Highly fluorescent and photostable organic- and water-soluble CdSe/ZnS core-shell quantum dots capped with thiols†
Jordi
Aguilera-Sigalat
a,
Simon
Rocton
a,
Juan F.
Sánchez-Royo
b,
Raquel E.
Galian
*ac and
Julia
Pérez-Prieto
*a
aMolecular Science Institute (ICMOL), University of Valencia, Catedrático José Beltrán 2, 46980 Paterna, Valencia, Spain. Fax: 34-963543576; Tel: 34-963543050E-mail: julia.perez@uv.es
bMaterials Science Institute, University of Valencia, Dr. Moliner 50, 46100 Burjassot, Valencia, Spain
cDepartment of Analytical Chemistry, ICMOL/Research Building, University of Valencia, Dr. Moliner 50, 46100 Burjassot, Valencia. Fax: 34-963543273; Tel: 34-963544307E-mail: raquel.galian@uv.es
First published on 22nd December 2011
Abstract
Highly fluorescent organic- and water-soluble CdSe/ZnS core-shell quantum dots (QDs) with thiol ligands chemisorbed on the QD surface were synthesized by the replacement of amine ligands by alkyl thiols under very mild conditions. The QDs exhibited an even greater photostability than the initial core-shell amine capped QDs.
1. Introduction
Core-shell CdSe/ZnS quantum dots (QDs) are highly fluorescent systems compared with organic dyes which makes them of interest for biological, medical, and engineering applications1 The ZnS shell plays a crucial role in their emission properties;2 it decreases non-radiative pathways associated with the trapping of the electron or the hole generated after the absorption of light by the CdSe core. In addition, the shell enhances the chemical- and photo-stability of the QDs.CdSe/ZnS nanoparticles are further passivated with organic ligands to allow them to remain stable as colloidal solutions in organic solvent and water. Organic-soluble QDs are required for optoelectronic applications, while water-soluble QDs are needed for biological applications. Trioctylphosphine (TOP) and trioctylphosphine oxide (TOPO), as well as fatty amines, are the most common ligands used in the synthesis of QDs.2,3
Theoretical calculations have shown that thiols cannot bind to the ZnS surface.4 By contrast, functional deprotonated thiols (thiolates) have been used as a capping material to make QDs water soluble and to add functionality to the nanoparticle. Thiolate ligands can replace TOPO and amine ligands and thus bind to the surface of CdSe/ZnS QDs more strongly, but such exchange produces QDs with drastically diminished fluorescence quantum yield (ΦF of more than 50% reduction).5 This has been explained as being due to a spontaneous binding of thiolates to the ZnS surface, causing a significant alteration of the surface.4 In addition, it has been reported that QDs capped with thiolates are vulnerable to photooxidation.6
Here we report that the chemisorption of thiols to CdSe/ZnS core-shell QDs helped by the amine ligands of the QDs can even improve their emission performance. This strategy was applied to the preparation of highly fluorescent organic-and water-soluble QDs that exhibited a higher photostability than the initial amine-capped QDs.
Model heterobifunctional ligands, 11-mercaptoundecanol (MU), 11-mercaptoundecanoic acid (MUA), 3-mercaptopropionic acid (MPA), and the ester of ketoprofen with MU (KP-SH)7 were used for these studies (Scheme 1). In addition, different sized CdSe/ZnS core-shell QDs capped with long-chain primary amines were used. These QDs were homemade or were purchased from different commercial sources (Table S1, ESI†).
 | ||
| Scheme 1 Heterobifunctional ligands used in this work and schematic preparation of the organic- and water-soluble QDs. | ||
2. Experimental section
2.1 Materials
All reagents were commercially available and used as received. The 11-mercaptoundecanol (MU), 11-mercaptoundecanoic acid (MUA), and 3-mercaptopropionic acid (MPA) were purchased from Sigma-Aldrich. Core-shell QDs capped with long-chain primary amine were purchased from Evident Technologies (www.evidenttech.com) and from Ocean NanoTech (www.oceannanotech.com). Solvents for chromatography (ethyl acetate and hexane) were reagent grade and used without further purification. 1H-NMR (CDCl3) spectra were recorded on a 300 MHz spectrometer. The sample was dissolved in deuterated chloroform. Data of 1H-NMR are reported as follows: chemical shift (ppm), multiplicity [singlet (s), doublet (d), triplet (t), quartet (q) or multiplet (m)], and coupling constant (Hz).2.2 Characterization
UV-vis spectra of the samples were recorded using quartz cuvettes in a UV-visible spectrophotometer: Agilent 8453E. Steady-state fluorescence spectra were measured on a spectrofluorometer PTI, equipped with a lamp power supply (LPS-220B, motor driver (MD-5020), Brytebox PTI and working at room temperature. The Felix 32 Analysis software was used to register the data. The excitation wavelength for the emission spectra was fixed at 400 nm. Images from the QDs were obtained by high resolution tunnelling electron microscopy (HRTEM, FEI Tecnai G2 F20) at an accelerating voltage of 200 kV. Samples were prepared by dropping the colloidal solution on a Lacey Formvar/carbon-coated copper grid. The digital analysis of the HRTEM micrographs was done using digital MicrographTM 1.80.70 for GMS by Gatan. X-ray photoemission (XPS) measurements were performed in an ESCALAB 210 multianalysis system (base pressure 1.0 × 10−10 mbar) from Thermo VG Scientific available in our laboratory. Photoelectrons were excited with the Mg-Kα line. The hemispheric photoelectron analyzer worked with pass energy of 20 eV. In order to compare all spectra recorded, we selected the C 1s core level attributable to C–C or C–H bonds as a reference, whose binding energy was fixed to 284.5 eV. Infrared measurements were performed on a Fourier Transformation-Infrared Spectrometer NICOLET 5700 (Thermo Electron Corporation).2.3 Synthesis of 11-mercaptoundecyl 2-(3-benzoylphenyl)propanoate (KP-SH)
The KP-SH ligand was prepared following an esterification method described in the literature.7 In brief, ketoprofen (500 mg, 2.0 mmol) and 11-mercapto-1-undecanol (402 mg, 2.0 mmol) were dissolved in anhydride toluene (24 mL). A complex of hafnium/THF (1.8 mg) was added and the mixture was heated for 48 h under azeotropic reflux conditions to remove water through a Soxhlet thimble with 3 Å molecular sieves. In order to quench the reaction, 1 mL of water was added. The product was purified by column chromatography performed on silica gel 60 (230–400 mesh) using a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of hexane
1 mixture of hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate. The compound was obtained in a 81% yield. 1H NMR (300 MHz, CDCl3): δ 1.00–1.34 (m, 14H), 1.25 (t, J = 7.7 Hz; 1H), 1.45–1.57 (m, 7H), 2.43 (q, J = 7.1 Hz; 2H), 3.73 (q, J = 7.2 Hz; 1H), 3.99 (t, J = 6.7 Hz; 2H), 7.33–7.55 (m, 5H), 7.60 (dt, J = 6.2 Hz, J = 1.5 Hz; 1H), 7.67–7.75 (m, 3H) ppm.
ethyl acetate. The compound was obtained in a 81% yield. 1H NMR (300 MHz, CDCl3): δ 1.00–1.34 (m, 14H), 1.25 (t, J = 7.7 Hz; 1H), 1.45–1.57 (m, 7H), 2.43 (q, J = 7.1 Hz; 2H), 3.73 (q, J = 7.2 Hz; 1H), 3.99 (t, J = 6.7 Hz; 2H), 7.33–7.55 (m, 5H), 7.60 (dt, J = 6.2 Hz, J = 1.5 Hz; 1H), 7.67–7.75 (m, 3H) ppm.
2.4 Steady-state irradiations
Photochemical irradiations were carried out in a Luzchem photoreactor equipped with 10 lamps of λ > 400 nm (λmax at 420 nm) under either nitrogen or air atmosphere using toluene or water as the solvent. The samples were placed in Pyrex tube, dissolved in the adequate solvent and purged with dry nitrogen over a period of 10–15 min prior to irradiation.2.5 Synthesis of QD-CS6
Fluorescent CdSe nanocrystals were synthesized using the procedure described by Peng et al. with some modifications.8 Briefly, a mixture of 0.2 mmol of CdO, 0.7 mmol of tetradecylphosphonic acid (TDPA) , and 9.1 mmol of TOPO was heated gradually up to 320 °C in a three-neck flask under N2 flow. The reaction was maintained at this temperature for 10–15 min, until the solution was clear. Then, the reaction was cooled to 270 °C, and 1.2 mL of the SeTOP was quickly injected (SeTOP mixture was prepared by the addition of 0.3 mmol of Se and 3.4 mmol of TOP under N2 flow at 70 °C). When the reaction turned orange (indicative of a size of ca. 2.5 nm), the nanocrystals were precipitated in cool MeOH and purified several times by centrifugation with MeOH to remove the excess of starting materials. Finally, the QDs were re-dispersed in toluene.The synthesis of core-shell QDs (CdSe/ZnS) was done following M. G. Bawendi's procedure with some modifications.2 For a typical reaction, 5 g of TOPO was heated up to 190 °C in a three-neck flask for 2 h under Ar flow. Then, the solution was cooled to 60 °C and 0.5 mL of TOP was added into the flask. After that, 2 mL of CdSe QD was added and the reaction was heated to 140 °C. Then, the shell was added drop-wise for 10 min.
For the shell, 440 μL of ZnEt2, 65 μL of (TMS)2S, and 2600 μL of TOP were mixed in a glove box under N2 flow. Then, the reaction was cooled to 90 °C and kept at this temperature for 3 h. For the purification, the CdSe/ZnS QDs were precipitated in MeOH several times. Finally, QD-CS6 was redissolved in toluene.
2.6 Synthesis of QD-CS7
In order to obtain a core-shell QD covered with amine ligands (QD-CS7) a ligand exchange methodology was applied. Typically, 2 mL of QD-CS6 and 431 mg of ODA (QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand molar ratio of 1
ligand molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) were added in a flask and the mixture was heated under reflux in chloroform for 48 h using a N2 flow and in absence of light. Then, the reaction was cooled down to room temperature. For the purification, the nanocrystals were precipitated in MeOH several times. Finally, the QDs were dissolved in toluene.
5000) were added in a flask and the mixture was heated under reflux in chloroform for 48 h using a N2 flow and in absence of light. Then, the reaction was cooled down to room temperature. For the purification, the nanocrystals were precipitated in MeOH several times. Finally, the QDs were dissolved in toluene.
2.7 Thiol binding to QD-CS5
QD-CS5 (0.5 mL) and 68 mg of KP-SH (QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand molar ratio of 1
ligand molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) were added in a flask and heated up to reflux in 25 mL of chloroform for 48 h, under N2 flow in an absence of light. Then, the reaction was cooled down to room temperature. For the purification, the nanocrystals were precipitated in MeOH several times. Finally, the QDs were dissolved in toluene.
5000) were added in a flask and heated up to reflux in 25 mL of chloroform for 48 h, under N2 flow in an absence of light. Then, the reaction was cooled down to room temperature. For the purification, the nanocrystals were precipitated in MeOH several times. Finally, the QDs were dissolved in toluene.
2.8 Ligand exchange of the QD-CS5 ligand by thiolate
KP thiolate (KP-S−) was prepared by stirring a mixture of 68 mg of MU and 56 mg of tetramethylammonium hydroxide (TMOH) in 1 mL of chloroform at room temperature for 1 h. This solution was added to 0.5 mL of QD-CS5 (QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiolate molar ratio of 1
thiolate molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) in 25 mL of chloroform and the mixture was heated under reflux, in N2 flow and absence of light, for 48 h. Then, the reaction was cooled down to room temperature. For the purification, the nanocrystals were precipitated in MeOH several times. Finally, the QDs were dissolved in toluene.
5000) in 25 mL of chloroform and the mixture was heated under reflux, in N2 flow and absence of light, for 48 h. Then, the reaction was cooled down to room temperature. For the purification, the nanocrystals were precipitated in MeOH several times. Finally, the QDs were dissolved in toluene.
3. Results and discussion
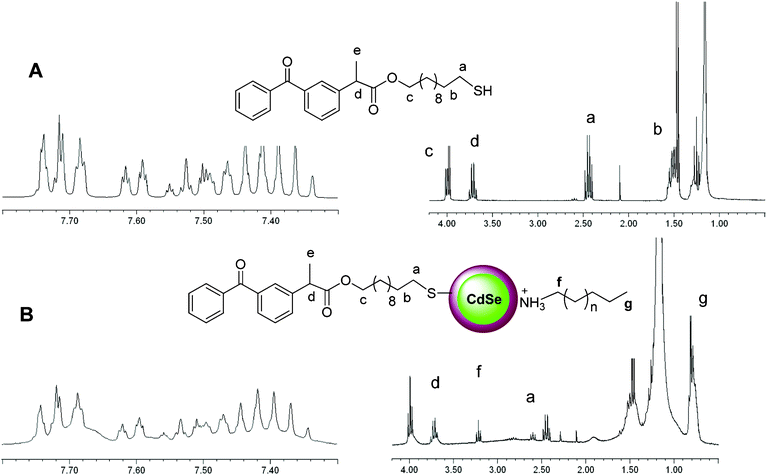
First, different sized CdSe/ZnS core-shell QDs capped with a fatty amine (QD-CS1, QD-CS2, and QD-CS3 with λmax exciton peaks at 509, 521, and 564 nm, respectively (Table S1, ESI†)), were purchased from Evident and used in these studies.A chloroform solution (25 mL) of QD-CS1 (5.46 × 10−7 mmol) and KP-SH (2.65 × 10−3 mmol, [thiol]/[QD] = 4859 molar ratio) was heated to reflux under nitrogen atmosphere for 48 h. After almost total solvent evaporation (2 mL) and the addition of methanol (30 mL), the samples were centrifuged at 8000 rpm for 20 min at 25 °C and the supernatant was decanted. The nanoparticles (CS1@KP) were dissolved in toluene (1 mL) and the purification process was repeated three times. The 1H-NMR spectrum of CS1@KP (Fig. 1B) showed a broadening of the ligand signals together with those of freely soluble ligands; the presence of a triplet at 3.3 ppm, ascribed to the CH2 attached to the N of protonated amine ligands (Fig. 1) was detected. It is well known that a ligand strongly bound to colloidal nanocrystals shows broad 1H-NMR signals, especially those of the protons close to the QD surface.9
The UV-visible spectrum of CS1@KP QDs exhibited the first exciton peak at the same position as the QD-CS1 QDs, i.e., QD functionalization did not produce any change in the first exciton peak (Fig. 2). High resolution transmission electron microscopy (HRTEM) images showed that CS1@KP maintained the size and the crystallinity of QD-CS1 (Fig. 3 and Fig. S1 in the ESI†). Interestingly, these QDs exhibited a higher ΦF (+ 50%) than the amine capped precursor (Fig. 4, Table S1, ESI†),10,11,12 demonstrating that the chemisorption of the thiol to the QD surface does not have a detrimental effect on the QD emission.
 | ||
| Fig. 2 UV- visible absorption spectrum of QD-CS1 and CS1@KP in toluene. | ||
 | ||
| Fig. 3 High Resolution Transmission Electron Microscopy images of CS1@KP, (2.3 ± 0.2 nm). | ||
 | ||
| Fig. 4 Comparative fluorescence spectrum of A: a deaerated toluene solution of QD-CS1 (■) and CS1@KP (•). | ||
Similar results were observed when reacting QD-CS2 (48.1 × 10−9 moles) with KP-SH (2.4 × 10−4 moles) to produce CS2@KP (Fig. S2, ESI,†ΦF increase of 16.5%) and when using MU as a capping ligand (Fig. S3, ESI,†). The increase of the ΦF was higher for the smaller QDs, which is probably associated with a higher surface ligand-to-QD size ratio in the case of smaller-sized nanoparticles.
The same strategy was applied for the preparation of QD-CS nanoparticles decorated with carboxy groups. Thus, a chloroform solution of QD-CS3 (1.4 × 10−7 moles) was heated under reflux in the presence of MUA and MPA (7.0 × 10−4 moles) for 48 h. After the above-mentioned work-up, the QDs were placed in water and their solubility was increased by addition of tetramethylammonium hydroxide (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/vwater: salt solution 0.1M) and mild heating (40 °C) for several minutes, leading to stable colloids of CS3@MUA and CS3@MPA. The absorption spectrum of CS3@MUA showed a clear red-shifted exciton peak (7 nm shift), while such variation was only of 2 nm for CS3@MPA. The solvatochromic effect is expected to induce a redshift as the solvent dielectric constant (ε) increases from 4.8 (chloroform) to 78 (water).13 The HRTEM images showed an average size of 3.1 ± 0.3 nm and 3.3 ± 0.3 nm for QD-CS3 and CS3@MPA QDs, respectively, (Fig. S4, ESI†). The emission bandwidth of the new water-soluble QDs was narrower than that of their precursor (Table S1). In addition, the Stokes shift of the thiol capped QDs was greater than that of the amine capped QDs.
1 v/vwater: salt solution 0.1M) and mild heating (40 °C) for several minutes, leading to stable colloids of CS3@MUA and CS3@MPA. The absorption spectrum of CS3@MUA showed a clear red-shifted exciton peak (7 nm shift), while such variation was only of 2 nm for CS3@MPA. The solvatochromic effect is expected to induce a redshift as the solvent dielectric constant (ε) increases from 4.8 (chloroform) to 78 (water).13 The HRTEM images showed an average size of 3.1 ± 0.3 nm and 3.3 ± 0.3 nm for QD-CS3 and CS3@MPA QDs, respectively, (Fig. S4, ESI†). The emission bandwidth of the new water-soluble QDs was narrower than that of their precursor (Table S1). In addition, the Stokes shift of the thiol capped QDs was greater than that of the amine capped QDs.
Interestingly, the water soluble QDs exhibited a ΦF of 54% (CS3@MUA, Fig. 5) and 45% (CS3@MPA, Fig. S5, ESI,†); i.e. ligand exchange and water solubilization led to a decrease in the ΦF of less than 29% (Table S1).
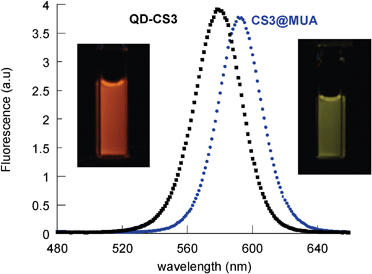 | ||
| Fig. 5 Comparative fluorescence spectra of a deaerated toluene solution of QD-CS3 (■) and a water solution of CS3@MUA(•). | ||
These results indicated that the replacement of the amine ligands by thiols under the described conditions resulted in well-passivated QDs. Lee et al.4 have reported that the replacement of TOPO ligands by MPA thiolates in considerably larger sized CdSe/ZnS QDs (5.2 nm, λmax at 610 nm) can give rise to QDs with a high photoluminescence performance if using an MPA thiolate soluble in chloroform and avoiding the presence of water. However, this only applied to small thiolate concentrations. In fact, high concentrations of thiolate were detrimental to ΦF and produced non-fluorescent QDs. We performed control experiments to determine the effect of the addition of such organic-soluble thiolates to QD-CS3 QDs, and found a drastic decrease of the QD fluorescence. Therefore, we reason that the exchange of the amine ligands by thiols in our studies could benefit from the basicity of the amines after their release from the QD surface, polarizing the thiol S–H bond14 and increasing the nucleophilicity of the thiol (Scheme 1). Consequently, chemisorption of the thiol to the core-shell QD surface would occur under very mild conditions.
To confirm the chemisorption of thiol to the core-shell QDs under the described mild conditions, the infrared spectra of KP-SH, CS2@KP, QD-CS2, and that of a freshly-prepared mixture of QD-CS2 and KP-SH were compared (Fig. S6, ESI,†). The stretching vibration mode of the thiol group of KP-SH at ca. = 2569 cm−1 was also present in the QD-CS/KP-SH mixture, but vanished in CS2@KP.
In addition, X-ray photoelectron spectroscopy (XPS) was used, since it provides the binding energy of a core-level electron of an atom in the solid.15 This energy depends on the potential energy at that position, which in turn depends on the chemical environment of the atom. Hence, an organic-soluble QD, CS2@KP, and a water-soluble QD, CS3@MUA, were analyzed by XPS and the data were compared with those for KP-SH, MUA, and a core-shell QD (QD-CS2), as well as those for a core-shell QD prepared by reacting QD-CS2 (48.1 × 10−9 moles) with KP-SH (2.4 × 10−4 moles) at room temperature, CS2@KPrt .
The Cd 3d spectrum splits by the spin-orbital coupling into the 3d 3/2 (404.7 eV) and 3d 5/2 (411.4 eV) components, with a typical splitting magnitude of 6.7 eV. The binding energy of the Cd was very similar for all the core-shell QDs, QD-CS2, CS2@KPrt , CS2@KP, and CS3@MUA (Fig. 6). Except for QD-CS2, deconvolution of the spectrum gave, besides the 3d 3/2 Cd contribution, a new component with a binding energy at ca. 403 eV. This was the main contribution for CS3@MUA, and it was ascribed to the presence of N1s of ammonium salts.16 In agreement with this, while the C 1s spectrum (not shown) of MUA exhibited a component at 288.8 eV that was attributed to the carboxylic moiety, CS3@MUA presented a peak at 287.8 eV, ascribed to the carboxylate group.17
 | ||
| Fig. 6 XPS spectra of Cd 3d and N 1s for QD-CS2, CS2@KPrt, CS2@KP, and CS3@MUA. | ||
Regarding the S 2s spectrum, KP-SH presented a peak at 227.4 eV ascribed to the thiol group,17 while QD-CS2 exhibited a peak with binding energy of 225.7 eV, brought about by the ZnS shell (Fig. 7).18 This band was very similar in the case of CS2@KPrt, but shifted to a lower binding energy (225.3 eV) in the case of CS2@KP (Fig. 7). This can be explained by the contribution of the thiol ligand, chemisorbed on the QD shell. Therefore, this peak was deconvoluted into two separate peaks by using the S 2s spectrum of the initial amine-capped core-shell QD as the reference. The new component shifted considerably to a lower value than that of the unbound thiol (at 224.4 eV vs. 227.4 eV) and this may be attributed to the chemisorption of KP-SH to the QD surface;18 its high contribution (ca. 30%) showed a large coverage of the QD surface by KP-SH.
 | ||
| Fig. 7 Top to bottom: XPS spectra of S 2s for QD-CS2, CS2@KPrt, CS2@KP, and KP-SH. | ||
The generality of the methodology to prepare highly fluorescent thiol-capped CdSe/ZnS core-shell QDs to QDs different from those manufactured by Evident was also studied, using homemade QDs as well as QDs purchased from another supplier, specifically from Ocean Nano Tech (QD-CS4: λmax at 503 nm, ΦF of 58% and QD-CS5: λmax at 544 nm, ΦF of 55%), see Table S1, ESI.†
Thus, a homemade TOPO-capped CdSe/ZnS core-shell QD (QD-CS6, 2.8 nm, λmax at 535 nm) was prepared following a previously reported procedure with some modifications (see experimental section). The TOPO ligands of QD-CS6 were replaced by amine ligands by heating a deaerated chloroform solution (25 mL) of QD-CS6 5.46 × 10−7 mmol and octadecylamine (ODA) ([amine]/[QD] = 4859 molar ratio) under reflux for 48 h. The amine-capped QDs (QD-CS7) exhibited its first exciton peak at 535 nm and a ΦF of 31%. The replacement of the capping ligands on QD-CS6 and QD-CS7 by KP-SH was carried out using the above-mentioned strategy to lead to CS6@KP (ΦF of 35%) and CS7@KP (ΦF of 30%), respectively. The S 2s spectrum of CS7@KP (not shown) presented a peak at 224.4 eV ascribed to the chemisorption of KP-SH to the QD, while neither CS6@KP, nor QD-CS6, nor QD-CS7 exhibited a peak with this binding energy. As expected, CS6@KP presented a peak at 227.4 eV ascribed to the thiol group.
In addition, the replacement of the capping ligands on QD-CS5 was performed with the thiol and thiolate form of KP-SH, leading to CS5@KP1 and CS5@KP2, respectively. The 1H-NMR spectrum of both QDs showed the characteristic broadening of the ligand signals. But, while the replacement of the QD capping ligand with the thiol led to a decrease in the ΦF of only 8%, the thiolate produced a drastic decrease (39%) of the QD emission. For further details of these and additional experiments regarding the effect on the emission properties of QD-CS4, QD-CS5, QD-CS6, and QD-CS7 after adding thiols, see Table S2 in ESI.†
The photostability of nanoparticles is of great importance in their applications; therefore, the photostability of several of the prepared thiol capped QDs was compared with that of their amine capped precursor. The samples were irradiated (400 nm < λ < 700 nm, λmax at 420 nm, 70 W/m2) in toluene or water, depending on the solubility of the corresponding QD, under nitrogen or air atmosphere for ca. five hours.
Surprisingly, the emission maximum of the QDs experienced only a small blue-shift (up to 6 nm) after 5 h irradiation under air atmosphere (Table 1).19 Blue-shifts up to 40 nm have been reported for TOPO capped CdSe/ZnS QDs and attributed to photooxidation of the CdSe core.20 For oxidation to take place, oxygen has to diffuse through the ZnS shell. Therefore, our data demonstrated that the CdSe core was well passivated by the ZnS shell in the QDs studied in this research work. The diminished ΦF of the organic-soluble QDs after their irradiation suggested photooxidation of mainly the ZnS layer,21 since it was accompanied by only a slight blue-shift of the emission maximum. In addition, the intensity decrease can be caused by the formation of lattice defects in the core-shell QDs.21
| λ max exciton | λ max emission | Φ F | |||||||
|---|---|---|---|---|---|---|---|---|---|
| before irradiation | after irradiation under N2 Air | before irradiation | after irradiation under N2 Air | before irradiation | after irradiation under N2 Air | ||||
| QD-CS2 | 521 | 520 | 520 | 539 | 537 | 538 | 0.61 | 0.31 | 0.22 |
| CS2@KP | 520 | 515 | 515 | 540 | 535 | 533 | 0.70 | 0.44 | 0.47 |
| QD-CS3 | 564 | 564 | 563 | 582 | 581 | 581 | 0.63 | 0.53 | 0.42 |
| CS3@MUA | 571 | 572 | 570 | 595 | 594 | 592 | 0.54 | 0.52 | 0.39 |
| CS3@MPA | 566 | 566 | 567 | 586 | 588 | 587 | 0.45 | 0.46 | 0.49 |
In addition, the photostability in air of the QDs covered with chemisorbed thiol ligands was higher than that of the amine-capped CdSe/ZnS QDs (see comparison between QD-CS3 (toluene) and CS3@MPA (water) in Fig. 8, and between QD-CS2 (toluene) and CS2@KP (toluene) in Fig. 9. Moreover, CS3@MPA exhibited an improved fluorescence (10%) after 5 h irradiation. This could be explained by the removal of recombination centers, created during the ligand exchange, by illumination.22
 | ||
| Fig. 8 Normalized fluorescence spectra of aerated solutions of CS3@MPA (water), before (■) and after (•) 270 min irradiation at λ > 400 nm. Inset: comparative fluorescence spectra of QD-CS3 (toluene). | ||
 | ||
| Fig. 9 Normalized fluorescence spectra of aerated toluene solutions of CS2@KP, before (■) and after (•) 270 min irradiation at λ > 400 nm. Inset: comparative fluorescence spectra of QD-CS2. | ||
The fluorescence of the amine-capped QDs also drastically decreased after irradiation under anaerobic conditions (though less than under air atmosphere conditions). However, irradiation of CS2@KP, CS3@MUA, and CS3@MPA in the presence or absence of oxygen made little difference and only slightly affected the QD fluorescence performance (Fig. 10, Fig. S7 and S8, ESI,† and Table 1).
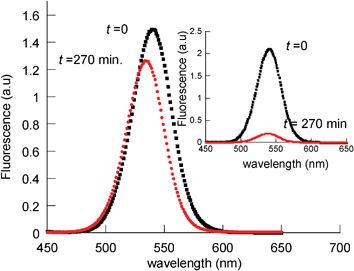 | ||
| Fig. 10 Normalized fluorescence spectra of deaerated toluene solutions of CS2@KP, before (■) and after (•) 270 min irradiation at λ > 400 nm. Inset: comparative fluorescence spectra of QD-CS2. | ||
4. Conclusions
In summary, we have demonstrated that the replacement of amine ligands by thiols to lead to either organic-soluble or water-soluble QDs can be performed under mild conditions, preserving or enhancing not only the emission properties of the nanoparticles but also their photostability. The QDs remain stable over the six-month study period. These results may help researchers to design new functional QDs for future applications in which highly fluorescent, photostable, and small-sized QDs are required.Acknowledgements
We thank MEC (Project CTQ2008-06777-CO2-01, contract granted to J. A-S, and RyC contract granted to R.E.G), GVA (Project ACOMP/2009/334), and UVEG (Project UV-AE-09-5805) for their support.References
- (a) M. G. S. Hyldahl, T. Bailey and B. P. Wittmerhaus, Sol. Energy, 2009, 83, 566 Search PubMed; (b) X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS; (c) A. P. Alivisatos, Nat. Biotechnol., 2004, 22, 47 CrossRef; (d) F. Chen and D. Gerion, Nano Lett., 2004, 4, 1827 CrossRef CAS.
- (a) O. Dabbousi, J. F. Rodriguez-Viejo, V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem. B, 1997, 101, 9463 CrossRef CAS; (b) B. M. A. Hines and P. Guyot-Sionnest, J. Phys. Chem., 1996, 100, 468 CrossRef CAS.
- H. Borchert, D. V. Talapin, C. McGiney, S. Adam, A. Lobo, A. R. B. Castro, T. Möller and H. Weller, J. Chem. Phys., 2003, 119, 1800 CrossRef CAS.
- B. K. Pong, B.L. Trout and J. Y. Lee, Langmuir, 2008, 24, 5270 CrossRef CAS.
- (a) K. Susumu, E. Oh, J. B. Delehanty, J. B. Blanco-Canosa, B. J. Johnson, V. Jain, W. J. Hervey IV, W. R. Algar, K. Boeneman and P. E. Dawson, J. Am. Chem. Soc., 2011, 133, 9480 CrossRef CAS; (b) I. L. Medintz; N. D. Abazovic, J. Z. Kuljanin-Jakovljevic and M. I. Comor, Russ. J. Phys. Chem. A, 2009, 83, 1511 Search PubMed; (c) W. Liu, H. S. Choi, J. P. Zimmer, E. Tanaka, J. V. Frangioni and M. Bawendi, J. Am. Chem. Soc., 2007, 129, 14530 CrossRef CAS; (d) V. V. Breus, C. D. Heyes and G. U. Nienhaus, J. Phys. Chem. C, 2007, 111, 18589 CrossRef CAS; (e) R. Gill, I. Wilner, I. Shweky and U. Banin, J. Phys. Chem. B, 2005, 109, 23715 CrossRef CAS.
- J. Aldana, Y. A. Wang and X. Peng, J. Am. Chem. Soc., 2001, 123, 8844 CrossRef CAS.
- KP-SH was synthesized following the Yamamoto procedure: K. Ishihara, M. Nakayama, S. Ohara, H. Yamamoto, Synlett. 2001, 7, 1117. See ESI for further details.†KP-SH was chosen to prepare thiol-capped QDs since it exhibits 1H-NMR signals well separated from those of ligands of the commercial or home-made QDs used for these studies. In addition, the benzophenone chromophore of KP-SH does not absorb at 400 nm > λ > 700 nm used for studies of the QD photostability . In addition, as the fluorescence studies show, the benzophenone did not significantly quench the QD emission.
- Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 183 CrossRef CAS.
- (a) X. Ji, D. Copenhaver, C. Sichmeller and X. Peng, J. Am. Chem. Soc., 2008, 130, 5726 CrossRef CAS; (b) J. S. Owen, J. Park, P. E. Trudeau and A. P. Alivisatos, J. Am. Chem. Soc., 2008, 130, 12279 CrossRef CAS.
- Yoon. et al. have reported an enhanced emission after binding of cysteine to CdSe/ZnS QDs, but they found indications that cysteine was actually bound to the QD-surface by both its thiol and its amine group. In addition, the enhancement disappeared at high cysteine concentration10.
- C. Park and T. H. Yoon, Colloids Surf., B, 2010, 75, 472 CrossRef CAS.
- For comparison, a chloroform solution (25 mL) of QD-CS1 and KP-SH ([thiol]/[QD] = 5000 molar ratio) was maintained at room temperature under nitrogen atmosphere for 48 h. After almost total solvent evaporation (2 mL) and the addition of methanol (30 mL), the samples were centrifugated at 8000 rpm for 20 min at 25 °C and the supernatant was decanted. The nanoparticles (CS1@KPrt) were dissolved in toluene (1 mL) and the purification process was repeated three times. The QDs exhibited a lower ΦF than the amine capped precursor. See also, J. Aguilera-Sigalat, S. Rocton, R. E. Galian and J. Pérez-Prieto, Can. J. Chem., 2011, 89, 359 Search PubMed.
- C. A. Leatherdale and M. G. Bawendi, Phys. Rev. B: Condens. Matter, 2001, 63, 165315 CrossRef.
- G. Zundel, J. Mol. Struct., 1982, 84, 205 CrossRef CAS.
- A. Lobo, T. Möller, M. Nagel, H. Borchert, S. G. Hickey and H. J. Weller, J. Phys. Chem. B, 2005, 109, 17422 CrossRef CAS.
- (a) G. K. Olivier, D. Shin, J. B. Gilbert, L. M. A. Monzon and J. Frechette, Langmuir, 2009, 25, 2159 Search PubMed; (b) A. Adenier, M. M. Chehimi, I. Gallardo J. Pinson and N. Vilà, Langmuir, 2004, 20, 8243 CrossRef CAS; (c) E. Uchida and Y. Ikada, J. Polymer Sci., 1996, 61, 1365 Search PubMed.
- J. J. Park, SHDP. Lacerda, S. K. Stanley, B. M. Vogel, S. Kim, J. F. Douglas, D. Raghavan and A. Karim, Langmuir, 2009, 25, 443 CrossRef CAS.
- This peak was also present in the S 2s spectrum of CS2@KPrt . By contrast, CS2@KP showed the signal of the disulfide..
- For one example of a slight hypsochromic shift upon photooxidation see L.I. Gurinovich, M.V. Artemév and A.A. Lyutich, J. Appl. Spectrosc., 2006, 73, 572 Search PubMed.
- W. G. J. H. M. Van Sark, P. L. T. M. Frederix, D. J. Van den Heuvel, H. C. Gerritsen, A. A. Bol, J. N. J. Van Lingen, C. de Mello Donegá and A. Meijerink, J. Phys. Chem. B, 2001, 105, 8281 CrossRef CAS.
- W. G. J. H. M. Van Sark, P. L. T. M. Frederix, A. A. Bol, H. C. Gerritsen and A. Meijerink, ChemPhysChem, 2002, 3, 871 CrossRef CAS.
- C. Carrillo-Carrión, S. Cárdenas, B. M. Simonet and M. Valcárcel, Chem. Commun., 2009, 5214 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: additional emission spectra of the QDs before and after irradiation under nitrogen and air atmosphere, UV-visible spectra, HRTEM images, XPS spectra, and physical data of the QDs. See DOI: 10.1039/c1ra01005k |
| This journal is © The Royal Society of Chemistry 2012 |