Core–shell α–Fe2O3@SnO2/Au hybrid structures and their enhanced gas sensing properties
Xianghong
Liu
,
Jun
Zhang
,
Xianzhi
Guo
,
Shurong
Wang
and
Shihua
Wu
*
Department of Chemistry, TKL of Metal- and Molecule-Based Material Chemistry and Key Laboratory of Advanced Energy Materials Chemistry (MOE), Nankai University, Tianjin, 300071, China.. E-mail: wushh@nankai.edu.cn
First published on 22nd December 2011
Abstract
Hybrid nanostructures with controlled morphology and tunable compositions are of vital significance to both fundamental studies and practical applications. A novel core–shell heterostructure of α-Fe2O3@SnO2/Au ternary hybrid nanospindles has been successfully fabricated, which were composed of α-Fe2O3 nanospindles as the cores and Au-decorated SnO2 coatings as the shells. The obtained core-shell structures have been characterized by means of XRD, SEM, TEM and XPS. By virtue of their special architectures and multiple compositions, the α-Fe2O3@SnO2/Au hybrid structures demonstrated enhanced gas sensing performances such as higher sensitivity and faster response-recovery, in comparison to pristine α-Fe2O3 nanospindles. This work proposes an example for the design of complex nanostructures with multiple functional compositions for high performance gas sensors.
Introduction
The fabrication of hybrid nanostructures is of vital significance to both fundamental studies and practical applications, as the hybrid materials integrate the different physical and chemical properties of two or more compositions.1–5 The obtained hybrid nanomaterials usually exhibit superior device performance or functional properties over their single components due to the synergistic interaction or the combination of the merits of individual components.2,4,6,7 To date, versatile composite nanostructures have been reported by many groups, such as ZnO@SnO2 core–shell tetrapods,2 SnO2-on-α-Fe2O3,3,4,8α-Fe2O3-on-SnO26 and ZnO-on-SnO27 hierarchical architectures CuO@SnO2,9α-Fe2O3@ZnO10 and α-Fe2O3@SnO211 core–shell nanorods, α-Fe2O3@SnO2 core-shell nanorattles,12–14MnO2@SnO2 nanowires,15 and ZnO/SnO2 nanosheets.16 More importantly, these hybrid nanostructures have demonstrated better performances in comparison to their single counterparts for various applications including optics,2,7 gas sensors,8–12lithium-ion batteries,6,12 photocatalysis,4,16 and supercapacitors.15 Besides metal oxides, noble metal nanoparticles such as Au, Ag, Pt and Pd are also frequently combined with metal oxides to form metal-oxide hybrid nanomaterials for enhanced applications such as gas sensors,17–21 photocatalysis,22–24 low-temperature CO oxidation25–30 and electrocatalysts31 by virtue of the unique electronic and catalytic properties of noble metals25,32 and the synergic metal-support interaction.29,30 To further expand the functionalities of composite nanomaterials, it is highly attractive to functionalize the hetero-nanostructures based on metal oxides with noble metals to generate more complex nanostructures with multiple compositions. To our knowledge, such hybrid structures as well as their advanced applications have been rarely reported.In this work, we report a novel hybrid structure of ternary α-Fe2O3@SnO2/Au core–shell nanorattles, which are constructed by α-Fe2O3 nanospindles as the inner cores and Au-functionalized SnO2 coatings as the outer shells. The obtained α-Fe2O3@SnO2/Au core–shell structures are expected to provide enhanced device performance for gas sensors due to their complex architecture and multiple compositions.
Experimental
Materials
Chemicals such as FeCl3·6H2O, Na2SnO3·3H2O, NaH2PO4, urea, ethanol, lysine, and NaBH4 were of analytical grade and purchased from Guangfu Fine Chemical Research Institute (Tianjin, China). HAuCl4·4H2O was obtained from Yingdaxigui Chemical Reagent Company (Tianjin, China). Distilled water was used throughout the experiments.Synthesis of α-Fe2O3@SnO2 core–shell nanospindles
α-Fe2O3@SnO2 core–shell nanospindles were prepared following the literature method with some modifications.14 First, α-Fe2O3 nanospindles were synthesized according to the ref. 33. Typically, 80 mL of aqueous solution containing 2.0 × 10−2 M FeCl3 and 4.5 × 10−4 M NaH2PO4 was placed in a 100 mL Teflon-lined autoclave and kept at 100 °C for 72 h. The red precipitate was centrifuged and washed several times with distilled water and absolute ethanol, and then dried at 80 °C. Subsequently, 0.053 g of α-Fe2O3 nanospindles was dispersed into a mixture of 15 mL ethanol and 25 mL distilled water by ultrasonication for 15 min. Then, 1.2 g of urea and 0.125 g of Na2SnO3·3H2O were completely dissolved in the above dispersion under stirring. The dispersion was transferred into a 50 mL Teflon-lined autoclave and kept at 160 °C for 24 h. The products was centrifuged and washed several times with distilled water and absolute ethanol, and then dried at 80 °C.Synthesis of Au-functionalized α-Fe2O3@SnO2 core–shell nanospindles
The deposition of Au nanoparticles onto α-Fe2O3@SnO2 is performed according to our previous work.20,21,34 0.0514 g of α-Fe2O3@SnO2 core–shell nanospindles was dispersed into 30 mL distilled water by ultrasonication for 15 min, followed by the addition of 1.5 mL of 0.01 M HAuCl4 and 2 mL of 0.01 M lysine solution. The dispersion was stirred for 30 min and then 3 mL of 0.1 M fresh NaBH4 (excess) solution was added to reduce HAuCl4 to Au nanoparticles. After further stirring for 40 min, the precipitate was collected by centrifugation and washed several times with distilled water and absolute ethanol, and dried at 80 °C. The product was calcined at 300 °C in air for 1 h to remove lysine.Characterizations and gas sensing tests
The crystalline structure of the product was characterized by powder X-ray diffraction (XRD, Rigaku D/max-2500, Cu Kα, λ = 1.5418 Å). The morphology was observed by scanning electron microscopy (SEM, Shimadzu SS-550, 15 kV) and transmission electron microscopy (TEM, Philips FEI Tecnai 20ST, 200 kV). The chemical composition of the sample was analyzed by X-ray photoelectron spectroscopy (XPS, Kratos Axis Ultra DLD spectrometer, Al Kα X-ray monochromator).Gas sensing tests were performed on a commercial HW-30A Gas Sensing Measurement System (HanWei Electronics Co., Ltd., Henan, China). The gas sensor was fabricated by coating an aqueous slurry of the materials onto an alumina tube with a diameter of 1 mm and length of 4 mm, positioned with a pair of Au electrodes and four Pt wires on both ends of the tube. A Ni–Cr alloy coil through the tube was employed as a heater to control the operating temperature by tuning the heating voltage. The working voltage for gas sensing test is 5 V. The analyte gas such as ethanol was introduced into the testing chamber on HW-30A by a microsyringe, using air as the diluting and reference gas. The sensor sensitivity is defined as the ratio of Ra/Rg, where Ra and Rg are the electrical resistances of the sensor in air and in test gas, respectively. The response time (τres) or recovery time (τrec) is defined as the time for the sensor to reach 90% of its maximum response. Details of the sensor fabrication, photograph, and gas sensing test principle can be seen in our previous work.34,35
Results and discussion
The α-Fe2O3@SnO2/Au core–shell structures were created by a three-step procedure, as shown in Fig. 1. Hydrothermally derived α-Fe2O3 nanospindles were used as the template to grow the SnO2 shells by a second hydrothermal method. An in situreduction route was used to deposit Au nanoparticles onto the binary α-Fe2O3@SnO2 core–shell nanospindles. Fig. 2a and b display the SEM images of the pristine α-Fe2O3 and α-Fe2O3@SnO2 core–shell nanospindles. By comparison, it can be seen that the pristine α-Fe2O3 nanospindles have two sharp tips, while the two tips of α-Fe2O3@SnO2 nanospindles become relatively round, which is caused by the outer SnO2 coatings. In addition the α-Fe2O3@SnO2 nanospindles have a little larger diameter compared with the pristine α-Fe2O3 nanospindles, as indicated by the white arrows, which apparently results from the SnO2 coatings on the α-Fe2O3 nanospindles. The crystal phase and composition of the samples were analyzed by XRD. Fig. 3 compares their XRD patterns. The pattern of α-Fe2O3 agrees well with the hematite phase (JCPDS No. 33-0644). Besides the diffraction peaks of hematite, α-Fe2O3@SnO2 also contains the peaks of SnO2 corresponding to the tetragonal rutile structure (JCPDS No.41-1445). As for α-Fe2O3@SnO2/Au structures, three additional peaks are detected, corresponding to Au (111), (200), and (220) planes. The average crystallite size of SnO2 is calculated to be 5.1 nm by the Scherer equation based on the (110) peak. | ||
| Fig. 1 (a) Schematic procedure for the synthesis of α-Fe2O3@SnO2/Au structures. | ||
 | ||
| Fig. 2 SEM images of (a) α-Fe2O3 and (b) α-Fe2O3@SnO2 nanospindles. | ||
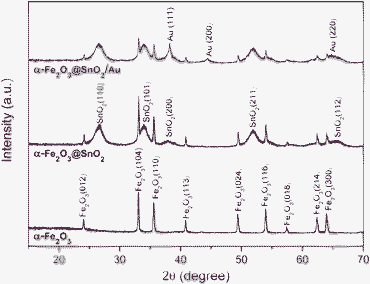 | ||
| Fig. 3 XRD patterns of α-Fe2O3, α-Fe2O3@SnO2 and α-Fe2O3@SnO2/Au nanospindles. | ||
Detailed structural information of the α-Fe2O3@SnO2/Au structures was observed by TEM. Fig. 4a shows the TEM image of pristine α-Fe2O3 nanospindles, which have a relatively smooth surface compared with the α-Fe2O3@SnO2/Au composite nanospindles shown in Fig. 4b. From Fig. 4b, one can see that each particle exhibits a typical core–shell structure. The α-Fe2O3 nanospindles are well encapsulated in the center by the SnO2 shells with a thickness of 16–18 nm and the tips of these core–shell nanospindles are not as spiculate as that of pristine α-Fe2O3. Interestingly, the diameter and length of the α-Fe2O3 nanospindle cores are slightly decreased compared with those in Fig. 4a. This phenomenon may be caused by the partial dissolution of α-Fe2O3 in the hydrothermal growth process of SnO2 shells. The dispersion of Au nanoparticles on the α-Fe2O3@SnO2/Au hybrid nanospindles could be observed by the TEM images in Fig. 4c and d. It is seen that Au nanoparticles are highly dispersed on the surface of α-Fe2O3@SnO2 nanospindles, suggested by their dark contrast against the support. The inset in Fig. 4d displays the size distribution histogram for the Au nanoparticles in Fig. 4d, indicating a particle size range of 2–11 nm with a mean size of 5.9 nm. TEM observation indicates that Au nanoparticle-decorated α-Fe2O3@SnO2 core–shell nanospindles were successfully synthesized.
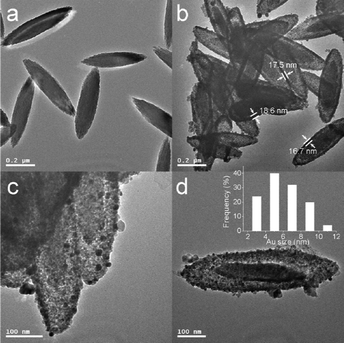 | ||
| Fig. 4 TEM images of (a) α–Fe2O3 and (b–d) α–Fe2O3@SnO2/Au nanospindles. Inset of (d) is the size distribution histogram of Au nanoparticles. | ||
The surface composition of α-Fe2O3@SnO2/Au core–shell nanospindles were further characterized by XPS analysis. The high resolution XPS spectra of Fe, Sn and Au are shown in Fig. 5a. In the spectrum of Fe 2p in Fig. 5a, the two peaks at 710.7 and 724.1 eV are assigned to Fe 2p3/2 and Fe 2p1/2. The interference peak at 715.9 eV is from Sn 3p. Fig. 5b displays two peaks with a binding energy of 486.3 and 494.7 eV corresponding to Sn 3d5/2 and Sn 3d3/2, respectively. The spectrum of Au 4f in Fig. 5c presents significant signals for Au 4f7/2 at 83.3 eV and Au 4f5/2 at 87.1 eV. Compared with the binding energy of bulk Au at 83.8 eV, the Au nanoparticles decorated herein displays a negative shift of ca. 0.5 eV. This negative shift of binding energy has been reported in previous publications, and was attributed to the electronic interaction between noble metal and metal oxide supports.30,36–38 Note that this kind of metal–support interaction has been taken as an important factor in the mechanism study of catalysts.36,37,39 The mass content of Au in the α-Fe2O3@SnO2/Au core–shell hybrids was 4.80% detected by XPS analysis.
 | ||
| Fig. 5 XPS spectra of (a) Fe 2p, (b) Sn 3d and (c) Au 4f. | ||
As important n-type metal oxides, both α-Fe2O3 and SnO2 have been widely utilized in applications such as catalysis and gas sensors. A combination of the two materials to form a hetero structure could provide more enhanced performances.4,6,8,12 Due to its exceptional catalytic activity, Au nanoparticles have been loaded onto various semiconductors as a promoter to improve catalyst and gas sensor properties. Herein, the α-Fe2O3@SnO2/Au core–shell nanospindles are expected to have excellent gas sensing performances. Therefore, gas sensing tests were conducted to examine the sensor performance of this novel hybrid structure.
In order to find the optimum operating temperature of the sensor, the sensitivities of α-Fe2O3@SnO2/Au core–shell nanospindles to 50 ppm ethanol at different operating temperature are collected in Fig. 5. One can see that the sensor has the highest sensitivity at 320 °C, therefore, all the following sensor tests were performed at this temperature. Fig. 6a displays the dynamic response-recovery curves of pristine α-Fe2O3, α-Fe2O3@SnO2 and α-Fe2O3@SnO2/Au sensors to ethanol with gas concentrations of 2, 10, 50, 100, 200, 500 and 800 ppm at 320 °C. It shows that the response of the three sensors increased with the increasing gas concentration. However, the increase amplitudes in the response of α-Fe2O3@SnO2/Au with gas concentration are more prominent than the other two sensors. To each gas concentration, the α-Fe2O3@SnO2/Au sensor shows the highest response. Furthermore, by comparison, it is seen that the response-recovery speed of α-Fe2O3@SnO2 is faster than that of pristine α-Fe2O3, while that of α-Fe2O3@SnO2/Au was further increased. Take the response-recovery curves of the three sensors to 100 ppm ethanol for example, as shown in Fig. 6b, the response time (τres = 5 s) and recovery time (τrec = 5 s) of α-Fe2O3@SnO2/Au are much shorter than that of α-Fe2O3@SnO2 (τres = 9 s and τrec = 9 s) and pristine α-Fe2O3 (τres = 15 s and τrec = 12 s). The relationship between sensitivities and ethanol concentrations of the three sensors are compared in Fig. 7a. The sensitivities of α-Fe2O3@SnO2/Au to each ethanol concentration are much higher than those of α-Fe2O3@ SnO2 and pristine α-Fe2O3. In order to further exploit the sensing properties of α-Fe2O3@SnO2/Au, other gases such as methanol, acetone, chloroform, and H2S were also tested to examine the selectivity of the sensor. All the analyte gases are of the same concentration (100 ppm). From Fig. 7b, it can be seen that the α-Fe2O3@SnO2/Au hybrid structure shows the highest sensitivity to ethanol (3.11), higher than that of acetone (2.23) and chloroform (2.14) and much higher than that of methanol (1.75) and H2S (1.64), implying that the α-Fe2O3@SnO2/Au nanospindles are more sensitive to ethanol gas and could be a very promising candidate for gas sensor materials.
 | ||
| Fig. 6 (a) Dynamic response-recovery curves to different ethanol concentrations, and (b) response-recovery curves to 100 ppm ethanol of α-Fe2O3, α-Fe2O3@SnO2 and α-Fe2O3@SnO2/Au nanospindles. (F denotes α-Fe2O3, FS is α-Fe2O3@SnO2 and FSA is α-Fe2O3@SnO2/Au.) | ||
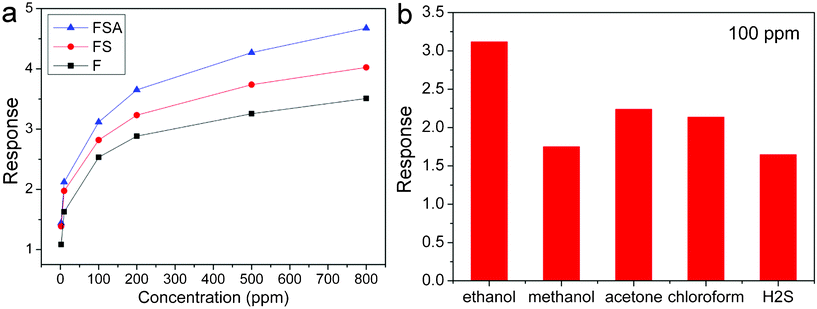 | ||
| Fig. 7 (a) Sensor sensitivity as a function of ethanol concentration of α-Fe2O3, α-Fe2O3@SnO2 and α-Fe2O3@SnO2/Au nanospindles, and (b) sensor sensitivity of α-Fe2O3@SnO2/Au to different gases. (F denotes α-Fe2O3, FS is α-Fe2O3@SnO2 and FSA is α-Fe2O3@SnO2/Au.) | ||
From the sensing results discussed above, we can see that the sensing performance of the α-Fe2O3@SnO2/Au structures have been significantly improved by coating SnO2 shells on α-Fe2O3 nanospindles and further functionalization of Au nanoparticles. Consequently, the enhanced sensing performance should be ascribed to at least the following factors, including the unique core–shell structure, synergic effect between α-Fe2O3 and SnO2 components, catalytic activity of Au nanoparticles and the metal-support interaction. However, deep efforts are needed to reveal a clear sensing mechanism for the complex nanostructures.
Conclusions
In conclusion, α-Fe2O3@SnO2/Au core–shell hybrid nanospindles were successfully fabricated via two steps of hydrothermal procedures and subsequent decoration of Au nanoparticles. Gas sensing studies demonstrate that the ternary hybrid structures exhibited significantly enhanced sensing performance in terms of higher sensitivity and faster response-recovery speed in comparison to pristine α-Fe2O3 nanospindles. This work has shed some new light on the synthesis of complex nanostructures combined with multiple functional compositions, which are expected to be promising for many other applications such as photocatalysis.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 20871071), and the Applied Basic Research Programs of Science and Technology Commission Foundation of Tianjin (Nos. 09JCYBJC03600 and 10JCYBJC03900).References
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- Q. Kuang, Z. Y. Jiang, Z. X. Xie, S. C. Lin, Z. W. Lin, S. Y. Xie, R. B. Huang and L. S. Zheng, J. Am. Chem. Soc., 2005, 127, 11777–11784 CrossRef CAS.
- D. F. Zhang, L. D. Sun, C. J. Jia, Z. G. Yan, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2005, 127, 13492–13493 CrossRef CAS.
- M. T. Niu, F. Huang, L. F. Cui, P. Huang, Y. L. Yu and Y. S. Wang, ACS Nano, 2010, 4, 681–688 CrossRef.
- Y. Zhao and L. Jiang, Adv. Mater., 2009, 21, 3621–3638 CrossRef CAS.
- W. Zhou, C. Cheng, J. Liu, Y. Y. Tay, J. Jiang, X. Jia, J. Zhang, H. Gong, H. H. Hng, T. Yu and H. J. Fan, Adv. Funct. Mater., 2011, 21, 2439 CrossRef CAS.
- C. W. Cheng, B. Liu, H. Y. Yang, W. W. Zhou, L. Sun, R. Chen, S. F. Yu, J. X. Zhang, H. Gong, H. D. Sun and H. J. Fan, ACS Nano, 2009, 3, 3069–3076 CrossRef CAS.
- Y. J. Chen, C. L. Zhu, X. L. Shi, M. S. Cao and H. B. Jin, Nanotechnology, 2008, 19, 205603 CrossRef.
- X. Y. Xue, L. L. Xing, Y. J. Chen, S. L. Shi, Y. G. Wang and T. H. Wang, J. Phys. Chem. C, 2008, 112, 12157–12160 CrossRef CAS.
- J. Zhang, X. H. Liu, L. W. Wang, T. L. Yang, X. Z. Guo, S. HuaWu, S. RongWang and S. M. Zhang, Nanotechnology, 2011, 22, 185501 CrossRef.
- Y. J. Chen, C. L. Zhu, L. J. Wang, P. Gao, M. S. Cao and X. L. Shi, Nanotechnology, 2009, 20, 045502 CrossRef.
- J. S. Chen, C. M. Li, W. W. Zhou, Q. Y. Yan, L. A. Archer and X. W. Lou, Nanoscale, 2009, 1, 280–285 RSC.
- X. W. Lou, C. Yuan and L. A. Archer, Adv. Mater., 2007, 19, 3328–3332 CrossRef CAS.
- X. W. Lou, C. Yuan and L. A. Archer, Small, 2007, 3, 261–265 CrossRef CAS.
- J. Yan, E. Khoo, A. Sumboja and P. S. Lee, ACS Nano, 2010, 4, 4247 CrossRef CAS.
- W.-W. Wang, Y.-J. Zhu and L.-X. Yang, Adv. Funct. Mater., 2007, 17, 59–64 CrossRef CAS.
- A. Kolmakov, D. O. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Nano Lett., 2005, 5, 667–673 CrossRef CAS.
- R. L. Vander Wal, G. W. Hunter, J. C. Xu, M. J. Kulis, G. M. Berger and T. M. Ticich, Sens. Actuators, B, 2009, 138, 113–119 CrossRef.
- Y. Zhang, Q. Xiang, J. Q. Xu, P. C. Xu, Q. Y. Pan and F. Li, J. Mater. Chem., 2009, 19, 4701–4706 RSC.
- X. H. Liu, J. Zhang, X. Z. Guo, S. H. Wu and S. R. Wang, Nanoscale, 2010, 2, 1178–1184 RSC.
- J. Zhang, X. H. Liu, X. Z. Guo, S. H. Wu and S. R. Wang, Chem-Eur J, 2010, 16, 8108–8116 CAS.
- Y. H. Zheng, L. R. Zheng, Y. Y. Zhan, X. Y. Lin, Q. Zheng and K. M. Wei, Inorg. Chem., 2007, 46, 6980–6986 CrossRef CAS.
- Q. Xiang, G. F. Meng, H. B. Zhao, Y. Zhang, H. Li, W. J. Ma and J. Q. Xu, J. Phys. Chem. C, 2010, 114, 2049–2055 CrossRef CAS.
- M. Miyauchi, Phys. Chem. Chem. Phys., 2008, 10, 6258–6265 RSC.
- A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef.
- B. K. Min and C. M. Friend, Chem. Rev., 2007, 107, 2709–2724 CrossRef CAS.
- Z. Y. Zhong, J. Y. Lin, S. P. Teh, J. Teo and F. M. Dautzenberg, Adv. Funct. Mater., 2007, 17, 1402–1408 CrossRef CAS.
- Z. Zhong, J. Ho, J. Teo, S. Shen and A. Gedanken, Chem. Mater., 2007, 19, 4776–4782 CrossRef CAS.
- N. F. Zheng and G. D. Stucky, J. Am. Chem. Soc., 2006, 128, 14278–14280 CrossRef CAS.
- B. H. Wu, H. Zhang, C. Chen, S. C. Lin and N. F. Zheng, Nano Res., 2009, 2, 975–983 Search PubMed.
- S. Guo, S. Dong and E. Wang, Chem.–Eur. J., 2009, 15, 2416–2424 CrossRef CAS.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS.
- M. Ozaki, S. Kratohvil and E. Matijevi, J. Colloid Interface Sci., 1984, 102, 146–151 CrossRef CAS.
- X. H. Liu, J. Zhang, L. W. Wang, T. L. Yang, X. Z. Guo, S. H. Wu and S. R. Wang, J. Mater. Chem., 2011, 21, 349–356 RSC.
- J. Zhang, S. R. Wang, M. J. Xu, Y. Wang, B. L. Zhu, S. M. Zhang, W. P. Huang and S. H. Wu, Cryst. Growth Des., 2009, 9, 3532–3537 CrossRef CAS.
- Z. Zhong, J. Ho, J. Teo, S. Shen and A. Gedanken, Chem. Mater., 2007, 19, 4776–4782 CrossRef CAS.
- K. Yu, Z. Wu, Q. Zhao, B. Li and Y. Xie, J. Phys. Chem. C, 2008, 112, 2244–2247 CrossRef CAS.
- C. Wang, H. Daimon and S. H. Sun, Nano Lett., 2009, 9, 1493–1496 CrossRef CAS.
- M. Haruta, CATTECH, 2002, 6, 102–115 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
