PCBs and PBDEs in environmental samples from King George Island and Ardley Island, Antarctica†
Pu
Wang
,
Qing-hua
Zhang
*,
Thanh
Wang
,
Wei-hai
Chen
,
Dai-wei
Ren
,
Ying-ming
Li
and
Gui-bin
Jiang
State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085, China. E-mail: qhzhang@rcees.ac.cn
First published on 16th December 2011
Abstract
The levels and distribution of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) were investigated in various environmental matrices including soil, sediment, lichen (Usnea aurantiaco-atra) and moss (Sanionia uncinata) from Fildes Peninsula at King George Island and Ardley Island, west Antarctica. In general, PCBs and PBDEs were detected at very low levels in the samples collected during December 2009 to February 2010. The mean concentrations of total PCBs were 410 pg g−1 dry weight (dw) (range 60.1–1436 pg g−1 dw) in soil and sediment, 544 pg g−1 dw (404–745 pg g−1 dw) in lichen and 670 pg g−1 dw (406–952 pg g−1 dw) in moss. The lower chlorinated CBs dominated in all the samples except for the dropping-amended soils from Ardley Island, where hexachlorinated congeners were more abundant. Notably, CB-11 was detected at significant levels, accounting for about 20% of total PCBs in most samples, this higher ratio compared to that in the technical mixture might suggest unidentified sources. Average levels of PBDEs were 24.0 pg g−1 dw (2.76–51.4 pg g−1 dw) in soil and sediment, 14.2 pg g−1 dw (7.51–22.3 pg g−1 dw) in lichen and 15.8 pg g−1 dw (6.54–36.7 pg g−1 dw) in moss. BDE-47 dominated the detected congeners, whereas BDE-99 and 71 were more abundant in the dropping-amended soils from Ardley Island. These results indicated that long-range atmospheric transport could be the main pathway of POPs to King George Island although anthropogenic influence (e.g., from research station, tourism and biotic activities) could also influence the spatial distribution of POPs.
1. Introduction
The Polar Regions are considered a final sink for many persistent organic pollutants (POPs).1 Their occurrence in these pristine areas has been explained by the global distillation or fractionation processes,2 and long-range atmospheric transport (LRAT) is identified as a main pathway for POPs to reach the Polar Regions. Ever since chlorinated pesticides were first detected in Antarctic wildlife in the 1960s,3 various studies have been conducted to study the contamination of POPs in the environment and wildlife of the Arctic and Antarctic regions. These early findings provided impetus for further and extensive study on the ubiquity of contaminants in these remote areas.4,5There is a large international presence of research activities in the Antarctic area, with up to 4000 individuals from 26 countries occupying 82 bases in the summer,6 which brings escalating pressures to the pristine environment relating to the increasing visits by tourists and scientists.
Two typical POPs, polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) were mainly produced for industrial and commercial purposes, with production and emissions centered on the Northern mid-latitude regions.7 However, they have also been widely detected in the polar environment and the presence of these toxic industrial contaminants suggests that long-range transport occurred due to their physical-chemical properties. Recently, additional influence of anthropogenic activities in the local areas has been illustrated by elevated levels of PCBs and PBDEs in the Antarctic environment.4,8,9 CB-11, a byproduct in the manufacture of paint pigments and one of the predominant congeners in refuse-derived fuel and automobile shredder residue,10 was also observed at a relatively high level in air samples from Antarctica, suggesting an unusual source in the southern hemisphere.11 PBDEs, especially BDE-209 which has limited environmental mobility, was quantitatively detected in sludge and dust, as well as aquatic sediments near the McMurdo station wastewater outfall, suggesting inputs from local sources.9
These studies suggest that both LRAT and local source play a key role in determining the level and pattern of POPs at the Polar Regions. The objective of this study was to investigate PCBs and PBDEs in the environment around the Fildes Peninsula at King George Island, west Antarctica, where many international research stations are located, including the Chinese Great Wall Station, and the adjacent Ardley Island which is an important settlement for penguins and migrating birds. The levels and distribution patterns of these contaminants were discussed and possible sources were also suggested.
2. Materials and methods
2.1 Materials
Pesticide grade reagents dichloromethane (DCM) and n-hexane were purchased from Tedia Company Inc. (Fairfield, OH, USA) and nonane from Sigma (St. Louis, USA). Silica gel 60 (0.063–0.100 mm particle diameter) was obtained from Merck (Darmstadt, Germany), and anhydrous sodium sulfate, concentrated sulfuric acid and sodium hydroxide were of guaranteed grade. Prior to use, silica gel and anhydrous sodium sulfate were baked at 550 °C for 12 h and 660 °C for 6 h, respectively. The preparation of acid silica gel and basic silica gel was described elsewhere.12 The carbon mixture (18% w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) was produced with 9 g carbon (Carbopak C, Supelco 10258, USA) dispersed into 41 g celite (545 coarse, Fluka 22140, USA), and then baked at 130 °C for 6 h. All PCBs and PBDEs internal (68A-LCS, 13C-BDE-LCS) and recovery (68A-IS) standards were purchased from Wellington Laboratories (Ontario, Canada).
w) was produced with 9 g carbon (Carbopak C, Supelco 10258, USA) dispersed into 41 g celite (545 coarse, Fluka 22140, USA), and then baked at 130 °C for 6 h. All PCBs and PBDEs internal (68A-LCS, 13C-BDE-LCS) and recovery (68A-IS) standards were purchased from Wellington Laboratories (Ontario, Canada).
2.2 Sampling
The sampling period was arranged during the 26th Chinese Antarctic Expedition in the austral summer between December 2009 and January 2010. Seven natural soil, three dropping-amended soil, one sediment, six lichen and eight moss samples were collected around Fildes Peninsula at King George Island, west Antarctica, where many international research stations are situated, and Ardley Island which is an important settlement for the penguins and migrating birds. The sampling sites are located between 62°12'19′′S and 62°13'48′′S, 58°55'13′′W and 58°59'59′′W (Fig. 1) and sample details are listed in Table 1. All the samples were sealed in clean plastic bags and transported back to the laboratory in Beijing, China and stored frozen (−20 °C) until analysis. The details about sampling are presented in the ESI.†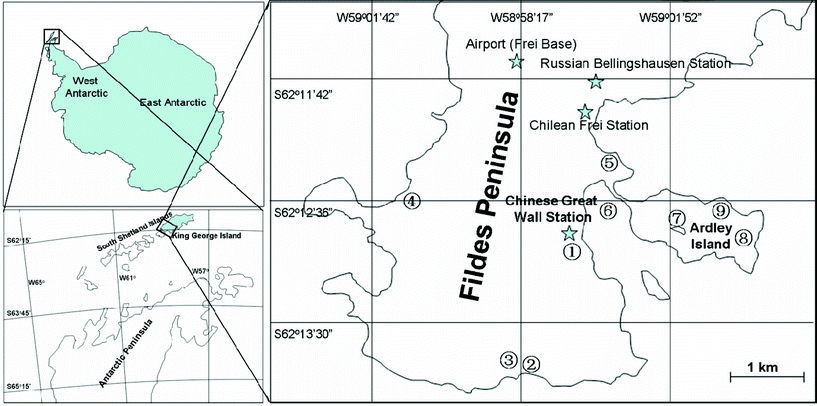 | ||
| Fig. 1 Distribution of the sampling sites in west Antarctic Peninsula. Sampling sites: 1. Badaling, 2. Biyutan, 3. Xiangjiao Mt., 4. Seal Bay, 5. Banbian Mt., 6. Great Wall Bay, 7. Yueya Lake, 8. East Ardley, 9. Dengta. | ||
| No. | Sampling site | Sample type | Latitude (S) | Longitude (W) | TOC/Lipid (%) | PCB concentrations (pg g−1 dw) | PBDE concentrations (pg g−1 dw) |
|---|---|---|---|---|---|---|---|
| 1 | Badaling | soil | 62°13'12′′S | 58°57'48′′W | 0.33 | 60.1 | 6.1 |
| lichen (Usnea aurantiaco-atra) | 0.72 | 745 | 18.8 | ||||
| moss (Sanionia uncinata) | 1.68 | 764 | 36.7 | ||||
| 2 | Biyutan | soil | 62°13'48′′S | 58°59'07′′W | 0.48 | 120 | 2.8 |
| moss (Sanionia uncinata) | 2.71 | 409 | 9.0 | ||||
| 3 | Xiangjiao Mt. | soil | 62°13'45′′S | 58°59'29′′W | 2.45 | 179 | 16.8 |
| lichen (Usnea aurantiaco-atra) | 1.21 | 410 | 8.8 | ||||
| 4 | Seal Bay | soil | 62°12'30′′S | 58°59'59′′W | 0.40 | 186 | 21.7 |
| lichen (Usnea aurantiaco-atra) | 2.95 | 568 | 19.0 | ||||
| moss (Sanionia uncinata) | 1.47 | 881 | 13.0 | ||||
| 5 | Banbian Mt. | lichen (Usnea aurantiaco-atra) | 62°12'19′′S | 58°57'17′′W | 2.01 | 582 | 22.3 |
| moss (Sanionia uncinata) | 1.34 | 406 | 16.4 | ||||
| 6 | Great Wall Bay | sediment | 62°12'35′′S | 58°57'24′′W | 1.32 | 182 | 3.2 |
| 7 | Yueya Lake | soil | 62°12'45′′S | 58°56'26′′W | 20.0 | 171 | 51.2 |
| lichen (Usnea aurantiaco-atra) | 0.96 | 404 | 8.6 | ||||
| moss (Sanionia uncinata) | 2.40 | 896 | 19.1 | ||||
| 8 | East Ardley | soil | 62°12'50′′S | 58°55'13′′W | 6.85 | 141 | 13.0 |
| dropping-amended soil | 34.0 | 1433 | 30.7 | ||||
| moss (Sanionia uncinata) | 2.43 | 760 | 9.6 | ||||
| 9 | Dengta | soil | 62°12'37′′S | 58°55'37′′W | 7.11 | 205 | 20.3 |
| dropping-amended soil 1 | 52.7 | 778 | 51.4 | ||||
| dropping-amended soil 2 | 55.9 | 1547 | 47.3 | ||||
| lichen (Usnea aurantiaco-atra) | 1.45 | 553 | 7.5 | ||||
| moss 1 (Sanionia uncinata) | 2.52 | 952 | 16.0 | ||||
| moss 2 (Sanionia uncinata) | 2.36 | 596 | 6.5 |
2.3 Sample extraction and cleanup
The soil and sediment samples were freeze-dried, homogenized and sieved through 16-mesh sieve. About 5 g of dry samples were weighed and extracted with DCM: n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) using Accelerated Solvent Extraction (ASE300, Dionex, USA). Prior to extraction, 68A-LCS (for PCBs) and PBDE-LCS (13C-BDE-47, 99, 153) were concurrently spiked to the samples. The extracts were fractioned using auto-gel permeation chromatography (GPC, AccuPrepTM, J2-Scientific, USA) and then loaded to multilayer silica columns for further cleanup. The eluates were concentrated into 20 μL nonane in a GC vial. Prior to instrumental analysis, the samples were spiked with 68A-IS to calculate the recoveries of both PCBs and PBDEs.
v) using Accelerated Solvent Extraction (ASE300, Dionex, USA). Prior to extraction, 68A-LCS (for PCBs) and PBDE-LCS (13C-BDE-47, 99, 153) were concurrently spiked to the samples. The extracts were fractioned using auto-gel permeation chromatography (GPC, AccuPrepTM, J2-Scientific, USA) and then loaded to multilayer silica columns for further cleanup. The eluates were concentrated into 20 μL nonane in a GC vial. Prior to instrumental analysis, the samples were spiked with 68A-IS to calculate the recoveries of both PCBs and PBDEs.
Different from the above extraction and cleanup procedures, 3.0 g lichen or moss samples were extracted using ASE. After lipid determination, the extracts were treated with acidic silica and then purified by multilayer silica column, basic alumina column and carbon column successively. The eluate was finally concentrated into 20 μL nonane and then spiked with a recovery standard (68A-IS). Details about the extraction and cleanup procedures can be found in the ESI.†
2.4 Instrumental analysis
The targets were analyzed using high-resolution gas chromatography coupled with high-resolution mass spectrometry (HRGC/HRMS). For PCBs, the GC instrument is Agilent 6890N (Wilmington, USA) with a CTC PAL autosampler, and the HRMS is AutoSpec Ultima (Waters Micromass, UK) with an electron impact (EI) ion source. Exactly 1 μL solution was injected into GC with a 60 m DB-5 ms fused silica capillary column (J&W, Scientific, 0.25 μm film thickness, 0.25 mm i.d.) in splitless mode. The HRMS was operated in VSIR mode at R ≥ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the electron emission energy was set to 35 eV and the source temperature was 270 °C. The carrier gas was helium at a constant flow of 1.0 mL min−1. The temperature conditions of chromatographic analysis were as follows: the initial column temperature was held at 120 °C for 1 min and increased to 150 °C at 30 °C min−1, then reached to 300 °C at 2.5 °C min−1 and maintained for 1 min. Dioxin-like and indicator PCBs were specified and quantified, the total concentration of PCB congeners in a homologue group was calculated as sum of detected peaks based on GC retention-time window definition.
000, the electron emission energy was set to 35 eV and the source temperature was 270 °C. The carrier gas was helium at a constant flow of 1.0 mL min−1. The temperature conditions of chromatographic analysis were as follows: the initial column temperature was held at 120 °C for 1 min and increased to 150 °C at 30 °C min−1, then reached to 300 °C at 2.5 °C min−1 and maintained for 1 min. Dioxin-like and indicator PCBs were specified and quantified, the total concentration of PCB congeners in a homologue group was calculated as sum of detected peaks based on GC retention-time window definition.
PBDEs were analyzed using a DFS system (Thermo Fisher, USA) with a 30 m DB-5 ms column (0.10 μm film thickness, 0.25 mm i.d.) for congener separation. The HRMS was operated in MID mode at R ≥ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the electron emission energy was set to 45 eV and the source temperature was 250 °C. Helium was the carrier gas with a constant flow of 1.0 mL min−1. The GC temperature program was: 100 °C initially for 2 min, then increased to 230 °C at 15 °C min−1 and increased again to 270 °C at 5 °C min−1, finally increased to 330 °C at 10 °C min−1 and maintained for 8 min. Quantification was processed for 14 PBDE congeners (BDE-17, 28, 47, 66, 71, 85, 99, 100, 138, 153, 154, 183, 190 and 209).
000, the electron emission energy was set to 45 eV and the source temperature was 250 °C. Helium was the carrier gas with a constant flow of 1.0 mL min−1. The GC temperature program was: 100 °C initially for 2 min, then increased to 230 °C at 15 °C min−1 and increased again to 270 °C at 5 °C min−1, finally increased to 330 °C at 10 °C min−1 and maintained for 8 min. Quantification was processed for 14 PBDE congeners (BDE-17, 28, 47, 66, 71, 85, 99, 100, 138, 153, 154, 183, 190 and 209).
Total organic carbon (TOC) in the soil and sediment samples was analyzed using a solid TOC Analyzer (O.I Analyzer, USA). About 0.1 g sample was weighed and loaded into the combustion house for 6 min at 900 °C. The signal was detected by non-dispersed infrared (NDIR) detector. Prior to combustion, the samples were wetted with 5% phosphoric acid and heated at 250 °C for 1 min to purge inorganic carbon.
2.5 Quality assurance/quality control (QA/QC)
All the target compounds were analyzed using isotope-dilution method. US EPA defined 68A-LCS and PBDE-LCS (13C-BDE-47, 99, 153) standards were utilized for specification and quantification, and 68A-IS was added for recovery calculation. The recoveries of 68A-LCS and PBDE-LCS were in the range 62–104% and 64–92%, respectively. The limit of detection (LOD) was defined as signal-to-noise ratio (S/N) = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the isotopic ratios between the two main ion pairs were within ± 15% of the theoretical values. In this study, the LODs for PCBs and PBDEs were in the range 0.03–0.34 pg g−1 and 0.01–0.38 pg g−1, respectively. A sample of XAD resins was used as travel blank to monitor the contamination, and two laboratory blanks were processed paralleled to the samples analysis for quality control. The analytical results showed that no target contaminants were detected in the XAD, and some indicator PCBs (e.g., CB-28, 52, 118 and 153) were found at relatively low level in the laboratory blanks. The reported concentrations of PCBs were therefore corrected for the laboratory blanks.
1 and the isotopic ratios between the two main ion pairs were within ± 15% of the theoretical values. In this study, the LODs for PCBs and PBDEs were in the range 0.03–0.34 pg g−1 and 0.01–0.38 pg g−1, respectively. A sample of XAD resins was used as travel blank to monitor the contamination, and two laboratory blanks were processed paralleled to the samples analysis for quality control. The analytical results showed that no target contaminants were detected in the XAD, and some indicator PCBs (e.g., CB-28, 52, 118 and 153) were found at relatively low level in the laboratory blanks. The reported concentrations of PCBs were therefore corrected for the laboratory blanks.
3. Results and discussion
The analytical results showed that both PCBs and PBDEs were detected in all the 25 samples. The concentrations and sample characteristics (TOC and lipid content) are listed in Table 1.3.1 The overall concentrations in the soil and sediment samples
Total PCB concentrations in the soil and sediment were in the range 60.1–1436 pg g−1 dry weight (dw) and with an average of 410 pg g−1 dw, which were consistent with many other results from Antarctica. Borghini et al.13 reported that PCB concentrations were 0.36–0.59 ng g−1 dw in soil from Victoria Land, Antarctica. Klánová et al.14 investigated PCBs, OCPs and PAHs in the soil and sediment from James Ross Island, Antarctica, and the results showed that indicator PCB concentrations ranged between 0.51–1.82 ng g−1 dw in soil and 0.32–0.83 ng g−1 dw in sediment. Fuoco et al.15 measured PCBs in marine sediment, lake sediment and soil samples collected at Terra Nova Bay, Wood Bay and Victoria Land during the 1989–1990 Italian expedition, and the concentrations ranged 45–361 pg g−1, 102–560 pg g−1 and 61–120 pg g−1, respectively. A follow-up study on marine sediment samples from Terra Nova Bay and Ross Sea, lake sediment and soil samples from Victoria Land reflected the same level of PCB concentrations with ranges of 30–160 pg g−1, 60–120 pg g−1 and 40–70 pg g−1, respectively, and were shown to strongly depend on the particle size distribution of each sample, as found in previous expeditions.16 However, Park et al.17 found lower PCB concentrations (8.0–33.8 pg g−1) in the same area (King George Island, South Shetland Islands, Antarctica), which could be due to the different sampling sites and sampling period.PCBs were generally of higher concentration in Ardley Island than in Fildes Peninsula, King George Island. Especially in the dropping-amended soils, the level was up to 1087 pg g−1 dw on average. Ardley Island is considered an important settlement for penguins in west Antarctic Peninsula, and many migrating birds also reside there during the summer. About 70–80% of the island is covered by vegetation such as moss and lichen around the penguin habitat. TOC analysis showed that the dropping-amended soils contained abundant organic matter, indicating that the soils could mostly be made up of penguin guano and plant residues. Studies indicated that biotic focusing of POPs can cause elevated contamination levels and become more significant than the contaminant input via abiotic pathways (air or water) on a local scale.18 Therefore, the relatively high contamination in soil from Ardley Island could be attributed to a constant input of contaminants by the migrating birds and their related biotic processes (nesting and excrement), which has also been illustrated in a study on PCBs in the eastern coast of Antarctica.8
For PBDEs, the average concentration was 24.0 pg g−1 dw (range 2.76–51.4 pg g−1 dw), which was at the same level as our previous results from Tibetan Plateau (4.3–34.9 pg g−1 dw with an average 11.1 pg g−1 dw in the surface soil),19 but much lower than other remote areas. Results showed that PBDEs were in the range 0.16–0.23 ng g−1 dw in the Russian Arctic20 and 0.065–12.0 ng g−1 dw in European background soils;21 Hale et al.9 reported a wide range of Σ6PBDE concentrations (< d.l. −677 ng g−1 TOC) in sediment samples from McMurdo Sound, Antarctic, and suggested that local source from the research station and tourism could be the major contributor. Similar to the spatial distribution of PCBs, relatively higher PBDE levels were detected in the soil from Ardley Island than from Fildes Peninsula, King George Island.
3.2 Overall concentrations in lichen and moss samples
The average concentrations of total PCBs were 544 pg g−1 dw (range 404–745 pg g−1 dw) and 670 pg g−1 dw (range 406–952 pg g−1 dw) in the lichen and moss samples, respectively, both of which were lower than those found in many other studies (Negoita et al., 3.3 ng g−1 in lichen from Russian station, Antarctica;8 Fuoco et al., < 5–34 ng g−1 dw in moss from Victoria Land, Antarctica;4 Borghini et al., 23–34 ng g−1 dw in moss from Victoria Land;13 Focardi et al., < 5–16 ng g−1 dw in moss from Victoria Land;22Leadet al., 6.1–52 ng g−1 dw in moss from Norway.23).For PBDEs, the concentrations were in the range of 7.51–22.3 pg g−1 dw (average 14.2 pg g−1 dw) in lichen and 6.54–36.7 pg g−1 dw (average 15.8 pg g−1 dw) in moss, which were lower than other studies. Mariussen et al.24 reported that PBDE concentrations (BDE-28, 47, 99, 100, 153, 154 and 183) ranged between 0.03–0.109 ng g−1 dw, with additional concentrations of BDE-209 ranging from 0.052 to 0.64 ng g−1 dw (contributing to 80% of the sum of 8 congeners) in moss from different sites across Norway. Yogui and Sericano25 found that PBDEs were 818 ± 270 pg g−1 dw in moss and 168 ± 75 pg g−1 dw in lichen along the shore of Admiralty Bay, King George Island, and significant difference was observed between these two sample types. However, no such difference was exhibited on both PCB and PBDE concentrations between lichen and moss (p > 0.05) in this study, as well as spatial distribution in the sampling area.
3.3 Homologue profiles of PCBs and PBDEs
As shown in Fig. S1–S3 in the ESI,† the lower chlorinated compounds (mono-, di-, tri- and tetra-CBs) showed high contributions to the total PCBs in all the samples, which is consistent with the greater long-range atmospheric transport potential for these lower chlorinated congeners. Furthermore, MoCBs showed high concentration in the sediment from Great Wall Bay (sampling site No.6) close to the research station, followed by DiCBs and TrCBs; whereas HxCBs dominated in the three dropping-amended soils from Ardley Island, accounting for about 30% of total PCBs, indicating significant influence of animal activities in the island as Fuoco et al.4 and Negoita et al.8 suggested. The indicator PCBs were evidently detected in all the samples (Table S1, ESI†), accounting for about 20% of the total PCBs. CB-28 was the dominant congener (accounting for about 40% of the indicator PCBs), followed by CB-138, 153 and 118, the relatively high abundance of mid-chlorinated congeners might indicate a local source rather than atmospheric transport.8 Notably, CB-11 was detected in all the samples, averaging 41.7 pg g−1 dw in the soil, 5.1 pg g−1 dw in sediment, 95.4 pg g−1 dw in lichen and 131 pg g−1 dw in moss, accounting for about 20% of the total PCBs in all the samples except for the dropping-amended soils (6%) and sediment (3%). The higher relative abundance of CB-11 suggested a different source from biotic activities. CB-11 has been considered one of the predominant congeners in refuse-derived fuel and automobile shredder residue,10 it was also observed during the manufacture of organic pigments near the New York/New Jersey Harbor, with CB-11 contributing to 5–20% of the total PCBs.26–28 The high composition ratio is significantly different from those in technical PCB mixtures (only Aroclor 1221 contained < 0.5% CB-11,29 and even less than 0.08% in Aroclors 1016, 1242, 1254, and 122130), indicating that it is not directly derived from commercial Aroclor products. Rodenburg et al.26 even considered that CB-11 could be used as a tracer for wastewater, stormwater, and combined sewer overflows. However, high levels of CB-11 were also observed in the atmosphere at the Korean Antarctic research station.11 As the authors indicated, it is difficult to explain why CB-11 is more abundant in the Antarctic environment and challenging to apportion sources of this congener under current knowledge, but might indicate unidentified local sources.For PBDEs (Table S1, ESI†), BDE-47 was the main congener in all samples, accounting for about 30% of the total concentrations, followed by BDE-99 (20%). This is slightly different from the observation of Yogui and Sericano,25 where BDE-99, 47 and 100 dominated in the lichen and moss samples from King George Island. In addition, the presence of BDE-183 suggests that other technical formulations (e.g., Octa-BDE and Deca-BDE) have reached Antarctica as Yogui and Sericano indicated.25 In the dropping-amended soils, BDE-99 and 71 were relatively more abundant compared to that in the other samples. BDE-209 was not detected in any sample, which is different to some other reports, e.g., Hale et al.9 found abundant BDE-209 in sludge and dust, as well as sediments collected near the McMurdo wastewater outfall, Antarctic, suggesting its main contributions are from local sources considering its limited volatility and long-range transport potential.
3.4 The effect of lipid and total organic carbon (TOC) contents
The lipid contents of lichen and moss were in the range of 0.72–2.95%, which is consistent with the study by Yogui and Sericano.25 There was no relationship observed between the lipid content and PCB or PBDE concentrations in each sample.TOC contents differed widely among the samples. In the sediment, it was 1.32%, comparable to 0.91% (0.33–2.45%) in the soil around the peninsula, which is consistent with the results of Borghini et al.13 However, in Ardley Island, the TOC content was up to 17.0% in the soil and even 48.0% on average in the three dropping-amended soils, which is much higher than others from penguin guano (Sun et al., 14.65 ± 1.36%;31 Wang et al., < 9%.32). As discussed previously, the abundant TOC contents in the dropping-amended soils indicated that the soils could mostly be made up of penguin guano and plant residues.
Soil organic matter is generally considered a preferential site for the sorption of hydrophobic pollutants.33 Both POPs concentration and TOC were log-transformed before correlation in order to have normal distributed variables according to the Kolmogorov–Smirnov test. Statistical significance was found for most detected congeners (p < 0.05, Table S2, ESI†) expect for CB-11, 169, BDE-85, 99 and 138. This good correlation between POPs concentration and TOC is in agreement with studies encompassing the global distribution of PCBs in background surface soils,34organochlorine compounds in mountain soils from the subtropical Atlantic region (Teide, Tenerife Island)35 and our previous investigation on PCBs and PBDEs in surface soil from the Tibetan Plateau.19
Regarding spatial distribution, the TOC normalized concentrations of PCBs and PBDEs showed a reversed pattern to that based on dry weight. Significantly higher concentrations were obtained in the sites around Fildes Peninsula than in Ardley Island, and the highest concentration was found at the west coast area (sampling site No.4, Seal Bay) (Fig. 2). No significant relationship was observed between the POPs concentrations and distances from the Chinese Great Wall Station. The higher TOC normalized concentrations around the peninsula might reflect local influences (e.g., human activities and tourism) on the POPs distribution rather than long-range atmospheric transport.
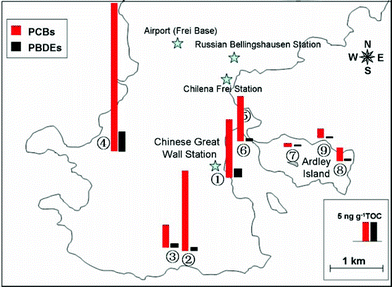 | ||
| Fig. 2 Spatial distribution of the TOC-normalized concentrations of PCBs and PBDEs in the soil and sediment samples. | ||
Acknowledgements
This study was jointly supported by the State Oceanic Administration, P.R. China (09/10GW08 and 10/11GW02), National Natural Science Foundation of China (20897011), and National Basic Research Program of China (2009CB421600). We also greatly thank Chinese Arctic and Antarctic Ministration for the arrangement during 26th Chinese Antarctic Expedition.References
- Arctic Monitoring and Assessment Programme, Workshop on Persistent Organic Pollutants (POPs) in the Arctic: Human Health and Environmental Concerns. AMAP Report 2000:1, http://www.amap.no. Search PubMed.
- F. Wania and D. Mackay, Ambio, 1993, 22, 10–18 Search PubMed.
- W. J. L. Sladen, C. M. Menzie and W. L. Reichel, Nature, 1966, 210, 670–673 CrossRef CAS.
- R. Fuoco, G. Capodaglio, B. Muscatello and M. Radaelli, A SCAR Publication, 2009 Search PubMed.
- D. C. G. Muir and C. A. de Wit, Sci. Total Environ., 2010, 408, 3044–3051 CrossRef CAS.
- Council of Managers of National Antarctic Programs, Antarctic Facilities in Operation, http://www.comnap.aq/operations/facilities. 2006.
- K. Breivik, A. Sweetman, J. M. Pacyna and K. C. Jones, Sci. Total Environ., 2007, 377, 296–307 CrossRef CAS.
- T. G. Negoita, A. Covaci, A. Gheorghe and P. Schepens, J. Environ. Monit., 2003, 5, 281–286 RSC.
- C. Hale, S. L. Kim, E. Harvey, M. J. La Guardia, T. M. Mainor, E. O. Bush and E. M. Jacobs, Environ. Sci. Technol., 2008, 42, 1452–1457 CrossRef.
- Y. Ishikawa, Y. Noma, T. Yamamoto, Y. Mori and S. Sakai, Chemosphere, 2007, 67, 1383–1393 CrossRef CAS.
- S. D. Choi, S. Y. Baek, Y. S. Chang, F. Wania, M. G. Ikonomou, Y. J. Yoon, B. K. Park and S. Hong, Environ. Sci. Technol., 2008, 42, 7125–7131 CrossRef CAS.
- H. X. Liu, Q. H. Zhang, Z. W. Cai, A. Li, Y. W. Wang and G. B. Jiang, Anal. Chim. Acta, 2006, 557, 314–320 CrossRef CAS.
- F. Borghini, J. O. Grimalt, J. C. Sanchez-Hernandez and R. Bargagli, Chemosphere, 2005, 58, 271–278 CrossRef CAS.
- J. Klánová, N. Matykiewiczová, Z. Mácka, P. Prosek, K. Láska and P. Klán, Environ. Pollut., 2008, 152, 416–423 CrossRef.
- R. Fuoco, M. P. Colombini and C. Abete, Int. J. Environ. Anal. Chem., 1994, 55, 15–25 CrossRef CAS.
- R. Fuoco, M. P. Colombini, C. Abete and S. Carignani, Int. J. Environ. Anal. Chem., 1995, 61, 309–318 CrossRef CAS.
- H. Park, S. H. Lee, M. Kim, J. H. Kim and H. S. Lim, Antarct. Sci., 2010, 22, 31–38 CrossRef.
- F. Wania, WECC-Report, 1998, 3 Search PubMed.
- P. Wang, Q. H. Zhang, Y. W. Wang, T. Wang, X. M. Li, Y. M. Li, L. Ding and G. B. Jiang, Chemosphere, 2009, 76, 1498–1504 CrossRef CAS.
- C. A. de Wit, M. Alaee and D. C. G. Muir, Chemosphere, 2006, 64, 209–233 CrossRef CAS.
- A. Hassanin, K. Breivik, S. N. Meijer, E. Steinnes, G. O. Thomas and K. C. Jones, Environ. Sci. Technol., 2004, 38, 738–745 CrossRef CAS.
- S. Focardi, C. Gaggi, G. Chemello and E. Bacci, Polar Rec., 1991, 162, 241–244 CrossRef.
- W. A. Lead, E. Steinnes and K. C. Jones, Environ. Sci. Technol., 1996, 30, 524–530 CrossRef CAS.
- E. Mariussen, E. Steinnes, K. Breivik, T. Nygård, M. Schlabach and J. A. Kålås, Sci. Total Environ., 2008, 404, 162–170 CrossRef CAS.
- G. T. Yogui and J. L. Sericano, Chemosphere, 2008, 73, 1589–1593 CrossRef CAS.
- L. A. Rodenburg, S. Du, B. Xiao and D. E. Fennell, Chemosphere, 2011, 83, 792–798 CrossRef CAS.
- M. Panero, S. Boehme and G. Munoz, New York Academy of Science, 2005 Search PubMed.
- S. Litten, B. Fowler and D. Luszniak, Chemosphere, 2002, 46, 1457–1459 CrossRef CAS.
- G. M. Frame, R. E. Wagner, J. C. Carnahan, J. F. Brown, R. J. May, L. A. Smullen and D. L. Bedard, Chemosphere, 1996, 33, 603–623 CrossRef CAS.
- D. Hu, A. Martinez and K. C. Hornbuckle, Environ. Sci. Technol., 2008, 42, 7873–7877 CrossRef CAS.
- J. J. Sun, R. B. Zhu, Y. S. Liu, Z. J. Gong and L. G. Sun, Chin J Polar Res, 2009, 21, 167–176 CAS.
- J. J. W. Y. H. Wang, X. M. Wang and L. G. Sun, Polar Biol., 2007, 30, 1475–1481 CrossRef.
- B. Marschner, J. Plant Nutr. Soil Sci., 1999, 162, 1–14 CrossRef CAS.
- S. N. Meijer, W. A. Ockenden, A. Seetman, K. Breivik, J. O. Grimalt and K. C. Jones, Environ. Sci. Technol., 2003, 37, 667–672 CrossRef CAS.
- A. Ribes, J. O. Grimalt, C. J. Torres Garcia and E. Cuevas, Environ. Sci. Technol., 2002, 36, 1879–1885 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: sampling and shipment of the samples, extrection and cleanup precedures of the experiment, relative distribution patterns of PCBs in all the samples (Fig. S1-S3), average concentrations of PCB and PBDE congeners (ranges) in each sample type (Table S1), Pearson correlation coefficients between the concentrations and TOC in the soil and sediment samples (Table S2). See DOI: 10.1039/c1ra00462j/ |
| This journal is © The Royal Society of Chemistry 2012 |
