New polyoxometalate-functionalized cellulosic fibre/silica hybrids for environmental applications
J. A. F.
Gamelas
*a,
M. G.
Evtyugina
b,
I.
Portugal
c and
D. V.
Evtuguin
*c
aCIEPQPF and Department of Chemical Engineering, University of Coimbra, Pólo II—R. Sílvio Lima, 3030-790, Coimbra, Portugal. E-mail: jafgas@eq.uc.pt (J. A. F. Gamelas)
bCESAM and Department of Environment and Planning, University of Aveiro, Campus Universitário de Santiago, 3810-193, Aveiro, Portugal
cDepartment of Chemistry and CICECO, University of Aveiro, Campus Universitário de Santiago, 3810-193, Aveiro, Portugal. E-mail: dmitrye@ua.pt (D. V. Evtuguin)
First published on 24th November 2011
Abstract
Cellulosic fibre/silica hybrid materials functionalized with Keggin-type polyoxometalates ([PV2Mo10O40]5−, [PVMo11O40]4−, ([PMo12O40]3− or [PW12O40]3−) were prepared by a sol–gel method at room temperature. The novel materials are composed of ca. 56 wt% of polysaccharides, ca. 37 wt% of propylamine-modified silica, 2 wt% of polyoxometalate, and 5% of hydration water. The silica network of these hybrids exhibits relatively high degree of condensation being distributed mainly on the surface of the cellulosic fibres as a dense film. The functionalization of silica with polyoxometalates via electrostatic interactions with protonated propylamino groups of modified silica was unambiguously confirmed. Despite their high silica content cellulose/silica hybrids retained basic cellulosic pulp properties—supramolecular and fibrous structure, porosity, relatively low density, etc. The novel bio-based material functionalized with 2% of [PVMo11O40]4− shows particularly high activity towards the oxidation of volatile organic compounds (VOCs) present in urban air thus anticipating future environmental applications.
Introduction
The combination of an inorganic phase with organic polymers at nano-, meso- or micro-scale affords organic/inorganic hybrid materials. These hybrids have received a great deal of attention in the last decade due to the unique characteristics obtained by the synergistic combination of inorganic and organic counterparts, with complementary properties.1–3 Furthermore, hybrids based on natural polymers are especially attractive because of the biodegradability and renewable properties of the latter that reduce end-of-life disposal problems. In particular, cellulose is the most abundant natural polymer with numerous technical applications from papermaking and plastics to biomedicine.4 Value-added applications of cellulosic materials are substantially extended upon appropriate chemical modification of native polymer (etherification, esterification, silylation, etc.).4–11 Among recent trends, cellulose derivatives of particular interest also include cellulose-based hybrids comprising an inorganic phase chemically linked or physically bound to cellulose.12,13Hybrid materials of cellulose or chemically modified cellulose with silica are the most widely reported cellulose-based hybrids.14–26Silica, a bioactive and biocompatible inorganic material, can improve the thermal stability of cellulose, its lipophilic behavior, and its affinity towards specific substrates.17–19,24–26 The incorporation of silica into a cellulosic matrix has been reported for hydroxypropyl cellulose14 and cellulose acetate,15 affording higher mechanical resistance of the ensuing materials. A silica based hybrid material with mechanical properties similar to those of cortical bones has been produced from cellulose acetate, which has bioresorption properties.16
Recently, cellulose/silica hybrids with potentially attractive mechanical and thermal properties have been synthesized by a mild sol–gel method, using heteropolyacids as catalysts.18 Bleached kraft cellulosic pulp was used as cellulose source and tetraethoxysilane as the silica precursor. Silica was deposited on the fibres in the form of a thin film or as discrete particles attached to the cellulose surface predominantly by non-covalent bonds. Under optimized synthesis conditions roughly 40–60 wt% of silica was incorporated into the cellulosic material considerably diminishing its hydrophilicity and improving thermal stability. These were considered as prospective materials for thermal insulation or packing purposes.19
The surface modification of cellulose with pre-hydrolysed/condensed alkoxysilanes having variable functions (propylamine, alkyl, and others) is expected to influence the hydrophilicity/hydrophobicity balance and the electron donor/acceptor properties of the ensuing hybrid materials.21–25 Particularly, hydrophobization of cellulose has been reported by using alkyl-substituted silanes19,25 whereas the modification of silica with triazine derivatives has been reported to improve target fixation of reactive dyes in cellulose/silica hybrids.26 The introduction of propylamino moieties on the surface of cellulose-silica hybrids, which can be achieved applying tetraethoxysilane and triethoxypropylamine silane in the sol–gel synthesis, could be useful to enable the electrostatic surface anchoring of polyoxometalates (POM).27–29 In this way, POMs with oxidative or acid catalytic properties,30–32 may be incorporated thus conferring catalytic properties to the cellulose-based hybrids.
To the best of our knowledge, cellulose fibres have never been reported as support material for POMs, although cellulose acetate was used as support for Keggin heteropolyacids (H3PW12O40 and H3PMo12O40).33 Furthermore, Keggin polyoxometalate (H4SiW12O40) nanotubes have been prepared by calcining layer-by-layer structured hybrid films coated cellulose acetate nanofibres.34 In the present work, new cellulose/silica hybrid materials incorporating polyoxometalates, [PV2Mo10O40]5−, [PVMo11O40]4−, [PMo12O40]3− and [PW12O40]3− as redox functionalities were synthesized and characterized. The catalytic activity of some of these hybrids for the oxidation of volatile organic compounds (VOCs) present in polluted urban air is revealed.
Experimental
Materials and methods
“H5[PV2Mo10O40]·11H2O” (1) and “H4[PVMo11O40]·10H2O” (2) were prepared following known procedures.35 It is noteworthy that, following the literature procedures, the heteropolyacid 1, in addition to the several isomers of [PV2Mo10O40]5−, also contained some amount of α-[PVMo11O40]4− (25 mol%) and [PV3Mo9O40]6− (8 mol%), as determined by 31P NMR spectroscopy in aqueous solutions. The heteropolyacid 2, besides α-[PVMo11O40]4−, also contained [PV2Mo10O40]5− (15%). These are common chemical compositions for these heteropolyacids obtained by the ether-extraction method.35–37 Notwithstanding, since [PV2Mo10O40]5− and α-[PVMo11O40]4− are by far the dominant polyoxoanions present in 1 and 2, respectively, the latter compounds may be described by the formulas above in quotes as a matter of simplification. H3[PMo12O40]·nH2O (3), H3[PW12O40]·nH2O (4), tetraethoxysilane (TEOS, 98% w/w), 3-aminopropyltriethoxysilane (APTS, 95% w/w) and ethanol (99.5% w/w) were supplied by Sigma-Aldrich and were used as received. Eucalyptus globulus industrial bleached kraft pulp, used as cellulose fibre source, was supplied by Portucel (Portugal). This pulp (hereafter mentioned as cellulosic pulp or cellulose) contained about 85 wt% (on oven-dry pulp weight) of cellulose and 14 wt% of glucuronoxylan. Prior to its use, 1.5 g of air dried cellulosic pulp was disintegrated (swollen) in 150 mL of water for 48 h, filtered and then washed with two 50 mL portions of ethanol to remove the excess of water.29Si and 31P solid-state magic angle spinning (MAS) NMR spectra and 13C–1H solid-state MAS NMR with cross-polarization (13C CP/MAS NMR) spectra were recorded on a 9.4T Bruker Avance-400 spectrometer, as previously reported.38 Chemical shifts in the 29Si and 13C NMR spectra were referenced to TMS, using kaolinite and glycine, respectively, as external references. For the 31P NMR spectra, the chemical shifts are referenced to phosphoric acid (85%). A large number of scans were required to obtain 29Si and 31P NMR spectra of acceptable quality due to the low content of silica and polyoxometalate in the hydrid materials.
The analyses of Mo, W, V and P were carried out by ICP spectrometry. For that, the hybrid materials were previously digested in a mixture of HF/HNO3/H2O2. Elemental analysis (C, H and N) was done using a Leco CHNS-932 apparatus. Thermogravimetric analyses were carried out between 30 and 700 °C under nitrogen at a heating rate of 5 °C min−1 on a TGA-50 Shimadzu thermobalance. Infrared absorption spectra of samples in KBr pellets were recorded on a Mattson 7000 FTIR spectrometer. Diffuse reflectance spectra were registered on Jasco V-560 spectrophotometer, using MgO as reference.
Scanning electron microscopy (SEM) images were obtained on a FEG-SEM Hitachi S4100 microscope coupled with an energy dispersive X-ray spectrometer (EDS) and operating at 25 kV, using gold coated samples.
Mercury intrusion porosity was determined using the AutoPore IV 9500 instrument from Micromeritics. The pressure range varied between 0.5 psi and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi, which enabled the measurement of pore diameters between 350 μm and 0.006 μm, respectively. The total porosity was calculated as the ratio of the total intruded volume and the sample bulk volume.
000 psi, which enabled the measurement of pore diameters between 350 μm and 0.006 μm, respectively. The total porosity was calculated as the ratio of the total intruded volume and the sample bulk volume.
Wide angle X-ray diffraction data were collected using a Philips X'Pert diffractometer operating in the Bragg–Brentano configuration with Co-kα (λ = 1.79 Å) source at a current of 35 mA and an accelerating voltage of 40 kV. Data were collected by the step counting method (step 0.025° and time 1.0 s) in the 2θ range of 4–50°. Pellets of about 1 cm diameter and 1 mm thickness were prepared for the analysis by pressing the samples at 50 MPa during 3 min. The peak fitting was performed using the program Profile Fit (Philips Analytical X-ray Almelo, 1996, version 1.0).
Synthesis of cellulose/silica hybrid materials functionalized with polyoxometalates
A mixture of 37.5 mL of TEOS, 1.55 mL of APTS and 20.5 mL of ethanol was added to 10.2 mL of water acidified with 70 μl of concentrated nitric acid (65%) under mechanical stirring (200 rpm). Thereafter, while keeping the reaction flask in a water bath at room temperature (ca. 20 °C), 800 μL of concentrated nitric acid was added affording a pH ≅ 1. The resulting solution was left reacting for 40 min, the stirring rate was increased to 350 rpm and ammonia (2.5% w/w, ca. 3.5 mL) was slowly added in 250 μL portions until the pH ≅ 3. Subsequently the stirring rate was reduced to 200 rpm and the resultant sol was allowed to react for 10 min. To this formulation 1.5 g of previously swollen cellulosic pulp was added in small portions and the suspension left reacting for 5 min more. Thereafter, POM (1 or 2, 0.10 g) or (3 or 4, 0.30 g), dissolved in 2 mL of ethanol was added to the suspension and the reaction left running for further 15 min for POM 1 or 2 or 30 min for POM 3 or 4. The final material was filtered on a glass G3 filter and dried under vacuum at room temperature.An unmodified cellulose/silica hybrid (free of propylamino moieties) was prepared by adding 53.0 mL of TEOS to 16 mL of water containing 60 μl of concentrated nitric acid (65%). The emulsion was stirred at 350 rpm for 1 h, at room temperature, and then 200 μl of ammonia (2.5% w/w) was added affording a pH ≅ 3. After 5 min, the stirring rate was reduced to 200 rpm and 1.5 g of previously swollen cellulosic pulp was added. The suspension was left reacting for another 5 min and an aqueous solution of POM (1, 0.10 g in 2 mL) was added and allowed to react for 15 min more under stirring. The solid material was filtered on a glass G3 filter and dried under vacuum at room temperature.
Oxidation of volatile organic compounds (VOCs) present in urban air
Urban air-containing sample was collected in an urban zone near the North entrance of the University of Aveiro (Portugal), during the sunny day of 18 August 2009 from 16:00 to 17:00 h.Teflon test tubes (20 cm × 0.4 cm i.d.) were filled with a known amount of the cellulose/aminopropylated silica hybrid materials functionalized and non-functionalized (control experiment) with POMs. Urban air was pumped through the Teflon tubes at a flow rate of 200 mL min−1 and the C5–C11 VOCs in the effluent stream were collected in a stainless steel tube trap (15 cm × 6.0 mm o.d. × 5.2 mm i.d.) filled with 150 mg of Tenax-TA (60–80 mesh) and 150 mg of Carbopack B (60–80 mesh). The samples were desorbed and analysed by the thermal desorption/cryogenic concentration method, on a Crompack® CP 9000 gas chromatograph (GC) equipped with Chrompack Thermal Desorption–Cold Trap Injection (TCT) unit and a flame ionization detector (FID) as described previously.39
Results and discussion
Synthesis of cellulose/silica hybrids functionalized with polyoxometalates
Novel cellulose-based hybrid materials containing silica modified with propylamino groups and small amount of polyoxometalate were prepared by sol–gel technique in an aqueous-ethanolic medium at room temperature (ca. 20 °C). Two main steps for the formation of the silica network in the cellulosic material were involved: (i) hydrolysis of appropriate amounts of TEOS and APTS (molar ratio 96/4) precursors at pH ≅ 1; (ii) condensation of the hydrolysed precursors affording a sol at pH ≅ 3 followed by the addition of bleached cellulosic pulp to the suspension in order to obtain the cellulose/modified silica material. The polyoxometalate is thereby incorporated into the hybrid material via electrostatic interaction with the protonated amino groups of the modified silica. It should be noted that, for the polyoxoanions [PMo12O40]3− (PMo12) and [PW12O40]3− (PW12) a higher amount of POM was added to the reaction medium, taking into account the lower charge of these polyoxoanions when compared to the [PVMo11O40]4− (PVMo11) and [PV2Mo10O40]5− (PV2Mo10) analogues.The cellulose/silica functionalized materials containing PVMo11 and PV2Mo10 present a bright yellow colour, due to the presence of polyoxometalate, while those of PMo12 and PW12 were pale green and white, respectively (Fig. 1). The hybrid with PVMo11 was found to be highly sensitive to the atmosphere as slow change to green colour could be observed upon brief exposure to laboratory air. This was due to the reduction of PVVMo11 to PVIVMo11.40 However, when the latter solid was immediately stored in an exsiccator colour change was not observed. The extensive washing of the new hybrid materials with water or ethanol did not reveal significant leaching of the polyoxometalates (less than 1% of the POM was extracted into the solvent).
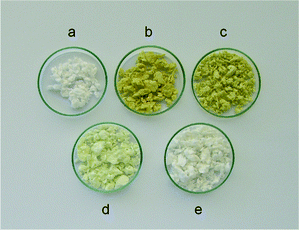 | ||
| Fig. 1 Photograph of cellulosic pulp (a) and cellulose/silica hybrids functionalized with PV2Mo10 (b), PVMo11 (c), PMo12 (d) and PW12 (e). | ||
For comparison, a hybrid material was prepared without APTS, i.e., using TEOS as the only precursor, and PV2Mo10 as polyoxometalate. Under these conditions, the material obtained was pale yellow with a very low amount of polyoxometalate (Table 1), which is easily leached. This confirms the role of the amino groups in promoting the chemical linkage between polyoxometalate and silica.38,41
| Material | Mo,W (%) | V (%) | Mo/V (molar ratio)c | POM (%)d | Cellulose (%)e | SilicaNH3 (%)f | N (%) | Propylamine (%)g |
|---|---|---|---|---|---|---|---|---|
| a Cellulose/propylamine-modified silica materials incorporating different polyoxometalates. b Hybrid material prepared without modified silica, i.e., experiment performed with no APTS. c In parenthesis, the value expected for the corresponding molybdovanadophosphate anion. d Based on the Mo (or W) analysis. e Based on the thermogravimetric analysis. f Obtained by difference (100-solvent (%)-cellulose (%)-POM (%)). g Based on the nitrogen elemental analysis and charge balance equations. h Amount of unmodified silica. | ||||||||
| Cell/SiO2(C3H6NH3)-POMa | ||||||||
| PV2Mo10 | 1.41 | 0.14 | 5 (5) | 2.5 | 54.3 | 37.0 | 1.12 | 2.5 |
| PVMo11 | 1.05 | 0.062 | 9 (11) | 1.8 | 55.0 | 37.7 | 1.08 | 2.4 |
| PMo12 | 0.65 | — | — | 1.0 | 58.1 | 35.4 | 1.02 | 2.2 |
| PW12 | 1.05 | — | — | 1.4 | 56.8 | 35.6 | 1.00 | 2.1 |
| Cell/SiO2-POMb | ||||||||
| PV2Mo10 | 0.39 | 0.042 | 5 (5) | 0.7 | 50.6 | 43.9h | — | — |
Characterization of polyoxometalate-functionalized cellulose/silica hybrids
The novel materials were characterized by several spectroscopic and analytical techniques. Characterization by solid-state NMR provided important information regarding the structural features of the different components of the materials. 13C CP/MAS NMR spectrum (Fig. 2) showed six high intensity signals in the 50–120 ppm range, assigned to known carbon resonances of cellulose framework.42 The position and relative intensity of these resonances in the hybrids were similar to those observed in the spectrum of initial cellulosic fibres. Hence, this suggests that the chemical structure of cellulose was maintained in the hybrid materials and that, apparently, no covalent bonding between the cellulose and silica framework has been established. In addition, three signals of low intensity were observed at 42.5, 21 and 9 ppm (Fig. 2). These are due to the C1, C2 and C3 carbon resonances, respectively, of the propylamino moieties (–H2N–C(1)H2–C(2)H2–C(3)H2–Si).38,43 According to previous studies of Caravajal et al.43 the C2 chemical shift at 21 ppm is an indication of protonation of the NH2 group. Otherwise, for neutral amino groups the C2 resonance would appear at 27 ppm.43 Thus, the 13C NMR results indicate the presence of propylamino moieties protonated at the NH2 group. This result is expected because the reactions were performed in acidic conditions (the pH values of the reaction filtrates were about 1.5–2.0).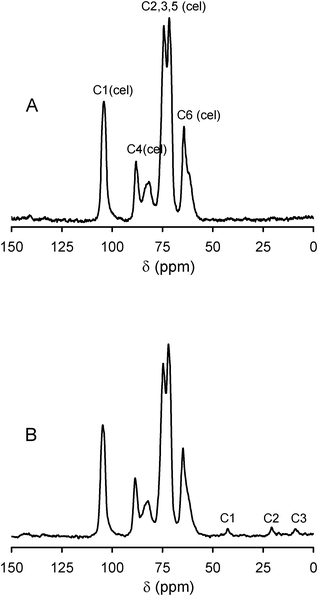 | ||
| Fig. 2 13C CP/MAS NMR spectra of initial cellulosic pulp (A) and of cellulose/silica hybrid functionalized with PVMo11 (B). | ||
Information on the silica counterpart of the hybrids was provided by 29Si NMR. 29Si MAS NMR spectra of the hybrid materials showed, in the −80 to −120 ppm region, three signals at −92 ppm (8%), −101 ppm (50%) and −110 ppm (42%) (Fig. 3), corresponding to (SiO)2Si(OH)2 (Q2), (SiO)3SiOH (Q3) and (SiO)4Si (Q4) groups, respectively.41,43,44 According to the relative abundance of Qn structures, the results here obtained indicate reasonably high degree of condensation of the bound silica frameworks (this being, however, incomplete), being similar to those reported for the cellulose/silica hybrid materials obtained by heteropolyacid catalysed sol–gel process18 and for silica materials containing POM clusters.44 In the 29Si MAS NMR spectra of the hybrids (Fig. 3), in addition to the Qn peaks, a signal centred at ca. −66 ppm was also observed. This signal assigned to RSi(OSi)3 (R = CH2−CH2−CH2−NH3+)43,44 confirms the linkage of propylamino moieties at the silica surface. No peak was observed at −45 ppm, which is the 29Si chemical shift that may arise from the 29Si resonance of non-reacted APTS physically sorbed at the silica surface.43
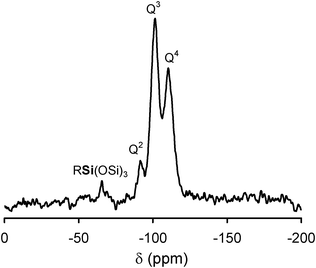 | ||
| Fig. 3 29Si MAS NMR spectrum of cellulose/silica hybrid functionalized with PV2Mo10. | ||
31P MAS NMR spectra showed single signals at −4.1 ppm for both PV2Mo10- and PVMo11-incorporated cellulose/silica materials (Fig. 4). Similar results were obtained after immobilization of the same polyoxometalates on propylamine-modified silica support,38 thus confirming the presence of the molybdovanadophosphate anions in the hybrid materials. As was found for other materials,38 no clear distinction between the two polyoxometalates, [PV2Mo10O40]5− and [PVMo11O40]4−, when immobilized in the cellulose/silica support could be made by this technique. In the case of [PV2Mo10O40]5−, which appears in solution mainly as a mixture of multiple positional isomers,36 a possible structural rearrangement of [PV2Mo10O40]5− to the most stable isomer/isomers accompanied by electrostatic interaction with the support material took place.38Cellulose/silica materials containing [PMo12O40]3− and [PW12O40]3− exhibited 31P NMR signals at −3.7 ppm and −15.2 ppm, respectively (Fig. 4). This is in agreement with reported values for the corresponding heteropolyacids, for composites involving these Keggin heteropolyanions in cellulose esters33 and with 31P NMR data for related materials,45 thus confirming the presence of the different Keggin species.
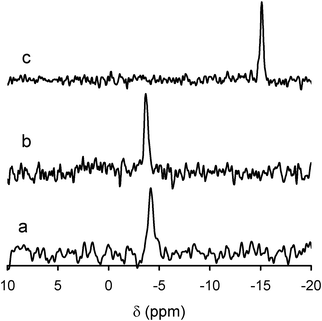 | ||
| Fig. 4 31P MAS NMR spectra of cellulose/silica hybrids functionalized with PVMo11 (a), PMo12 (b) and PW12 (c). Spectrum of PV2Mo10 hybrid (not shown) was very similar to the spectrum of PVMo11 hybrid (a). | ||
The Fourier transform infrared (FTIR) absorption spectra of the novel cellulose-based hybrid materials were identical to each other, i.e., no differences due to the presence of different polyoxometalates could be found. Thus, the absorption bands due to the Keggin-type polyoxometalate structure, which usually appear in the region of wavenumbers lower than 1100 cm−1,45 were masked by those of the cellulosic matrix and functionalized silica. This fact is not surprising taking into consideration the very low amount of POM in the hybrids (< 3 wt%, Table 1). However, notable differences were found when the spectra of the hybrids were compared to that of initial cellulosic pulp (Fig. 5). The FTIR spectrum of cellulosic pulp presents several characteristic bands, the most intense being between 1200–1000 cm−1 (mainly due to the C–O–C and C–O–H stretching vibrations46). For the hybrids, additional bands appeared at ca. 450 cm−1 and 795 cm−1 (Fig. 5, marked with *) assigned to δ (O–Si–O) and νs (Si–O–Si), respectively.44 The band expected at about 1080 cm−1 (νas (Si–O–Si)) was overlapped with the bands of cellulose in the same spectral region. In addition, a sharp band at 1385 cm−1 arose in the spectra of the hybrids, indicating the presence of nitrate anions.
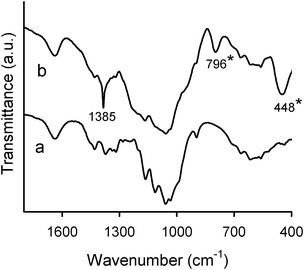 | ||
| Fig. 5 FTIR spectra of cellulosic pulp (a) and cellulose/silica hybrid functionalized with PV2Mo10 (b). | ||
Thermograms of hybrids showed three main steps of weight loss (Fig. 6): a first step up to about 120 °C corresponding to 5–8% weight loss, essentially due to moisture release; a second step between 150 and 230 °C (4.5–5.5% weight loss), which is tentatively attributed to the decomposition of the protonated propylamino moieties and nitrate anions (not observed in the hybrid without APTS); a third step accounting for more than 50% weight loss, beginning at about 250 °C and finishing at 600–700 °C, with maximum decay rate at ca. 295 °C for the hybrids with PV2Mo10 and PVMo11 and at 310 °C and 340 °C for those with PMo12 and PW12, respectively. It is noteworthy that cellulosic pulp presents its maximum decay rate at ca. 350 °C (Fig. 6). Thus, it seems, regarding the last decomposition step that silica(C3H6NH3+)-POM promoted, in general, the thermal decay of cellulose. Cellulosic pulp gave almost no char after the thermal degradation up to 800 °C (less than 1%) suggesting the presence of cellulose with relatively high crystallinity in the starting material.47 Accordingly, the thermogravimetric data enabled the approximate quantification of cellulose content in the cellulose/silica functionalized hybrids, which is of 54–58% (Table 1).
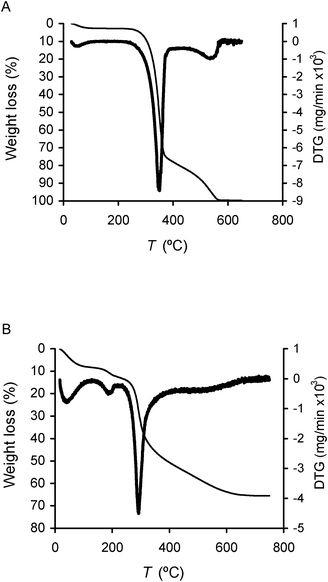 | ||
| Fig. 6 Thermogravimetric curve (thin line) and corresponding derivative curve (DTG, thick line) of cellulosic pulp (A) and cellulose/silica hybrid functionalized with PV2Mo10 (B). | ||
Based on the Mo (or W) elemental analysis the amount of polyoxometalate attached to the novel cellulose/silica functionalized materials was found to be of 1.0–2.5 wt% (Table 1). Moreover, the Mo/V molar ratio indicates that the PV2Mo10 and PVMo11 anions did not degrade under the experimental conditions used in the synthesis. On the other hand, the calculated amount of propylamine-modified silica was found to be of 35–38 wt% (Table 1). Based on the nitrogen elemental analysis and charge balance equations (i.e., considering that the charge of protonated propylamine is balanced by the charge of nitrate anions and polyoxoanions) the amount of propylamino moieties was estimated to be of 2.1–2.5 wt% (Table 1). The amount of nitrate anions was estimated to be 2.2–2.3 wt%.
The cellulose/silica functionalized materials containing PV2Mo10, PVMo11 or PMo12 showed significant absorption in the visible region of the diffuse reflectance spectra, with a characteristic shoulder at ca. 470 nm (PV2Mo10 and PVMo11 hybrids) and ca. 420 nm (PMo12 hybrid) (Fig. 7). The absorption of visible wavelength confirms the presence of polyoxometalates,45 since both initial cellulosic pulp (Fig. 7) and cellulose/modified silica hybrid without polyoxometalate do not absorb in the visible region (i.e., both samples are white). In the UV region, two bands centred at 320–330 nm and 240–250 nm were detected. These UV bands are mainly due to the presence of polyoxometalates, with some contribution of other components of the hybrids (cellulose and propylamine-modified silica). For the PW12 hybrid (white material) only a single band centred at 265 nm was observed (Fig. 7).
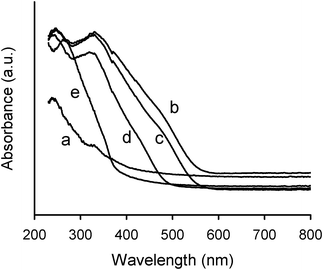 | ||
| Fig. 7 Diffuse reflectance spectra of initial cellulosic pulp (a) and cellulose/silica hybrids functionalized with PV2Mo10 (b), PVMo11 (c), PMo12 (d) and PW12 (e). | ||
It should be noted that heteropolyacids of PV2Mo10, PVMo11 and PMo12 present broad bands in the UV-Vis spectra with absorption maximum at 400–420 nm (O→Mo) and the corresponding heteropolyacid with PW12 presents two bands in the UV region with maxima near 330 nm and 260 nm (O→W).40,45,48. Therefore, in comparison to the Keggin heteropolyacids, a blue shifting of the O→Mo and O→W charge transfer bands occurred after POM immobilization in the cellulose-based hybrids. The different chemical environments of the polyoxoanions in the heteropolyacids, where a complex network of water molecules and hydrated protons surrounds the polyoxoanion49 and in the hybrids, in which the polyoxoanions are held in the silica support by electrostatic interactions with the protonated propylamino moieties, are responsible for the occurrence of the charge transfer bands at different energy. Similar behaviour occurred for PVMo11 immobilized on the silica surface modified with cationic quaternary ammonium moieties, [(CH2)3N(CH3)3]+.50
Wide angle X-ray diffractograms (Co–kα radiation) of cellulose/silica functionalized materials containing POM presented the non-resolved reflections from the 101 and 10ī planes around 18° and the reflection from the 002 plane at about 26° (Fig. 8). Therefore, the basic polymorph of cellulose I,51,52 present in the cellulose substrate (Fig. 8), was preserved in the hybrid materials. The degree of crystallinity was calculated by a procedure53 based on the integrated areas of the peaks from crystalline cellulose and amorphous halo as shown in Fig. 8. The value of crystallinity for the initial cellulosic matrix was 71.5% while for the cellulose/silica materials functionalized with POM degrees of crystallinity in the range of 70–72% were obtained. Since no significant decrease of the crystallinity of cellulose occurred in the hybrids, when compared to the initial cellulosic pulp, this suggests that the synthesis procedure did not significantly solvate the cellulose crystallites.
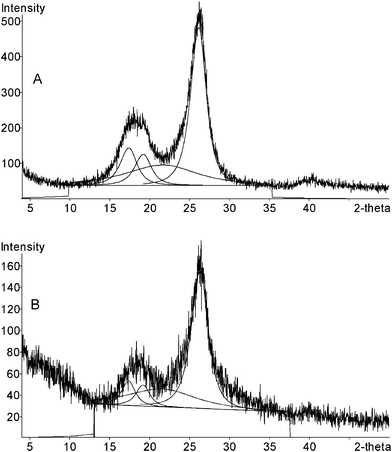 | ||
| Fig. 8 X-ray diffractograms of pellets of initial cellulosic pulp (A) and cellulose/silica hybrid functionalized with PV2Mo10 (B) showing fitted peaks from the crystalline reflections and the amorphous halo. | ||
SEM analysis of the novel materials showed that functionalized silica is coating cellulosic fibres predominantly in the form of a dense film (Fig. 9). The micrographs did not reveal discrete silica domains on the fibres surface or isolated silica between the fibres. EDS analyses confirmed the presence of C, O, and Si at the fibres surface as well as minor amounts of Mo. Overall, SEM-EDS analyses revealed, in general, fairly homogeneous coverage of cellulosic fibres with silica, although some regions might be only partially coated with silica as could be observed in high resolution micrographs (Fig. 9).
Mercury intrusion porosimetry results (Table 2) show that all cellulose-based materials presented in this work have very high porosity (>75%). The decrease of porosity in the hybrids, when compared to that of the initial cellulosic pulp, can be a consequence of the filling of some fibre pores by silica and POM. The high porosity of these materials is an important characteristic when considering their possible catalytic applications for solid-gas or solid-liquid heterogeneous oxidation reactions. In addition, the apparent skeletal density of the materials increased from 1.0 g cm−3 in cellulosic pulp to about 1.2–1.3 g cm−3 in the hybrids, mainly due to the silica coating on the fibres.
| Material | Porosity (%) | Skeletal density (g cm−3) |
|---|---|---|
| Cellulosic pulp | 83 | 1.04 |
| Cell/SiO2(C3H6NH3)-PV2Mo10 | 78 | 1.18 |
| Cell/SiO2(C3H6NH3)-PVMo11 | 76 | 1.25 |
| Cell/SiO2(C3H6NH3)-PMo12 | 80 | 1.30 |
Oxidation of urban air VOCs using polyoxometalate-functionalized cellulose/silica hybrids
The POM-functionalized cellulose/silica hybrids were tested for urban air VOCs removal, at ca. 25 °C, in order to evaluate the oxidative catalytic potential of these new materials in environmental applications. Polluted urban air was pumped through Teflon tubes filled with cellulose/silica hybrids without or with POMs (PVMo11 and PV2Mo10) and the treated air was analysed by GC/FID. Both catalysts showed reactivity with VOCs as revealed by change of colour of the starting hybrid materials. In fact, the yellow colour of POM-functionalized hybrids changed to green colour indicating the reduction of vanadium atoms in POMs (VV→ VIV) upon VOCs oxidation. Qualitative analysis of GC chromatograms (see example depicted in Fig. 10 for air treated with PVMo11-functionalized cellulose/silica hybrid) reveals the oxidation of the majority of C5–C11 VOCs towards low molecular weight degradation products (peak shift to low retention times) or oxygenated products (peak shift to high retention times). It is noteworthy that both catalysts could be regenerated by passing purified air (Air K) through the tubes, as revealed by colour change of hybrid materials (from green to yellow, VIV→ VV), being, however, the re-oxidation slower for the PVMo11 hybrid. Hence, aforementioned POMs incorporated in cellulose/silica hybrids may be suggested as effective and regenerable catalysts for VOCs oxidation. This opens new perspectives towards functional filtering materials for indoor and outdoor air purification. Further work on mechanistic studies and oxidation of particular air pollutants with cellulose/silica hybrids functionalized with POM is in progress.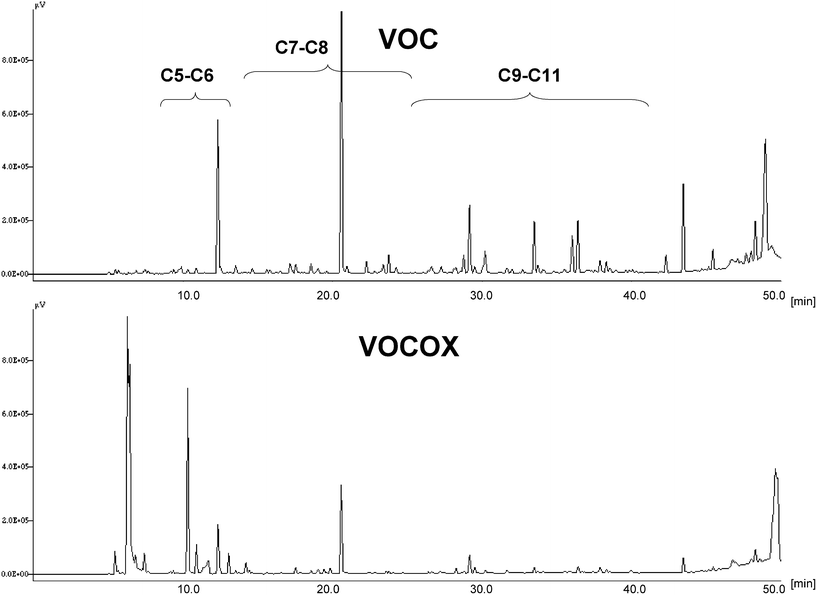 | ||
| Fig. 10 Normalised GC/FID chromatograms of VOCs in polluted urban air before (VOC) and after contact with PVMo11-functionalized cellulose/silica hybrid (VOCOX). | ||
Conclusions
The preparation of cellulose/silica hybrid materials functionalized with Keggin-type polyoxometalates employing sol–gel synthesis under mild conditions has been developed. The new bio-based materials are composed by ca. 37 wt% of propylamine-modified silica dispersed over cellulosic fibres (ca. 56 wt%) and a small amount (2%) of Keggin-type polyoxoanions attached electrostatically to the protonated amino groups (Fig. 11). Taking into account the larger excess of propylamino moieties to POM clusters (e.g.: estimated as 40/1 for [PVMo11O40]4−) only a small fraction of the protonated amino groups in modified silica should be involved in the interaction with POM cluster, with the major part being probably attracted to other counter-anions, namely nitrate anions. By analogy with other cellulose/silica hybrid materials obtained using similar procedure,18 it is presumed that the silica counterpart is bound to cellulosic fibres by hydrogen bonds between cellulose hydroxyl and siloxane groups (Fig. 11), since no covalent bonding (C–O–Si) between cellulose and silica has been detected. This covalent bonding, if it occurs, is of less importance and a subject of discussion. Tshabalala and co-workers,21 based on X-ray photoelectron spectroscopy results, proposed the covalent bonding of cellulose with siloxane groups in wood coated with polysiloxane by sol–gel process. However, in other studies of related cellulose/silica hybrid materials, the covalent bonding between cellulose and silica was not confirmed.17,18,20,22,25,26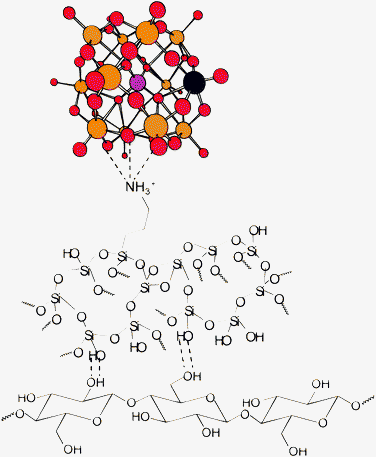 | ||
| Fig. 11 Schematic representation of the main plausible chemical interactions that occur in the novel cellulose/silica hybrids functionalized with POM (silica is bound to cellulose essentially by hydrogen bonding though minor frequency covalent bonding cannot be completely excluded). In the ball and stick representation of the Keggin structure of POM the P, Mo (or W) and O atoms are depicted as pink, orange and red spheres, respectively. The substituting heteroatom (vanadium) is shown in blue. | ||
Most of the original cellulose properties, namely its fibrous structure, crystallinity, high porosity, and low density, were preserved in the hybrid materials. Silica, that served as support for POMs, was intended to protect cellulose against “oxidative ageing” while avoiding the direct contact between POM and cellulose. However, this purpose was not completely fulfilled since some decrease in the thermal stability of cellulose was observed for the POM-functionalized hybrids (except for that containing [PW12O40]3−). Keggin-type POMs used in this study, [PVMo11O40]4− and [PV2Mo10O40]5−, in the form of cellulose/silica hybrids manifested, in general, redox behaviour comparable to that normally observed in liquid phase, i.e., POMs retained their catalytic properties upon immobilization. The preliminary results for VOCs oxidation in polluted urban air demonstrate the potential of these materials in gas-solid heterogeneous oxidative catalysis for eventual air purification purposes. Systematic studies on anthropogenic indoor and outdoor VOCs oxidation with the new POM-functionalized cellulose/silica hybrid materials are under way.
Acknowledgements
Authors thank Ana Paula Esculcas for precious help in the NMR spectra measurements.References
- A. P. Wight and M. E. Davis, Chem. Rev., 2002, 102, 3589 CrossRef CAS.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem. Int. Edit., 2006, 45, 3216 CrossRef.
- C. Sanchez, P. Belleville, M. Popall and L. Nicole, Chem. Soc. Rev., 2011, 40, 696 RSC.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem. Int. Edit., 2005, 44, 3358 CrossRef CAS.
- T. Heinze and T. Liebert, Prog. Polym. Sci., 2001, 26, 1689 CrossRef CAS.
- J. Kim, S. Yun and Z. Ounaies, Macromolecules, 2006, 39, 4202 CrossRef CAS.
- M. L Hassan, C. N. Moorefield and G. R. Newkome, Macromol. Rapid Commun., 2004, 25, 1999 CrossRef CAS.
- H. Tonami, H. Uyama and S. Kobayashi, Macromolecules, 2004, 37, 7901 CrossRef CAS.
- U. Bora, K. Kannan and P. Nahar, J. Membr. Sci., 2005, 250, 215 CrossRef CAS.
- C. Liu, R. Sun, A. Zhang, M. Qin, J. Ren and X. Wang, J. Agric. Food Chem., 2007, 55, 2399 CrossRef CAS.
- S. Boufi, M. R. Vilar, V. Parra, A. M. Ferraria and A. M. B. Rego, Langmuir, 2008, 24, 7309 CrossRef CAS.
- S. Yano, K. Iwata and K. Kurita, Mat. Sci. Eng. C-Bio S, 1998, 6, 75 Search PubMed.
- W. A. Daoud, J. H. Xin and Y. Zhang, Surf. Sci., 2005, 599, 69 CrossRef CAS.
- S. Yano, Polymer, 1994, 35, 5565 Search PubMed.
- R. A. Zoppi and M. C. Gonçalves, J. Appl. Polym. Sci., 2002, 84, 2196 Search PubMed.
- K. Tanaka and H. Kozuka, J. Mater. Sci., 2005, 40, 5199 Search PubMed.
- R. S. Gill, M. Marquez and G. Larsen, Micropor. Mesopor. Mat., 2005, 85, 129 Search PubMed.
- S. Sequeira, D. V. Evtuguin, I. Portugal and A. P. Esculcas, Mat. Sci. Eng. C-Bio S, 2007, 27, 172 Search PubMed.
- S. Sequeira, D. V. Evtuguin and I. Portugal, Polym. Compos., 2009, 30, 1275 Search PubMed.
- H. S. Barud, R. M. N. Assunção, M. A. U. Martines, J. Dexpert-Ghys, R. F. C. Marques, Y. Messaddeq and S. J. L. Ribeiro, J. Sol–Gel Sci. Technol., 2008, 46, 363 Search PubMed.
- M. A. Tshabalala, P. Kingshott, M. R. Vanlandingham and D. Plackett, J. Appl. Polym. Sci., 2003, 88, 2828 CrossRef CAS.
- M. Abdelmouleh, S. Boufi, M. N. Belgacem, A. Dufresne and A. Gandini, J. Appl. Polym. Sci., 2005, 98, 974 Search PubMed.
- B. Ding, C. Li, Y. Hotta, J. Kim, O. Kuwaki and S. Shiratori, Nanotechnology, 2006, 17, 4332 Search PubMed.
- A. Hou, Y. Shi and Y. Yu, Carbohydr. Polym., 2009, 77, 201 Search PubMed.
- X. Chen, Y. Liu, H. Lu, H. Yang, X. Zhou and J. H. Xin, Cellulose, 2010, 17, 1103 CrossRef CAS.
- J. Alongi, M. Ciobanu and G. Malucelli, Cellulose, 2011, 18, 167 Search PubMed.
- M. T. Pope and A. Muller, Angew. Chem. Int. Ed. Engl., 1991, 30, 34 CrossRef.
- M. T. Pope and A. Muller (ed.). Polyoxometalate Chemistry: From Topology Via Self Assembly to Applications, Kluwer, Dordrecht, 2001 Search PubMed.
- D. L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171 CrossRef CAS.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS.
- A. R. Gaspar, J. A. F. Gamelas, D. V. Evtuguin and C. Pascoal Neto, Green Chem., 2007, 9, 717 RSC.
- F. de C. Oliveira, J. Schneider, A. Siervo, R. Landers, A. M. G. Plepis, J. Pireaux and U. P. Rodrigues-Filho, Surf. Interface Anal., 2002, 34, 580 Search PubMed.
- B. Ding, J. Gong, J. Kim and S. Shiratori, Nanotechnology, 2005, 16, 785 CrossRef CAS.
- G. A. Tsigdinos and C. J. Hallada, Inorg. Chem., 1968, 7, 437 CrossRef CAS.
- L. Pettersson, I. Andersson, A. Selling and J. H. Grate, Inorg. Chem., 1994, 33, 982 CrossRef CAS.
- K. Nomiya, K. Yagishita, Y. Nemoto and T. Kamataki, J. Mol. Catal. A: Chem., 1997, 126, 43 CrossRef CAS.
- J. A. F. Gamelas, D. V. Evtuguin and A. P. Esculcas, Transition Met. Chem., 2007, 32, 1061 CrossRef CAS.
- M. G. Evtyugina, T. Nunes, C. Pio and C. S. Costa, Atmos. Environ., 2006, 40, 6277 Search PubMed.
- M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag, Berlin, 1983 Search PubMed.
- B. J. S. Johnson and A. Stein, Inorg. Chem., 2001, 40, 801 CrossRef CAS.
- K. Wickholm, P. T. Larsson and T. Iversen, Carbohydr. Res., 1998, 312, 123 CrossRef CAS.
- G. S. Caravajal, D. E. Leyden, G. R. Quinting and G. E. Maciel, Anal. Chem., 1988, 60, 1776 CrossRef CAS.
- R. C. Schroden, C. F. Blanford, B. J. Melde, B. J. S. Johnson and A. Stein, Chem. Mater., 2001, 13, 1074 CrossRef CAS.
- J. A. F. Gamelas, F. M. Santos, V. Felix, A. M. V. Cavaleiro, E. de Matos Gomes, M. Belsley and M. G. B. Drew, Dalton Trans., 2006, 1197 RSC.
- M. Kacurakova, A. C. Smith, M. J. Gidley and R. H. Wilson, Carbohydr. Res., 2002, 337, 1145 CrossRef CAS.
- H. Haykiri-Acma, S. Yaman and S. Kucukbayrak, Fuel Process. Technol., 2010, 91, 759 Search PubMed.
- J. A. F. Gamelas, A. M. V. Cavaleiro, E. de Matos Gomes, M. Belsley and E. Herdtweck, Polyhedron, 2002, 21, 2537 CrossRef CAS.
- G. M. Brown, M. R. Noe-Spirlet, W. R. Busing and H. A. Levy, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33, 1038 CrossRef.
- C. N. Kato, A. Tanabe, S. Negishi, K. Goto and K. Nomiya, Chem. Lett., 2005, 34, 238 Search PubMed.
- A. C. O' Sullivan, Cellulose, 1997, 4, 173 CrossRef CAS.
- D. Fengel and G. Wegener, Wood. Chemistry, Ultrastructure, Reactions, Walter de Gruyter, Berlin, 1984 Search PubMed.
- P. Ricou, E. Pinel and N. Juhasz, JCPDS—International Centre for Diffraction Data, Advances in X-ray Analysis, 2005, 48, 170 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |

