which are greatly superior at transferring images and storing information. Recently, despite many efforts in searching for other new other materials, including halides of alkali metals,19–21 elpasolites,22 glasses23 and glass ceramics,24–27 BaFBr:Eu2+ phosphors are still proven to be the most efficient XPS and still far from being replaced in the consideration of stability and optical performances.28,29 The irregular shaped BaFBr:Eu2+ particles with wide size distribution used for conventional X-ray intensifying screens and IPs are often prepared by the traditional high temperature solid phase methods.30–32 As a result, it is difficult to arrange them tightly in the process of making IPs. Under such conditions, the incidence and readout beams are significantly scattered, which results in inferior spatial resolution.In more common practical applications, the demand for a higher spatial resolution is essential, which means that the size and shape of the BaFBr:Eu2+ particles have to be reduced and well-dispersed.33 However, the emission efficiency will be limited by tiny BaFBr:Eu2+ grains. To circumvent this, Li et al. suggested polycrystalline BaFBr:Eu2+ sizes of several micrometer in diameter as the ideal size.34 Consequently, many efforts have been focused on the synthesis of appropriate and well-distributed BaFBr:Eu2+ particles to overcome these problems. von Seggern et al. reported a single step solid phase synthesis method for high-quality BaFBr:Eu2+ phosphors by using BaCO3, EuF3 and NH4X as reactants.35,36 Kostova et al. fabricated relatively uniform tabular BaFBr:Eu2+ particles using pin wood as templates.37 These solid state methods generally require a complicated process, higher temperature and longer reaction time. Therefore, a facile and cost-effective synthetic approach is still highly desirable in order to obtain high quality BaFBr:Eu2+ phosphors with a uniform size and well-defined morphology.
Among many preparations, due to the advantages of convenience in experimental equipment and particularly the capability to facilitate the fabrication of complex materials, the emulsion method has been widely used to prepare nano or micro-structures with a uniform size and shape.38–40 Reverse emulsion is a translucent and isotropic liquid medium with uniform “water pools” dispersed in a continuous oil phase, which is stabilized by surfactant and co-surfactant molecules at the water/oil interface. These “water pools” offer ideal microreactors that provide limited space and reactants for the proceeding reactions.41 To the best of our knowledge, there have been no reports on the synthesis of uniform square-like BaFBr:Eu2+ microplates through such a method. In this submission, a water/oil (W/O) emulsion synthesis route for fabricating uniform square-like BaFBr:Eu2+ microplates is introduced. The phase and morphology of the products have been comprehensively investigated. A possible formation mechanism has been discussed based on the time and temperature-dependent experimental results. In addition, the optical properties of the annealed samples were also studied. This method is facile, cost-effective and also suitable for large-scale preparations.
2. Experimental
2.1 Chemical reagents
Cetyltrimethyl ammonium bromide (CTAB, 99.0%), n-butanol (99.0%), n-octane (99.0%), NH4F (99.0%) were purchased from Aladdin reagent company; EuBr2 (99.99%), BaBr2 (99.9%) were obtained from Alfa Aesar. Other chemicals were analytical grade reagents purchased from Beijing Chemical Corporation. Deionized water was used throughout. All the initial chemicals in this work were used without further purification.2.2 Synthesis route
For preparing the W/O system, CTAB was used as the surfactant, with n-butanol as the cosurfactant and n-octane as the oil phase. The synthetic procedure of square-like BaFBr:Eu2+ microplates are as follows. Firstly, two aqueous solutions were obtained by dissolving a calculated amount of EuBr2, 0.5942 g BaBr2 and 0.037 g of NH4F in a certain volume of distilled water, respectively. Then, two identical oil solutions were obtained by dissolving 2 g of CTAB in the solvent mixtures containing a little butanol and 11 mL of octane. Secondly, the W/O emulsion I was prepared by dispersing a BaBr2, EuBr2 aqueous solution in the oil phase under stirring. Also, emulsion II was prepared by a similar method except dispersing NH4F solution instead. The two mixtures became translucent after 1 h of vigorous agitation separately at room temperature. Subsequently, emulsion II was added to I rapidly under stirring. Lastly, after agitating for some time at a certain temperature, the turbid solution was collected by centrifuging. The precipitates were washed several times alternately with methanol and ethanol to remove superfluous reactants and surfactant, followed by centrifugation and drying in vacuum at 50 °C for 8 h. To investigate the effects of the experimental conditions, the ratio of reactants, reaction time and reaction temperature were varied, as depicted in Table S1 (See ESI†).2.3 Characterization
The phase identification and crystallography of the products were characterized by X-ray powder diffraction (XRD) using a German Bruker AXS D8 focus X-ray diffractometer equipped with graphite monochromatized Cu-Kα radiation (wavelength λ = 1.5405 Å), operating at 40 kV and 30 mA. The XRD data for index and cell-parameter calculations were collected by a scanning mode with 0.02 in the 2θ range from 10° to 70°. Fourier-transform IR spectra were obtained via a Varian Excalibur 3100 IR spectrophotometer using KBr pellet technique in the range 500–4000 cm−1. Fourier transform Raman spectroscopy was carried out with a Bruker-RFS 100 (Germany) using 785 nm laser excitation from a diode laser. The dimension and morphology of the products were analyzed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM was performed with a Hitachi S-4800 Scanning Electron Field Emission Microscope equipped with an energy-dispersive X-ray spectrum (EDS, JEOL JXA-840). Prior to SEM analysis, very thin Au films were coated on the surface of powder with an E1045 sputtering coater (Hitachi). TEM was recorded on a JEOL JEM 2010F electron microscope operating with acceleration voltage of 200 kV. For TEM observation, the sample was dispersed in absolute ethanol by ultrasonic treatment and then dropped on a carbon-copper grid. Structural information of the crystals was inspected on a high-resolution transmission electron microscope (HRTEM). Elemental analysis of europium in the solid sample was measured by inductively coupled plasma optical emission spectrometry analysis (ICP, Varian 710-ES). The excitation and emission spectra of the products were obtained from a Varian Carry Eclips 500 fluorescence spectrophotometer equipped with a 60 W xenon lamp as the excitation source. Luminescence decay curve was analyzed by an Edinburgh Combined Fluorescence Lifetime and Steady State Spectrometer (F900) using a 220 nm laser as the excitation source. All measurements were performed at room temperature.3. Result and discussion
3.1 Phase, composition and structure of the products
The phase of and the compositions of BaFBr and BaFBr:Eu2+ were first inspected by X-ray diffraction patterns (XRD). The collected line profiles are analyzed with MDI Jade 5.0 and approximated with a pseudo-Voigt function after performing an instrumental broadening correction and background subtraction. Fig. 1 shows the XRD pattern of the square-like BaFBr and BaFBr:Eu2+ microplates, and a standard tetragonal BaFBr data was listed for comparing (JCPDS Card No. 24-0090). | ||
| Fig. 1 XRD patterns of the square-like BaFBr (a), BaFBr:Eu2+ (b) microplates prepared at 25 °C for 3 h. The standard data for BaFBr (JCPDS Card No. 24-0090) is presented in the figure for comparison. | ||
All peaks can be indexed to pure tetragonal phase (space group: P4/nmm) of BaFBr (JCPDS Card No. 24-0090) except for a slight shift of BaFBr:Eu2+ pattern. No traces of additional peaks related to other impurity phase such as BaF2, BaBr2 or EuF2 were detected, implying the high purity of the products. It can also be observed that the diffraction peaks are very sharp and strong, indicating that products with high crystallinity have been obtained. A slight shift of the diffraction peaks together indicates that Eu2+ ions may have been effectively doped into the BaFBr host lattices. The profile fitting was performed with a Rietveld structural method using the Fullprof program.42 A good agreement and acceptable reliability results have been found (See Fig. S1 and S2 in ESI†). The refined parameters are a = b = 4.5116(4) Å, c = 7.4439(3) Å for BaFBr, and a = b = 4.4971(1) Å, c = 7.4222(2) Å for BaFBr:Eu2+, which correspond well to the standard data of a = b = 4.5109 Å, c = 7.4430 Å. The slightly reduced lattice constants are related with the smaller ion radius of the doped Eu2+ (rEu2+ = 1.09 Å, rBa2+ = 1.35 Å). The average crystallite sizes of the particles were calculated from the Scherrer formula: Dhkl = Kλ/(βcosθ), where Dhkl is the crystalline size, K is a constant (0.89), λ is the X-ray wavelength (0.15405 nm), β and θ are the full width at half-maximum and the diffraction angle, respectively. The strongest four peaks (101) at 2θ = 23.03, (002) at 2θ = 23.89, (110) at 2θ = 27.945 and (102) at 2θ = 31.12 are used to calculate the average crystallite sizes. Finally, the estimated average crystal size of BaFBr and BaFBr:Eu2+ are 65.6 and 62.3 nm, respectively.
3.2 Morphology of the products
SEM and TEM were used to characterize the morphology and microstructure of the products. Fig. 2 illustrates the SEM images of the BaFBr:Eu2+ sample prepared under 25 °C for 3 h. From the low and high-magnification SEM images in Fig. 2 (A), it can be clearly observed that square-like BaFBr:Eu2+ micro-plates with sharp corners, edges and smooth surfaces have been fabricated. The average length is determined to be about 1.3 μm, measured on the basis of SEM images for about 500 particles (Fig. 2B). The thickness of the plates is about 0.2 μm, as determined from those plates that are perpendicular to the conducting resin in the SEM image. Moreover, the EDS result suggests that except for the element Au, which forms the coating, only Ba, F, Br and Eu elements have been detected, indicating that the as-prepared products are composed of pure phase BaFBr:Eu, as presented in Fig. 2(C). The average composition is determined for four random crystallites. The approximate surface composition extracted from the EDS analysis gives a Ba/F/Br/Eu atomic ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.925
0.925![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.953
0.953![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.012, which is closer to the theoretic stoichiometric composition. A small deviation from Ba
0.012, which is closer to the theoretic stoichiometric composition. A small deviation from Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F
F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br = 1
Br = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is due to the experimental error of EDS. The exact doping concentration of the europium was determined from ICP measurements. The actual doping concentration of europium is 0.92 mol%. The europium concentration is lower than in the initial solution, because the radius and properties of Eu2+ are different from those of Ba2+. It should be mentioned that doping a small amount of Eu2+ in the BaFBr host does not change the morphology.
1 is due to the experimental error of EDS. The exact doping concentration of the europium was determined from ICP measurements. The actual doping concentration of europium is 0.92 mol%. The europium concentration is lower than in the initial solution, because the radius and properties of Eu2+ are different from those of Ba2+. It should be mentioned that doping a small amount of Eu2+ in the BaFBr host does not change the morphology.
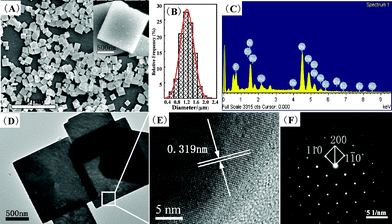 | ||
| Fig. 2 SEM images (A), the size distribution (B), EDS pattern (C), TEM (D), HRTEM images (E) and SAED pattern (F) of the square-like BaFBr:Eu2+ microplates prepared at 25 °C for 3 h. | ||
To further investigate the morphology and microstructure of the Eu-doped BaFBr microplates, TEM and HRTEM were performed as well. The results are depicted in Fig. 2(D) and 2(E). It can be seen that the product exhibits the well-defined square-like shape with a good size distribution, corresponding well with above SEM results. The spacing of the adjacent lattice planes is determined to be about 0.319 nm, which is consistent with the interplanar spacing of (110) lattice planes of tetragonal BaFBr. Furthermore, the selected area electron diffraction (SAED) pattern (Fig. 2F) exhibits a large and homogeneous regular diffraction dot array, which indicates the single crystalline feature of the synthesized BaFBr:Eu2+.
3.3 FT-IR and Raman spectra
In order to check the purity of products, FT-IR and Raman techniques were employed. Similar IR spectra were observed for square-like BaFBr and BaFBr:Eu2+ microplates (Fig. 3A). Water was found to be present in the samples, as shown by strong absorptions at 3440 cm−1 and 1630 cm−1 indicative of anti-symmetric and symmetric stretches of H–O–H bending modes.43 No distinctive C–H stretching vibration bands at about 2900 cm−1 and 1380 cm−1 can be detected, implying that CTAB can not be adsorbed on the surface of the BaFBr:Eu2+ microplates after washing process.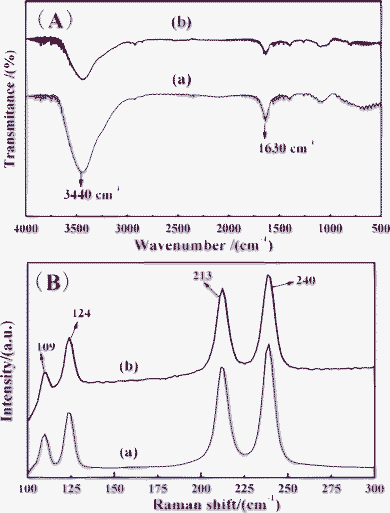 | ||
| Fig. 3 FT-IR (A) and Raman spectra (B) of the square-like (a) BaFBr, (b) BaFBr:Eu2+ microplates prepared at 25 °C for 3 h. | ||
Fig. 3(B) shows the Raman spectra of BaFBr and BaFBr:Eu2+ microplates (sample 4). It can be observed that the Raman peaks match well with the previous reports.44 BaFBr crystallizes in the matlockite structure, and possesses tetragonal P4/nmm symmetry.13 The local coordination environment of barium is characterized by C4v symmetry, where it is surrounded by four fluorine atoms and five bromine atoms (Fig. S2 in ESI†). According to group theoretical calculation, the crystalline BaFBr is composed of 12 Raman modes, as described in the following equation45
| Γ = 2Alg + Blg + 3Eg + 3A2u + 3Eu |
where A and B modes are non-degenerate and the E modes are doubly degenerate, the subscripts “g” and “u” are the parity under inversion in central symmetric crystals. There are six Raman active modes (2A1g, B1g and 3Eg), four IR active modes (2A2u and 2Eu) and two acoustic modes (A2u and Eu).44,46,47 The IR active modes are not observed, because they locate range from 100 to 400 cm−1.44 The Eg vibrations at 109 and 240 cm−1 are assigned as Ba–Br stretching modes. The bands at 213 cm−1 and 124 cm−1 are attributed to B1g and A1g symmetric vibrations.48 It is worth noting that the width of a BaFBr:Eu2+ crystal at half maximum (FWHM) is wider than that of the non-doped sample, which may be due to the following two reasons: the implantation of the heavier Eu2+ ion in the Ba2+ lattice may result in distortions in the lattice and changing the efficient mass of the oscillating atoms.49 On the other hand, Eu2+ doping refines the grain size of BaFBr crystals and increases the internal stress, which prevents lattice vibrations.50 Moreover, no other Raman modes of Eu-related compounds and other impurities were observed. It can be concluded that some microscopic structural disorders are introduced when some Ba2+ ions in the BaFBr lattices are substituted by Eu2+ ions. Above results of the XRD, EDS, HRTEM, SAED, IR and Raman investigations confirm that the products are pure phase BaFBr or BaFBr:Eu2+.
3.4 The factors affecting the formation of the products
It is well known that the phase and morphology of the products are highly correlative with the concentration of reactants, reaction time and reaction temperature. To get a better insight into the evolution process and the influence of factors on the morphology and size of products, a series of dependent experiments were carried out. The detail experimental parameters and the structure of products were summarized in Table 1 (see ESI†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, only BaFBr was obtained (to be discussed later).
2, only BaFBr was obtained (to be discussed later).
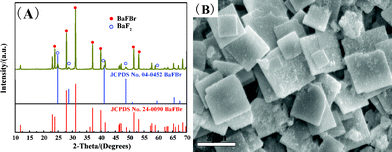 | ||
| Fig. 4 XRD pattern (A) and SEM image of sample S1. The standard data for cubic BaF2 (JPCDS Card No. 04-0452) and BaFBr (JPCDS Card No.24-0090) are also shown in the figure for comparison. | ||
 | ||
| Fig. 5 XRD patterns for the samples S2–S8. The standard data for BaFBr (JCPDS Card No. 24-0090) is also listed in the figure for comparison. | ||
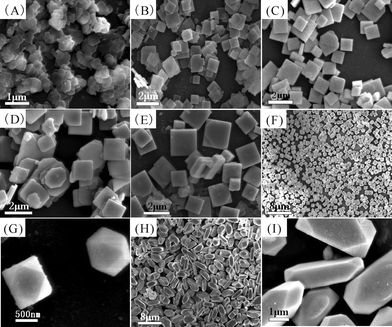 | ||
| Fig. 6 SEM and high-magnification SEM images of the samples S2 (A), S3 (B), S4 (C), S5 (D), S6 (E), S7 (F, G) and S8 (H, I). | ||
Fig. 6 presents the SEM images of samples S2–S8. The product (Sample 2) for 0.5 h primarily consists of nano-sheets, with a mean length of 0.3 μm and a thickness of 50 nm, as depicted in Fig. 6(A). After 1 h of reaction time, the length and the thickness of the square plates (Sample S3) are about 0.8 μm and 80 nm (Fig. 6B). When the reaction was performed for 3 h, the products were square-like plates (Sample 4) of 1.3 μm in length and 0.2 μm in thickness (Fig. 6C). There is no distinctive difference in the morphology compared with that of the sample subjected to 12 h of reaction time.
Temperature-dependent experiments were also performed to investigate the influence of reaction temperature on the products. The product (Sample 5) at 40 °C consisted of square microplates with a length and the thickness of about 1.4 μm and 0.4 μm (Fig. 6D). When the temperature was elevated to 60 °C, the length and thickness of square plates (Sample 6) increased to 1.7 μm and 0.5 μm (Fig. 6E). Interestingly, as the temperature was elevated to 70 °C (Sample 7), cuboctahedral BaFBr particles with the size about 1.5–2.0 μm were obtained (Fig. 6F and 6G). And the product for 80 °C possessed uniform corner cut cuboid like morphology with an average size of about 1.2 (height) × 1.2 (width) × 4.0 μm (length), as depicted in Fig. 6(H, I). Therefore, the phase, size and morphology of the products can be controlled by regulating the ratio of the reactants, reaction time and reaction temperature.
3.5 Possible formation mechanism
Throughout the whole experimental observation and analysis, a possible formation process is proposed, as illustrated in Scheme 1 (See ESI†). The typically evolution process can be described as follows. First of all, after the emulsified droplets containing NH4F was added into the BaBr2, EuBr2 emulsion, the reverse micelles colliding with each other results in the formation of a great number of transient dimmers. As the newly formed dimers were thermodynamically unstable, collisions between these dimers caused continuous coalescence, which resulted in the exchange of Ba2+, Eu2+, Br− and F− ions from one “water pool” to another. At the same time, amorphous BaFBr:Eu2+ nuclei formed rapidly at the micelles edges. As the reaction proceeded, the larger nuclei continued to grow at the consumption of the small ones, and simultaneously tended to aggregate together to minimize interfacial energy. However, the growth of nuclei may be confined within the “water pool”. Owing to the coulomb force action, the strong attractive interactions between CTAB and BaFBr grains allowed the assembly of this cationic surfactant molecules on a particular series of crystallographic surface, which restricted the lateral growth. As a result, anisotropic growth along the sides leads to the formation of tabular structures. Finally, the square-like BaFBr:Eu2+ microplates were obtained through further Ostwald ripening and a washing process. While this relatively stable emulsion system may be disrupted after increasing the temperature above 60 °C. Admittedly, the shape of the products relies on the relative growth rate of different crystallographic planes and their crystal habit. The faster growing rate along the [100], [010] and [001] directions, and crystal faces selective activated in the presence of CTAB results in the formation of cuboctahedron-like particles.51 When increasing the temperature to 80 °C, the excess CTA+ and Br− ions would preferentially adsorb on (001) planes of BaFBr crystal nuclei and inhibit their growth. After a long growth time, corner cut cuboid like BaFBr crystals were produced. Although the precise mechanism for the formation of the products is not yet fully understand, it can be can deduced from above investigations that the W/O emulsion system and the crystal habits of BaFBr are crucial factors. The detailed mechanism needs need to be further investigated in our future work.3.6 Fluorescence properties
During our PL measurements process, the square-like BaFBr:Eu2+ microplates showed too weak luminescence to be detected so we attempted to improve the emission by thermal processing. Annealing the sample for 0.5 h at 300 °C under reduced atmospheres leads to strong luminescence. Note that this heat treatment alters neither the phase nor the shape of the final products (see Fig. S3 in ESI†). Sample 4 was taken as a typical sample for detailed investigation. The PL excitation and emission spectra of the annealed square-like BaFBr:Eu2+ microplates are illustrated in Fig. 7(A). The excitation spectrum exhibits one broad peak at 280 nm with a shoulder at 270 nm. The band observed at 270 nm is assigned to the 8S7/2 → 7FJ (J = 0–6) transition. The band at 280 nm was expected due to the 8S7/2 → 4f65d1 (eg) transition of Eu2+. A single strong emission band with maximum at about 390 nm was observed. This band is associated with the 4f65d1 (t2g) → 8S7/2 transition of the Eu2+ activator ion. These results are basically in accordance with the literature.52 This single narrow band of the 4f65d1→ 8S7/2 emission can be understood by the simplified energy level scheme (inset in Fig. 7A). While the square-like BaFBr:Eu2+ microplates are irradiated by 280 nm UV lights, the Eu2+ ions are excited from the 4f7 ground state to the excited 4f65d1 levels, then relaxed to 6P7/2 and further relaxed to the lower 4f65d1 levels by nonradiative relaxation processes. The emission about 450–490 nm ascribed to the oxygen-vacancy centers can be generally observed in the emission spectra of europium doped BaFX (X = Cl, Br, I) crystals, but it can not appear in present phosphors even excited with 250 nm UV lights.36,53It can be concluded that trace or even no oxygen was incorporated into the lattices of BaFBr microplates.54 In addition, the color coordinates of the square-like BaFBr:Eu2+ microplates is calculated to be (0.30, 0.26), which indicates that the emission color of the BaFBr:Eu2+ phosphors is located in the purple blue light region (see Fig. S4 in ESI†). | ||
| Fig. 7 (A) PL excitation and emission spectra of the annealed square-like BaFBr:Eu2+ (0.92 mol%) microplates (sample 4). (B) The luminescence decay curve for the emission 390 nm. Inset shows the energy level diagram of Eu2+ ion as a function of crystal field. | ||
To disclose the crystal field environment of Eu2+ and energy transfer in BaFBr:Eu2+ microplates, the kinetic properties for the luminescence of the BaFBr:Eu2+(0.92 mol%) microplates were investigated by PL decay curves. The typical decay curve for the luminescence of Eu2+ ions (monitored by 4f65d1 → 4f7) in the present BaFBr:Eu2+ phosphors is shown in Fig. 7(B). The decay curves can be well fitted into a single-exponential function as I = I0 exp (−t/τ), in which I0 is the initial emission intensity at t = 0 and τ is the 1/e lifetime of the emission center respectively. The average lifetime for the Eu2+ is determined to be 600 ns, which is shorter than that of that of bulk materials.29 This is mainly due to the increase of the non-radiative transition rate caused by the much greater number of surface defects in the square-like BaFBr:Eu2+ microplates. Also, more electrons will be closely trapped on the exposed (001) faces which possessed the largest surface area. And this results in an increase of the trapping rate of charge carriers at grain boundaries and dislocations. More importantly, the faster decay in BaFBr:Eu2+ microplates shows promise for improving the imaging performance for IPs.18
The doping concentration of the activator plays an important role in the performance of luminescent materials. Therefore, in order to achieve the excellent luminescent performance, the optimal Eu2+ doping concentration for obtaining maximum luminescent intensity is determined by varying the concentration of the Eu2+ ion. From the PL spectra in Fig. 8, it can be observed that the peak positions of excitation and emission does not vary as Eu2+ concentrations changed. It suggests that crystal field around Eu2+ does not alter with the Eu2+ concentration. However, the emission intensity of the transitions of 4f65d1 → 4f7 of Eu2+ obviously changed. The inset in Fig. 8 shows the dependence of the emission integrated area of 4f65d1→ 8S7/2 on Eu2+ concentration. The intensity increases gradually and reaches the maximum at about 0.92 mol%, and then begins to decrease as the Eu2+ concentration increases to 1.34 mol%. The decrease in emission intensity is due to the concentration quenching effect caused by the cross-relaxation process between the Eu2+. The effect of doping concentrations on luminescence intensities is similar to previous literature.43,55–57 This fact demonstrates that the optimum doped concentration for Eu2+ is about 0.92 mol%. A more detailed investigation of the luminescent properties of the products with different morphologies and dimensions will be further studied in our future work.
 | ||
| Fig. 8 Dependence of PL spectra on Eu2+-doped concentration for (a) 3.02, (b) 0.13, (c) 0.45, (d) 0.76, (e) 1.34, (f) 0.92 mol%. The inset shows the relative integrated area of the emission spectra as a function of doping concentration in square-like BaFBr microplates host. | ||
4. Conclusions
In conclusion, square-like BaFBr:Eu2+ microplates with a narrow size distribution have been successfully synthesized via a simple W/O emulsion route. The length and thickness of the microplates can be tuned by adjusting the reaction parameters. The reaction time and temperature-dependent experiments allow us to propose a tentative evolution mechanism, which involves the function of the emulsion system, the growth habits of BaFBr crystal and the sequential Ostwald ripening process. Furthermore, cuboctahedron and corner cut cuboid-like BaFBr:Eu2+ particles could be obtained when the reaction temperature is increased above 60 °C. The resultant uniform square-like BaFBr:Eu2+ microplates annealed at 300 °C for 0.5 h under reduced atmosphere exhibit strong purple-blue emission due to the transitions of 4f65d1 → 4f7 of Eu2+. The luminescence intensities vary with Eu-doped concentration. The doping concentration for Eu2+ has been optimized to be about 0.92 mol% to Ba2+. The average lifetime of the emission at 390 nm is determined to be about 0.6 μs. The optical behaviors of these uniform square-like BaFBr:Eu2+ microplates make them good candidate for the applications in luminescence. This facile method offers new opportunities to prepare other functional materials with well-defined morphologies and structures.Acknowledgements
This work was financially supported by Laboratory of Special Photographic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences (CAS).References
- J. Xiong, G. Cheng, G. Li, F. Qin and R. Chen, RSC Advances, 2011, 1, 1542 RSC.
- Y. Ding, J. Gu, J. Ke, Y.-W. Zhang and C.-H. Yan, Angew. Chem. Int. Ed., 2011, 50, 12330 CrossRef CAS.
- B. T. Zhu, Z. Wang, S. Ding, J. S. Chen and X. W. Lou, RSC Advances, 2011, 1, 397 RSC.
- Y. G. Sun and Y. N. Xia, Science, 2002, 298, 2176 CrossRef CAS.
- F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat Mater, 2011, 10, 968 CrossRef CAS.
- J.-C. G. Bunzli, Chem. Rev., 2010, 110, 2729 CrossRef.
- J. Jiang, K. Zhao, X. Xiao and L. Zhang, J. Am. Chem. Soc., 2012, 134, 4473 CrossRef CAS.
- J. W. Hong, S.-U. Lee, Y. W. Lee and S. W. Han, J. Am. Chem. Soc., 2012, 134, 4565 CrossRef CAS.
- Y. C. Wu, Y. C. Chen, D. Y. Wang, C. S. Lee, C. C. Sun and T. M. Chen, J. Mater. Chem., 2011, 21, 15163 RSC.
- L. Zhou, Z. Gu, X. Liu, W. Yin, G. Tian, L. Yan, S. Jin, W. Ren, G. Xing, W. Li, X. Chang, Z. Hu and Y. Zhao, J. Mater. Chem., 2012, 22, 966 RSC.
- F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, Nature, 2010, 463, 1061 CrossRef CAS.
- X. Zhai, M. Yu, Z. Cheng, Z. Hou, P. a. Ma, D. Yang, X. Kang, Y. Dai, D. Wang and J. Lin, Dalton Trans., 2011, 40, 12818 RSC.
- H. P. Beck, Z. Anorg. Allg. Chem., 1979, 451, 73 CrossRef CAS.
- P. Comodi and P. F. Zanazzi, J. Appl. Crystallogr., 1993, 26, 843 CrossRef CAS.
- Y. R. Shen, T. Gregorian and W. B. Holzapfel, High Pressure Res., 1991, 7, 73 CrossRef.
- Y. R. Shen and W. B. Holzapfel, Phys. Rev. B, 1995, 51, 15752 CrossRef CAS.
- W. Chen, J. Nanosci. Nanotechnol., 2008, 8, 1019 CrossRef CAS.
- Y. Amemiya and J. Miyahara, Nature, 1988, 336, 89 CrossRef CAS.
- H. Vonseggern, A. Meijerink, T. Voigt and A. Winnacker, J. Appl. Phys., 1989, 66, 4418 CrossRef CAS.
- M. Thoms, H. Vonseggern and A. Winnacker, J. Appl. Phys., 1994, 76, 1800 CrossRef CAS.
- U. Rogulis, S. Schweizer, S. Assmann and J. M. Spaeth, J. Appl. Phys., 2000, 87, 207 CrossRef CAS.
- T. Pawlik and J. M. Spaeth, J. Appl. Phys., 1997, 82, 4236 CrossRef CAS.
- J. Qiu and K. Hirao, Solid State Commun., 1998, 106, 795 CrossRef CAS.
- Z. L. Pei, Y. S. Wang, D. W. He and X. G. Meng, J. Rare Earths, 2009, 27, 338 CrossRef.
- A. Edgar, G. V. M. Williams, S. Schweizer and J. M. Spaeth, Current Applied Physics, 2006, 6, 399 CrossRef.
- S. Schweizer, L. W. Hobbs, M. Secu, J. M. Spaeth, A. Edgar and G. V. M. Williams, Appl. Phys. Lett., 2003, 83, 449 CrossRef CAS.
- A. Edgar, G. V. M. Williams, P. K. D. Sagar, M. Secu, S. Schweizer, J. M. Spaeth, X. Hu, P. J. Newman and D. R. Macfarlane, J. Non-Cryst. Solids, 2003, 326, 489 CrossRef.
- H. von Seggern, Braz. J. Phys., 1999, 29, 254 CrossRef CAS.
- J. M. Spaeth, Radiat. Measur., 2001, 33, 527 CrossRef CAS.
- W. Chen, S. P. Wang, S. L. Westcott, J. Zhang, K. Dou, A. G. Joly and D. E. McCready, J. Appl. Phys., 2005, 97, 83506 CrossRef.
- X. G. Meng, Y. S. Wang, H. Jin and L. Sun, J. Lumin., 2007, 122, 385 CrossRef.
- S. Hesse and H. von Seggern, J. Phys. D: Appl. Phys., 2004, 37, 836 CrossRef CAS.
- M. Kaszuba, J. Nanopart. Res., 1999, 1, 405 CrossRef CAS.
- H. Li, P. Hackenschmied, E. Epelbaum and M. Batentschuk, Mater. Sci. Eng., B, 2002, 94, 32 CrossRef.
- H. von Seggern, S. Hesse, J. Zimmermann, G. A. Appleby, X. G. Meng, C. Fasel and R. Riedel, Radiat. Measur., 2010, 45, 478 CrossRef CAS.
- S. Hesse, J. Zimmermann, H. von Seggern, X. Meng, C. Fasel and R. Riedel, J. Appl. Phys., 2009, 105, 73511 CrossRef.
- M. H. Kostova, M. Batentschuk, F. Goetz-Neunhoeffer, S. Gruber, A. Winnacker, P. Greil and C. Zollfrank, Mater. Chem. Phys., 2010, 123, 166 CrossRef CAS.
- A. K. Ganguli, A. Ganguly and S. Vaidya, Chem. Soc. Rev., 2010, 39, 474 RSC.
- Y. P. Guo, T. S. Lin, Y. Zhou, D. C. Jia and Y. J. Guo, Microporous Mesoporous Mater., 2010, 127, 245 CrossRef CAS.
- C. J. Yang, D. Y. Li, Z. C. Guan, W. H. Zhang, X. Zhou, W. Y. Zhang, Z. X. Zhuang and X. R. Wang, ACS Appl. Mater. Interfaces, 2010, 2, 2711 Search PubMed.
- G. A. Kumar, C. W. Chen, R. Riman, S. Chen, D. Smith and J. Ballato, Appl. Phys. Lett., 2005, 86 Search PubMed.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65 CrossRef CAS.
- C. Yang, P. P. Yang, W. X. Wang, J. Wang, M. L. Zhang and J. Lin, J. Colloid Interface Sci., 2008, 328, 203 CrossRef CAS.
- M. Sieskind, M. Ayadi and G. Zachmann, Phys. Status Solid B., 1986, 136, 489 CrossRef CAS.
- V. D'Anna, L. M. L. Daku, H. Hagemann and F. Kubel, Phys. Rev. B, 2010, 82, 24108 CrossRef.
- B. Sundarakanan, T. R. Ravindran, R. Kesavamoorthy and S. V. M. Satyanarayana, Solid State Commun., 2002, 124, 385 CrossRef.
- M. Merawa, Y. Noel, B. Civalleri, R. Brown and R. Dovesi, J. Phys. Condens. Matter, 2005, 17, 535 CrossRef CAS.
- J. F. Scott, J. Chem. Phys., 1968, 49, 2766 CrossRef CAS.
- T. T. Basiev, A. A. Sobol, Y. K. Voronko and P. G. Zverev, Opt. Mater., 2000, 15, 205 CrossRef CAS.
- C. H. Yang, Z. Q. Ma, F. Xu, L. Zhao, F. Li and B. He, Acta Phys. Sinica, 2010, 59, 6549 CAS.
- J. D. H. Donnay and D. Harker, Am. Mineral., 1937, 22, 446 CAS.
- V. R. Kumar, K. V. Narasimhulu, N. O. Gopal, J. L. Rao and R. P. S. Chakradhar, Physica B., 2004, 348, 446 CrossRef CAS.
- A. R. Lakshmanan, N. Murase, T. Yazawa, J. Qiu, T. Mitsuyu, K. Hirao, A. Tomita and W. Hoffmann, Radiat. Measur., 2001, 33, 119 CrossRef CAS.
- R. S. Eachus, R. H. D. Nuttall, M. T. Olm, W. G. Mcdugle, F. K. Koschnick, T. Hangleiter and J. M. Spaeth, Phys. Rev. B., 1995, 52, 3941 CrossRef CAS.
- P. Hackenschmied, G. Schierning, M. Batentschuk and A. Winnacker, J. Appl. Phys., 2003, 93, 5109 CrossRef CAS.
- J. Liao, B. Qiu, H. Wen, W. You and Y. Xiao, J. Lumin., 2010, 130, 762 CrossRef CAS.
- Z. L. Wang, Z. W. Quan, P. Y. Jia, C. K. Lin, Y. Luo, Y. Chen, J. Fang, W. Zhou, C. J. O'Connor and J. Lin, Chem. Mater., 2006, 18, 2030 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra00047d |
| This journal is © The Royal Society of Chemistry 2012 |
