Phase selective carbohydrate gelator†‡
Somnath
Mukherjee
* and
Balaram
Mukhopadhyay
*
Department of Chemical Sciences, Indian Institute of Science Education and Research-Kolkata, Mohanpur, P.O. BCKV Campus Main Office, Nadia 741252, India. E-mail: carbo.som@gmail.com; sugarnet73@hotmail.com; Fax: +91 33 25873020; Tel: +91 9800278237
First published on 6th January 2012
Abstract
A simple eco-friendly carbohydrate gelator for phase selective gelation of oils from oil–water mixtures at room temperature, dye removal for water purification and X-ray crystal structure in the monohydrate form.
Low molecular weight organogelators (LMOGs) are emerging as attractive materials for cosmetics,1 sensors,2 template materials,3drug delivery agents,4 biomedical applications,5 phase selective gelation and dye-removal,6dye sensitized solar cells7 and soft optical devices.8 LMOGs are small organic compounds (Mw < 1000) that can immobilize organic solvents and have the ability to create 3D network through self-assembly of the monomers to higher order aggregates like fibrous, helical or tubular.9 Various non-covalent interactions like hydrogen-bonding, dipole–dipole, π–π staking, electrostatic and van der Waals forces are responsible for the supramolecular architectures.10
Among the LMOGs, those capable of gelating one solvent preferentially over the other in a given two-phase mixture are termed as phase selective organogelators (PSOGs). These small molecules self-assemble into fibers in liquid and form networks above the minimum gelation concentration (MGC) and thus convert the liquid to a gel. Despite a large number of LMOGs reported in the literature, PSOGs are still rare. Phase selective gelation was first demonstrated by Bhattacharya et al.6a with amino acid amphiphiles. PSOGs are becoming extremely relevant for water purification and the recovery of oil from water, especially when the World has seen several devastations of the marine ecology due the spillage of oil in recent times and the existing materials proved to be incapable of handling the situation well.11
Owing to their eco-friendly behaviour, carbohydrates are attractive scaffolds for designing LMOGs. With the development of modern synthetic techniques to handle carbohydrate structures, sugar derived LMOGs have gained considerable interest in recent years.12 Recently, John and co-workers13 have reported sugar-derived gelators as model oil-solidifiers. In this communication, we report a galactose-based organogelator capable of gelating a series of organic liquids, edible oils and fuel oils. Moreover, this organogelator exhibits phase selective gelation and it can gel (solidify) various fuel oils from an oil–water mixture at room temperature. After the phase selective gelation of the oil from the mixture, both oil and gelator can be recovered and reused.
Although the synthesis of the derivative is reported by Du et al.,14 the experimental protocol has been modified considerably to make it suitable for large scale preparation. In this case, the target derivative was prepared from known p-methoxyphenyl galactoside (1)15 instead of the corresponding thioglycoside as used by Du et al. Target compound 2 was obtained in 70% yield over three steps (Fig. 1a). However, chromatographic purification was carried out only in the final step (see ESI for details†).
 | ||
| Fig. 1 a) Chemical synthesis of the target galactoside amphiphile 2. b) DIC image of the diesel gel of 2 (1.2% w/v). c) TEM image of the toluene gel (0.8% w/v) of 2. d) SEM image of the mesitylene gel (2% w/v) and e) SEM image of the p-xylene gel (1.5% w/v). | ||
The galactoside amphiphile 2 was found to be an effective gelator of various organic liquids, diesel, kerosene, petrol and edible oil. The gelation capabilities of compound 2 are summarized in Table 1. The minimum gelation concentration (MGC) values in % w/v ranges from 1.0 to 5.0 in different oils and organic liquids. It is worth noting that compound 2 forms gel with diesel with a MGC of 1.2% w/v. The gelation processes were found to be thermoreversible i.e. they can be converted to solution above a certain temperature (Tgel) and be reformed upon cooling. The gelation abilities remain unaltered even after several repetitions of the sol–gel cycle proving the total thermoreversible nature of the gels. The Tgel value has increased with increasing concentration of the gelator, which indicates the stability enhancement of the gel at higher gelator concentrations (Fig. S2, ESI†). All gels were stable at room temperature and no noticeable change was observed when stored in a closed container for 2 months, pointing towards their temporal stability.
| Liquid or oil | MGC (% w/v) | Liquid or oil | MGC (% w/v) |
|---|---|---|---|
a Nature of the gels is in the parenthesis: TG = transparent gel; OG = opaque gel; S = soluble; P = precipitate; I = insoluble.
b Mixture of aliphatic (pentane, hexane, heptane) and aromatic (benzene, toluene, xylene) hydrocarbons in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. 1 ratio.
|
|||
| Petrol | 1.1 (OG) | Benzene | 2.0 (OG) |
| Diesel | 1.2 (OG) | Mesitylene | 2.0 (OG) |
| Kerosin | 1.1 (OG) | p-Xylene | 1.5 (OG) |
| Olive oil | 3.1 (OG) | o-Xylene | 2.5 (OG) |
| Coconut oil | 2.7 (OG) | m-Xylene | 2.0 (OG) |
| Sunflower oil | 1.5 (OG) | Carbon tetrachloride | 1.6 (OG) |
| Soyabean oil | 3.0 (OG) | Bromobenzene | 4.0 (TG) |
| Mineral oil | 2.2 (OG) | Chlorobenzene | 3.7 (OG) |
| Tetrahydrofuran | S | 1,2-Dichlorobenzene | 5.0 (OG) |
| 1,4 Dioxan | S | Water | I |
| Methanol | P | Hexadecane | 2.4 (OG) |
| Isopropanol | S | Dichloromethane | S |
| Ethyl acetate | S | Hexane | P |
| Toluene | 1.0 (TG) | Mixture of hydrocarbonsb | 2.4 (OG) |
Rheological analyses were done to study the strength of the organogels. Fig. 2a shows the variation of the storage modulus (G′) and the loss modulus (G′′) with frequency keeping the strain constant in the case of the diesel gel of amphiphile 2. The G′ is always higher than the G′′ and both remain almost constant over the frequency range, showing typical viscoelastic behaviour and indicating good tolerance performance to the external forces. Fig. 2b shows a variation of G′ and G′′ with shear stress at a constant frequency of 1 Hz and G′ is greater than the G′′ by more than one order of magnitude, indicating a dominant elastic character of the sample (Fig. 2). Both G′ and G′′ remain almost constant bellow their yield stress value (upper limit of linear regime above which the gel starts to flow). Similar rheological experiments were done with toluene and mesitylene gels (Fig. S5, ESI†). Yield stress values for diesel (3% w/v), toluene (2% w/v) and mesitylene (2% w/v) gels are 133 Pa, 1683 Pa and 407 Pa respectively, and those are high enough to bear its own weight in an inverted vial.
 | ||
| Fig. 2 Dynamic rheology of diesel gel (3% w/v): (a) frequency sweep, (b) stress sweep. | ||
Microscopic images (SEM, AFM, TEM, DIC) showed cross-linked fibrous morphologies of the gelator in the gels with different organic liquids and oils (Fig. 1b–e and ESI†). XRD studies revealed a layered structure in the gel state with an interlayer distance which is close to the calculated molecular length of the gelator. FT-IR and 1H NMR spectroscopic studies revealed the presence of intermolecular hydrogen bonding (Figs. S7 and S8, ESI†). Upon benzoylation of the two free hydroxyl groups of compound 2, the derivative loses its gelation ability with oils, showing the importance of intermolecular hydrogen bonding for the formation of the supramolecular architecture. UV-Vis study indicates that the CH–π or π–π stacking might be playing some role in the aggregation of the gelator (Fig. S9, ESI†). CD spectroscopy showed that observed CD signal in the gel state comes from the chirality of the supramolecular assembly, not from the molecule itself (Fig. S10, ESI†)
Next, we investigated the ability of compound 2 to gel oils selectively in the presence of water. Interestingly, the gelator 2 was found to be appropriate for selective gelation of various organic liquids, edible oils and fuel oils (diesel, kerosene, petrol, etc.) from the corresponding mixtures in water. In a typical procedure, compound 2 (10 mg) was added in a mixture of water (2 mL) and toluene (0.5 mL) in a vial and solubilized by heating. When the vial was cooled to room temperature, the toluene layer was found to be gelled leaving the water layer intact. However, the requirement of heating the phase-selective gelation limits the real life application of the gel, e.g. oil spill recovery. Therefore, phase-selective gelation of diesel in the presence of water at room temperature was studied. A concentrated solution of gelator 2 (25 mg) in tetrahydrofuran (100 μL) was injected by a syringe in the interface of a mixture of saline water (2 mL) and diesel (0.5 mL). Instantaneous gelation of the diesel layer was observed and after 45 min, when the vial was inverted, the diesel gel was strong enough to allow its own weight plus the weight of the water (Fig. 3a–c). Considering the real situation of oil spill, we have tested the phase-selective gelation of a thin layer of diesel floating on a huge amount of water taken in a Petri dish. When aliquots of the gelator 2 in THF was added to the diesel–water mixture, gelation of the diesel layer was observed and after 10 min it was strong enough so that it can be scooped out by a spatula from which diesel can be recovered by vacuum distillation and the gelator can be reused (Fig. 3d–f). Similar phase-selective gelations were observed with kerosene, hexadecane and a mixture of hydrocarbons (aromatic and aliphatic) indicating the potential application in the real oil spill scenario. It is worth noting, that the different oil–water ratios, the nature of the aqueous solutions (saturated NaCl, CuSO4, saturated CaCl2, KMnO4) and small impurities in the gelator did not influence the phase-selective gelation.
 | ||
| Fig. 3 Phase-selective gelation from a two phase system at room temperature: (a) mixture of 2 mL water and 0.5 mL diesel (b) selective gelation of the diesel layer (diesel gel floating on water) after addition of the solution of galactoside 2 (c) gel holding the water shown in inverted vial after 45 min (d) thin layer of diesel floating on large pool of water (in a Petri dish) (e) instantaneous selective gelation of diesel layer after addition of the solution of the galactoside 2 (f) diesel gel scooped out with a spatula. | ||
Removal of toxic dyes and other pollutants from contaminated water is an emerging field of research for obvious environmental reasons. Interestingly, the galactoside 2 was found to remove crystal violet efficiently (∼97%) from its aqueous solution in two different ways, a) adsorption of the dye by the xerogel of the galactoside 2 in toluene (Fig. 4a,b) and b) via phase-selective gelation (Fig. 4c,d) (see ESI for more details†). The efficient removal of crystal violet by the galactoside 2 can be suitably exploited in water purification.
 | ||
| Fig. 4 (a) 0.03 mM aqueous solution of CV before the addition of xerogel, (b) clear aqueous solution after the adsorption of dye by the xerogel of galactoside 2 after ∼24 h. (c) 0.5 mL toluene was added to 2 mL of aqueous solution of CV (d) removal of CV via phase-selective gelation method. | ||
Despite the significant progress in the field of LMOGs, designing a gelator is still a major challenge. It is advantageous if one can correlate between the single crystal structure and gelling/non-gelling behaviour for finding a promising gelator molecule.16 Interestingly, the galactoside 2 was crystallized as a monohydrate from hexane/ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture (Fig. 5a). Detailed crystallographic data and the key bond distances and angles are listed in Table S2 and S3 respectively (ESI†). X-Ray crystallography indicates that the pyranose ring of the gelator remains in the typical 4C1 conformation and the water molecule plays a very crucial role for the crystalline character of the galactoside 2 through the intermolecular hydrogen bond network. Fig. 5b shows the 1D hydrogen bonding network in which a tetrameric structure is formed by two water molecules and two gelator molecules (marked as W1, A, W2, B) via intermolecular (O–H⋯O) hydrogen bonds. The tetrameric structure is further extended or linked to the adjacent structure via intermolecular hydrogen bonds (O–H⋯O) and in such a way; a double stranded columnar type of packing arrangement is formed (Fig. 5, viewed down a-axis). Besides intermolecular O–H⋯O bonding, intramolecular C–H⋯O hydrogen bonding and aromatic π–π interaction (3.369 Å) between the π rings of two tetrameric structures are also evident.
1) mixture (Fig. 5a). Detailed crystallographic data and the key bond distances and angles are listed in Table S2 and S3 respectively (ESI†). X-Ray crystallography indicates that the pyranose ring of the gelator remains in the typical 4C1 conformation and the water molecule plays a very crucial role for the crystalline character of the galactoside 2 through the intermolecular hydrogen bond network. Fig. 5b shows the 1D hydrogen bonding network in which a tetrameric structure is formed by two water molecules and two gelator molecules (marked as W1, A, W2, B) via intermolecular (O–H⋯O) hydrogen bonds. The tetrameric structure is further extended or linked to the adjacent structure via intermolecular hydrogen bonds (O–H⋯O) and in such a way; a double stranded columnar type of packing arrangement is formed (Fig. 5, viewed down a-axis). Besides intermolecular O–H⋯O bonding, intramolecular C–H⋯O hydrogen bonding and aromatic π–π interaction (3.369 Å) between the π rings of two tetrameric structures are also evident.
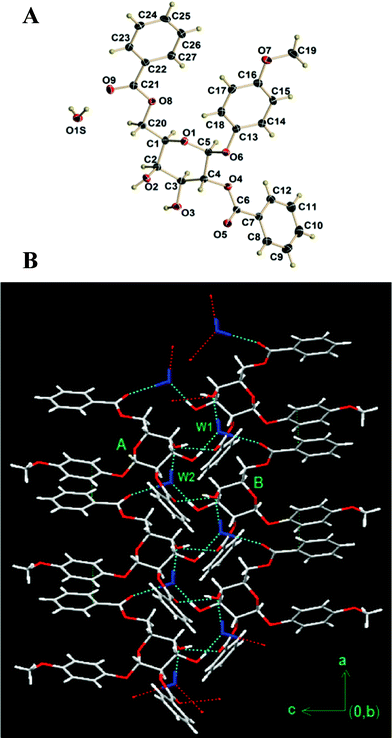 | ||
| Fig. 5 (a) ORTEP drawing of monohydrated compound 2 with 50% probability ellipsoids, showing the atomic numbering scheme. (b) A packing view along b direction. | ||
Conclusions
In summary, we have developed a new sugar derived thermoreversible organogelator that is able to gel various organic liquids and oils (commercial fuels and edible oils). It showed phase-selective gelation behaviour and interestingly, it was observed that the gelator 2 can gel (solidify) oils selectively from the biphasic mixtures of oil–water at room temperature. Both oil and gelator can be recovered through vacuum distillation and the gelator can be reused multiple times. Gelator 2 is definitely attractive because it is simple, eco-friendly and can be prepared easily in large scale. Strikingly, the gelator was found to remove crystal violet efficiently from its aqueous solution, indicating its significant role in water purification. Further more, the gelator crystallizes in its monohydrate form. Further studies on the correlation between the crystal structure and gelling behaviour of 2 are in progress for designing a smarter gelator scaffold as a more efficient oil solidifier.References
- (a) A. Wynne, M. Whitefield, A. J. Dixon and S. Anderson, J. Dermatol. Treat., 2002, 13, 61 CrossRef CAS.
- (a) K. Murata, M. Aoki, T. Nishi, A. Ikeda and S. Shinkai, J. Chem. Soc., Chem. Commun., 1991, 1715 RSC; (b) J. J. D. de Jong, L. N. Lucas, R. M. Kellogg, J. H. van Esch and B. L. Feringa, Science, 2004, 304, 278 CrossRef CAS.
- (a) K. J. C. van Bommel, A. Friggeri and S. Shinkai, Angew. Chem., Int. Ed., 2003, 42, 980 CrossRef CAS; (b) Y. Zhou, Q. Ji, M. Masuda, S. Kamiya and T. Shimizu, Chem. Mater., 2006, 18, 403–406 CrossRef CAS; (c) J. H. Jung and S. Shinkai, Topics Curr. Chem., 2004, 248, 223–260 Search PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869 CrossRef CAS.
- (a) K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869 CrossRef CAS; (b) Z. Yang, G. Liang, L. Wang and B. Xu, J. Am. Chem. Soc., 2006, 128, 3038 CrossRef CAS.
- (a) S. Bhattacharya and Y. Krishnan-Ghosh, Chem. Commun., 2001, 185–186 RSC; (b) M. Freemantle, Chem. Eng. News, 2001, 79, 12 Search PubMed; (c) S. Bhattacharya and A. Pal, J. Phys. Chem. B, 2008, 112, 4918–4927 CrossRef CAS; (d) S. Debnath, A. Shome, S. Dutta and P. K. Das, Chem.–Eur. J., 2008, 14, 6870–6881 CrossRef CAS; (e) D. R. Trivedi, A. Ballabh and P. Dastidar, Chem. Mater., 2003, 15, 3971–3973 CrossRef CAS; (f) D. Khatua and J. Dey, Langmuir, 2005, 21, 109–114 CrossRef CAS; (g) M. Xue, D. Gao, K. Liu, J. Peng and Y. Fang, Tetrahedron, 2009, 65, 3369–3377 CrossRef CAS; (h) P. Dastidar, Chem. Soc. Rev., 2008, 37, 2699–2715 RSC; (i) S. Ray, A. K. Das and A. Banerjee, Chem. Mater., 2007, 19, 1633–1639 CrossRef CAS.
- W. Kubo, T. Kitamura, K. Hanabusa, Y. Wada and S. Yanagida, Chem. Commun., 2002, 374 RSC.
- (a) A. Ajayaghosh, V. K. Praveen and C. Vijayakumar, Chem. Soc. Rev., 2008, 37, 109–122 RSC; (b) P. Xue, R. Lu, G. Chen, Y. Zhang, H. Nomoto, M. Takafuji and H. Ihara, Chem.–Eur. J., 2007, 13, 8231–8239 CrossRef CAS; (c) A. Vidyasagar, K. Handore and K. M. Sureshan, Angew. Chem., Int. Ed., 2011, 50, 8021–8024 CrossRef CAS.
- (a) P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133 CrossRef CAS; (b) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC; (c) O. Grownwald and S. Shinkai, Curr. Opin. Colloid Interface Sci., 2002, 7, 148 CrossRef; (d) M. Suzuki, T. Sato, A. Kurose, H. Shirai and K. Hanabusa, Tetrahedron Lett., 2005, 46, 2741–2745 CrossRef CAS; (e) M. Suzuki, S. Owa, M. Kimura, A. Kurose, H. Shiraib and K. Hanabusa, Tetrahedron Lett., 2005, 46, 303–306 CrossRef CAS.
- (a) M. Suzuki, M. Nanbu, M. Yumoto, H. Shiraib and K. Hanabusa, New J. Chem., 2005, 29, 1439–1444 RSC; (b) H. Yang, T. Yi, Z. Zhou, J. Wu, M. Xu, F. Li and C. Huang, Langmuir, 2007, 23, 8224–8230 CrossRef CAS; (c) M. Suzuki, T. Nigawara, M. Yumoto, M. Kimura, H. Shiraib and K. Hanabusa, Org. Biomol. Chem., 2003, 1, 4124–4131 RSC; (d) N. Mohmeyer and H. W. Schmidt, Chem.–Eur. J., 2007, 13, 4499–4509 CrossRef CAS.
- (a) http://www.nytimes.com/2010/05/03/us/03spill.html. ; (b) M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, J. Porous Mater., 2003, 10, 159–170 CrossRef CAS; (c) R. R. Lessard and G. Demarco, Spill Sci. Technol. Bull., 2000, 6, 59–68 CrossRef CAS; (d) L. Guterman, Science, 2009, 323, 1558–1559 CrossRef CAS; (e) W. L. Stanley, A. G. Pittman, US patent, 1975, 3869385 Search PubMed; (f) J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS.
- O. Grownwald and S. Shinkai, Chem.–Eur. J., 2001, 7, 4328 CrossRef.
- S. R. Jadhav, P. K. Vemula, R. Kumar, S. R. Raghavan and G. John, Angew. Chem., Int. Ed., 2010, 49, 7695–7698 CrossRef CAS.
- G. Gu, Y. Du and J. Pan, Carbohydr. Res., 2002, 337, 1313–1317 CrossRef CAS.
- Z. Zhang and G. Magnusson, J. Org. Chem., 1996, 61, 2383–2393 CrossRef CAS.
- (a) R. Luboradzki, O. Gronwald, M. Ikeda, S. Shinkai and D. N. Reinhoudt, Tetrahedron, 2000, 56, 9595 CrossRef CAS; (b) S. -I. Tamaru, R. Luboradzki and S. Shinkai, Chem. Lett., 2001, 336 CrossRef CAS; (c) P. Dastidar, Chem. Soc. Rev., 2008, 37, 2699–2715 RSC.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Details of the gel preparation, and gel characterization (Tgel, optical microscopy, FE-SEM, TEM, AFM, PXRD, IR, UV-Vis, NMR, CD and rheology) and single crystal X-ray diffraction of the monohydrated compound 2 are included. See DOI: 10.1039/c2ra00036a/ |
| ‡ Dedicated to Professor Richard R. Schmidt in the occasion of his 80th birthday. This work is funded by DST, New Delhi (India) through grant SR/S1/OC-67/2009. SM is thankful to CSIR, New Delhi (India) for the Senior Research Fellowship. |
| This journal is © The Royal Society of Chemistry 2012 |
