Synthesis of glycoconjugated poly(amindoamine) dendrimers for targeting human liver cancer cells†
Rui
Guo
ab,
Ying
Yao
c,
Guangcun
Cheng
c,
Su He
Wang
d,
Yong
Li
c,
Mingwu
Shen
a,
Yuehua
Zhang
d,
James R.
Baker
Jr.,
d,
Jianhua
Wang
*c and
Xiangyang
Shi
*ab
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai, 201620, People's Republic of China
bCollege of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai, 201620, People's Republic of China. E-mail: xshi@dhu.edu.cn; Fax: +86-21-67792306-804; Tel: +86-21-67792656
cDepartment of Biochemistry and Molecular & Cell Biology, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200025, People's Republic of China. E-mail: jianhuaw2007@gmail.com
dMichigan Nanotechnology Institute for Medicine and Biological Science, University of Michigan, Ann Arbor, MI 48109, USA
First published on 1st November 2011
Abstract
Amine-terminated generation 5 poly(amidoamine) dendrimers were utilized as a platform to conjugate fluorescein isothiocyanate and lactobionic acid. The conjugated lactobionic acid moieties enabled effective targeting to human liver cancer cells (HepG2) in vitro, which was demonstrated by both flow cytometry and confocal microscopy.
Dendrimers have been extensively applied in biomedical fields due to its unique structural characteristics, such as monodispersed nanoscale dimension, relatively hydrophobic internal cavity, and tunable surface functional groups.1–3 Particularly, in cancer diagnosis and therapy, dendrimers are employed not only as delivery vectors for anticancer drugs, genes and other therapeutic agents,4,5 but also as building blocks or platforms to form multifunctional imaging contrast agents.6,7 Although simple dendrimer-based carriers have been reported to increase the aqueous solubility of hydrophobic anticancer drugs, to improve the stability of nanoparticle-based imaging agents, to prolong their circulation time in bloodstream and passively target tumor tissues through enhanced permeation and retention (EPR effect), in some cases they still inevitably cause undesirable side effects and systemic toxicity due to the lack of tumor-specific delivery and accumulation. Therefore, target-specific ligands, such as folic acid,8–12antibodies,13,14aptamers,15,16 and RGD peptides,17–19 have been conjugated to the surface of dendrimers in order to obtain a high degree of selectivity to tumor tissues and to enhance the uptake of dendrimers into the target cells.
The detoxifying function of liver makes it possibly affected by a wide range of diseases, such as viral and bacterial infections, degenerative diseases, and tumors, etc. It was reported that asialoglycoprotein receptors (ASGPR) are present on the surface of hepatocytes and several human carcinoma cell lines with a high density, and show strong binding efficiency with galactose.20 Moreover, in addition to binding to galactose-containing ligands, ASGPR can also facilitate efficient intracellular uptake of the galactose ligands or the galactose ligand-modified conjugtaes.21,22 In a recent study, Yang et al. developed multifunctional hyperbranched poly(amidoamine) (PAMAM) nanoparticles bearing N-galactosamine moieties for liver cancer cell targeting and imaging.23 In another study, Medina et al. reported the synthesis of N-acetylgalactosamine-functionalized dendrimers as hepatic cancer cell-targeted carriers.24 These studies clearly indicate that the galactose derivative-functionalized nanoparticles are able to target liver cancer cellsviareceptor-mediated endocytosis.
For effective liver cancer cell targeting, lactobionic acid (La), a specific ligand bearing galactose, has recently been used in building nanocarriers that are able to selectively target liver cancer cellsvia the specific interactions between ASGPR on the cell surface and galactose residues.25–27 It is expected that the La moieties are also able to be covalently linked onto dendrimer surface for liver cancer cell targeting. In this communication, we attempted to covalently link both La moieties as targeting agents and fluorescein isothiocyanate (FI) as imaging agents onto partially acetylated generation 5 PAMAM dendrimers with a portion of amine termini. The synthesized conjugates were characterized by 1H NMR and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Moreover, the formed conjugates were demonstrated to have no cytotoxicity in vitro in the given concentration range, and could specifically target liver cancer cells, which was confirmed by flow cytometry and confocal microscopy. Compared with previous reports,22,24,28 this work presents a relatively simple preparation approach. To our knowledge, this is the first report related to the modification of La moieties onto dendrimers for effective liver cancer cell targeting.
Fig.1 illustrates the approach to synthesizing the multifunctional G5 PAMAM dendrimer-based conjugates. The number of terminal primary amine groups of each G5 dendrimer was estimated to be 110 according to our previous report.12 Firstly, 5 molar-equivalent FI moieties were covalently attached to the surface of each dendrimer, rendering the dendrimers with imaging capability. Then, 90 out of the remaining amino groups on the dendrimer surface were acetylated to minimize non-specific binding of the dendrimers to cell membranes and improve the biocompatibility of the dendrimer species. Finally, La molecules were conjugated to G5 dendrimerviaamide bonds between carboxyl groups of La molecules and the amine groups on the surface of G5 dendrimers to obtain the G5–FI–Ac–La product. In a similar manner, G5–FI–Ac was prepared as a control conjugate without La moieties, by acetylating all the remaining amino groups of G5 dendrimer after modification with FI.
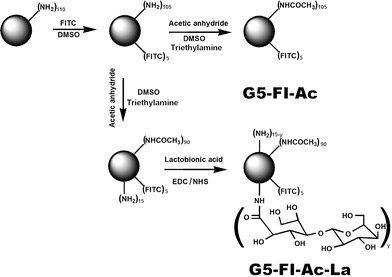 | ||
| Fig. 1 Schematic illustration of the synthesis of G5–FI–Ac–La conjugates. | ||
The chemical structures of the conjugates G5–FI–Ac and G5–FI–Ac–La were characterized by 1H NMR spectroscopy (Fig.2). In both samples, the characteristic proton peaks related to FI can be clearly seen in the aromatic region at 6.47 and 7.07 ppm, and the distinct peak at 1.87 ppm can be assigned to the –CH3protons of the acetyl group, confirming the successful modification of dendrimers. Compared to the spectrum of G5–FI–Ac, the new emergence of proton peaks from 3.40 ppm to 4.10 ppm in G5–FI–Ac–La indicated that La moieties were successfully conjugated. Based on NMR integration, the number of FI moieties attached to each dendrimer molecule was estimated to be 5.0, and all remaining amine groups of G5–FI–Ac were acetylated, which is consistent with our previous report.12 The number of acetyl groups in G5–FI–Ac–La was estimated to be 90, and the number of La modified on each dendrimer was calculated to be about 7.7, which is in agreement with our design. The successful modification of La molecules on G5 dendrimer was also confirmed by measuring the molecular weight of G5–FI–Ac–La using MALDI-TOF mass spectrometry (Fig. S1, Electronic Supplementary Information, ESI†). It is shown that after the modification of La, the molar mass of conjugates increases from 31703 to 32190. Further calculation proved that the number of La molecules conjugated onto each dendrimer was about 4.0, which somewhat deviates from the result deduced from NMR spectra. The deviation could be caused by the nature of the used method. NMR integration is based on the average number of moiety modification, whereas the MALDI-TOF mass spectrometry of dendrimers does not reflect the average molecular structure and often give a broad peak distribution, making precise identification of the molecular weight of dendrimers difficult. Overall, after the modification of La molecules, the G5–FI–Ac–La conjugates possess a proper density of galactose residues on their surface, which is essential for the efficient recognition and ASGPR-mediated cellular uptake (see below).
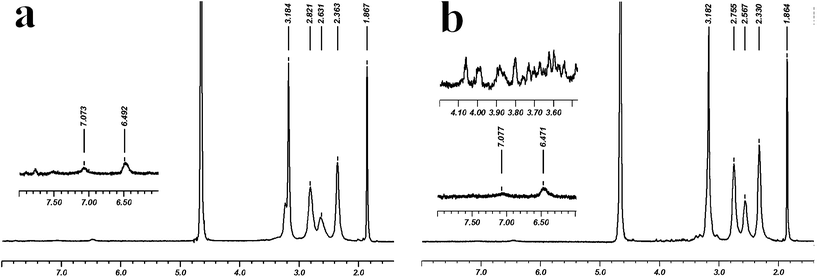 | ||
| Fig. 2 1H NMR spectra of (a) G5–FI–Ac and (b) G5–FI–Ac–La conjugates. | ||
For the application of liver cancer cell targeting, the synthesized multifunctional conjugates should be biocompatible in a given concentration range. The cytotoxicity of the synthesized conjugates of G5–FI–Ac and G5–FI–Ac–La were evaluated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay. Fig. 3 shows that the viability of human liver cancer cells (HepG2 cells) treated with the conjugates at different concentrations (0–2000 nM) is all above 95%, and no obvious inhibition of cell proliferation is observed. This indicates that the prepared conjugates are indeed biocompatible in the given concentration range, which is an important prerequisite for nanocarriers to be used in biomedical applications.
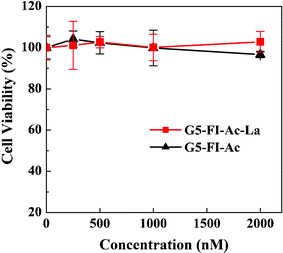 | ||
| Fig. 3 In vitro MTT cytotoxicity assay of the viability of HepG2 cells treated with G5–FI–Ac and G5–FI–Ac–La conjugates at different concentrations for 24 h. | ||
In order to examine the binding behavior of G5–FI–Ac–La to HepG2 cells, which have high-level ASGPR expression, the cells were incubated with multifunctional conjugates and assayed by flow cytometry (Fig. 4). At all tested dendrimer concentrations ranging from 50 nM to 1000 nM, the HepG2 cells treated with G5–FI–Ac–La exhibited a higher mean fluorescence than those treated with the control conjugate of G5–FI–Ac without La moieties under similar conditions. It is worth noting that although the control G5–FI–Ac conjugates do not have galactose residues, HepG2 cells incubated with them also showed a certain level of fluorescence. This can be explained by the fact that G5–FI–Ac can bind to cells through non-specific interaction.12,13,29 However, with the increase of conjugate concentration, the difference between these two groups enlarged, further confirming the significant role played by the modified La ligand on the dendrimer surface. At the concentration of 500 nM, the mean fluorescence of HepG2 cells treated with G5–FI–Ac–La was over 5-fold higher than that of the same cells treated with G5–FI–Ac. This result indicates that the La modification onto dendrimer surface strongly facilitates the uptake of conjugates by HepG2 cellsvia specific ligand-receptor interaction.
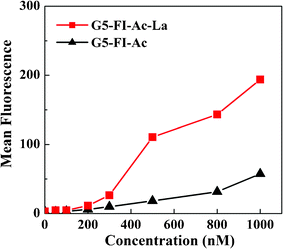 | ||
| Fig. 4 Dose-dependent binding of G5–FI–Ac and G5–FI–Ac–La conjugates to HepG2 cells after 2 h of incubation with HepG2 cells. | ||
The specific binding of G5–FI–Ac–La to HepG2 cells was also visually confirmed by confocal microscopic imaging (Fig. 5). The fluorescence images of HepG2 cells treated with G5–FI–Ac (Fig. 5b) showed almost no fluorescence signal which is quite close to PBS-treated HepG2 cells (Fig. 5a). In contrast, after being treated with the same concentration of G5–FI–Ac–La, HepG2 cells displayed prominent fluorescence signal (Fig. 5c). A further observation of Fig. 5c revealed that the fluorescence signal accumulated in the cytoplasm of the HepG2 cells. Therefore, it can be concluded that the G5–FI–Ac–La conjugates can be specifically endocytosed upon the incubation with HepG2 cells by virtue of the specific interactions between the ASGPR on cells and the La moieties on the dendrimer surface. It is worthwhile to note that flow cytometry data have actually shown that there is a certain level of non-specific binding of La-free dendrimers to HepG2 cellsvia passive endocytosis (Fig. 4). However, in comparison with the La-mediated specific cellular uptake, the non-specific binding was too low to be detected in the confocal microscopic images under similar operation conditions.
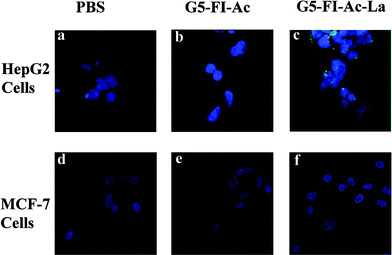 | ||
| Fig. 5 Confocal microscopic images of HepG2 cells (a, b, and c) and MCF-7 cells (d, e, and f) treated with PBS, G5–FI–Ac (500 nM) and G5–FI–Ac–La conjugates (500 nM) for 2 h, respectively. | ||
The targeting specificity of G5–FI–Ac–La conjugates to ASGPR-expressing cells was further confirmed by culturing a human breast adenocarcinoma cell line (MCF-7) with low level of ASGPR expression with the conjugates. Confocal microscopic images (Fig. 5d–e) showed that MCF-7 cells treated with G5–FI–Ac–La conjugates have almost no fluorescence signal, which is similar to those of the MCF-7 cells treated with PBS buffer and G5–FI–Ac conjugates. This implies that the specific cellular binding and uptake of G5–FI–Ac–La conjugates only occurs with ASGPR-expressing cancer cells. Obviously, their targeting ability will differ among different cell lines depending on the ASGPR expression level on the cell surface. It should be noted that healthy liver cells may also have a certain degree of ASGPR expression. In our future effort, the targeting efficacy of the La-modified dendrimers to both healthy liver cells and liver cancer cells will be compared to fully elaborate the dendrimer-based nanomedicine for specific liver cancer cell targeting.
In summary, we present a simple approach to synthesizing La–targeted dendrimer-based multifunctional conjugates. Flow cytometry and confocal microscopy demonstrated the in vitro targeting specificity of the multifunctional conjugates to human liver carcimona cells that have high-level ASGPR expression. With the unique features of dendrimers, these conjugates may be used as a multifunctional platform to construct liver cancer cell-targeted drug delivery systems or organic/inorganic hybrid imaging agents for a range of biomedical applications, especially in targeted cancer therapy and imaging.
Acknowledgements
Rui Guo and Ying Yao equally contributed to this work. This work was financially supported by the National Natural Science Foundation of China (20974019, 81071747, 81101150), the National Basic Research Program of China (973 Program, 2007CB936000, 2011CB510106, and 2011CB504300), the Program for Professor of Special Appointment (Eastern Scholar for X. S. and J. W.) at Shanghai Institutions of Higher Learning, the Fundamental Research Funds for the Central Universities (for R. G., M. S. and X. S.), and Shanghai Education Committee Key Discipline and Specialties Foundation (J50208). R. G. thanks the supports from Shanghai Natural Science Foundation (10ZR1400800).References
- A. R. Menjoge, R. M. Kannan and D. A. Tomalia, Drug Discovery Today, 2010, 15, 171–185 CrossRef CAS.
- C. C. Lee, J. A. MacKay, J. M. J. Frechet and F. C. Szoka, Nat. Biotechnol., 2005, 23, 1517–1526 CrossRef CAS.
- N. Joshi and M. Grinstaff, Curr. Top. Med. Chem., 2008, 8, 1225–1236 CrossRef CAS.
- X. Y. Shi, I. Lee, X. S. Chen, M. W. Shen, S. L. Xiao, M. F. Zhu, J. R. Baker, Jr. and S. H. Wang, Soft Matter, 2010, 6, 2539–2545 RSC.
- G. Thiagarajan, A. Ray, A. Malugin and H. Ghandehari, Pharm. Res., 2010, 27, 2307–2316 CrossRef CAS.
- M. W. Shen and X. Y. Shi, Nanoscale, 2010, 2, 1596–1610 RSC.
- S. Langereis, A. Dirksen, T. M. Hackeng, M. H. P. van Genderen and E. W. Meijer, New J. Chem., 2007, 31, 1152–1160 RSC.
- P. Singh, U. Gupta, A. Asthana and N. K. Jain, Bioconjugate Chem., 2008, 19, 2239–2252 CrossRef CAS.
- J. F. Kukowska-Latallo, K. A. Candido, Z. Cao, S. S. Nigavekar, I. J. Majoros, T. P. Thomas, L. P. Balogh, M. K. Khan and J. R. Baker, Jr., Cancer Res., 2005, 65, 5317–5324 CrossRef CAS.
- X. Shi, S. H. Wang, S. Meshinchi, M. E. Van Antwerp, X. D. Bi, I. H. Lee and J. R. Baker, Jr., Small, 2007, 3, 1245–1252 CrossRef CAS.
- T. P. Thomas, I. J. Majoros, A. Kotlyar, J. F. Kukowska-Latallo, A. Bielinska, A. Myc and J. R. Baker, Jr., J. Med. Chem., 2005, 48, 3729–3735 CrossRef CAS.
- Y. Wang, R. Guo, X. Y. Cao, M. W. Shen and X. Y. Shi, Biomaterials, 2011, 32, 3322–3329 CrossRef CAS.
- R. Shukla, T. P. Thomas, J. L. Peters, A. M. Desai, J. Kukowska-Latallo, A. K. Patri, A. Kotlyar and J. R. Baker, Jr., Bioconjugate Chem., 2006, 17, 1109–1115 CrossRef CAS.
- G. Wu, R. F. Barth, W. Yang, S. Kawabata, L. Zhang and K. Green-Church, Mol. Cancer Ther., 2006, 5, 52–59 CrossRef CAS.
- J. Zhou, B. Soontornworajit, J. Martin, B. A. Sullenger, E. Gilboa and Y. Wang, Macromol. Biosci., 2009, 9, 831–835 CrossRef CAS.
- J. Zhou, B. Soontornworajit and Y. Wang, Biomacromolecules, 2010, 11, 2087–2093 CrossRef CAS.
- R. Shukla, E. Hill, X. Shi, J. Kim, M. C. Muniz, K. Sun and J. R. Baker, Jr., Soft Matter, 2008, 4, 2160–2163 RSC.
- E. Hill, R. Shukla, S. S. Park and J. R. Baker, Jr., Bioconjugate Chem., 2007, 18, 1756–1762 CrossRef CAS.
- Z. Li, P. Huang, X. Zhang, J. Lin, S. Yang, B. Liu, F. Gao, P. Xi, Q. Ren and D. Cui, Mol. Pharmaceutics, 2010, 7, 94–104 CrossRef CAS.
- G. Ashwell and J. Harford, Annu. Rev. Biochem., 1982, 51, 531–554 CrossRef CAS.
- G. Ashwell and A. G. Morell, Adv. Enzymol. Relat. Areas Mol. Biol., 1974, 41, 99–128 CAS.
- J. Huang, F. Gao, X. X. Tang, J. H. Yu, D. X. Wang, S. Y. Liu and Y. P. Li, Polym. Int., 2010, 59, 1390–1396 CrossRef CAS.
- W. Yang, C. Y. Pan, X. Q. Liu and J. Wang, Biomacromolecules, 2011, 12, 1523–1531 CrossRef CAS.
- S. H. Medina, V. Tekumalla, M. V. Chevliakov, D. S. Shewach, W. D. Ensminger and M. E. H. El-Sayed, Biomaterials, 2011, 32, 4118–4129 CrossRef CAS.
- K. M. K. Selim, Y. S. Ha, S. J. Kim, Y. Chang, T. J. Kim, G. H. Lee and I. K. Kang, Biomaterials, 2007, 28, 710–716 CrossRef.
- D. Q. Wu, B. Lu, C. Chang, C. S. Chen, T. Wang, Y. Y. Zhang, S. X. Cheng, X. J. Jiang, X. Z. Zhang and R. X. Zhuo, Biomaterials, 2009, 30, 1363–1371 CrossRef CAS.
- Y. X. Yang, Z. W. Zhang, L. L. Chen, W. W. Gu and Y. P. Li, Biomacromolecules, 2011, 11, 927–933 CrossRef.
- D. Bhadra, A. K. Yadav, S. Bhadra and N. K. Jain, Int. J. Pharm., 2005, 295, 221–233 CrossRef CAS.
- T. P. Thomas, R. Shukla, A. Kotlyar, J. Kukowska-Latallo and J. R. Baker, Jr., Bioorg. Med. Chem. Lett., 2010, 20, 700–703 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, and MALDI-TOF mass spectra of G5-FI-Ac and G5-FI-Ac-La conjugates. See DOI: 10.1039/c1ra00320h/ |
| This journal is © The Royal Society of Chemistry 2012 |
