Study on the interfacial structures of Tin oxide/multiwalled carbon nanotube heterojunctions
Yong
Jia
ab,
Fan-Li
Meng
a,
Mei-Yun
Zhang
a,
Zheng
Guo
a,
Xing
Chen
a,
Tao
Luo
a,
Xu-Cheng
Fu
a,
Ling-Tao
Kong
a,
Jin-Huai
Liu
*a and
Xing-Jiu
Huang
*a
aResearch Center for Biomimetic Functional Materials and Sensing Devices, Institute of Intelligent Machines, Chinese Academy of Sciences, Hefei 230031, P. R. China. E-mail: xingjiuhuang@iim.ac.cn; jhliu@iim.ac.cn
bDepartment of Pharmacy, Anhui University of Traditional Chinese Medicine, Hefei 230031, P. R. China
First published on 6th January 2012
Abstract
Tin oxide/multiwalled carbon nanotube (SnO2/MWCNT) heterojunctions were synthesized using the SnCl2 solution method. The interfacial structures, especially the interactions between the SnO2 and MWCNTs, were investigated by field emission scanning electron microscopy, transmission electron microscopy, ultrasonic destruction experiments, Raman spectroscopy, and thermal analysis. At the initial reaction stage, chemical bonds were the predominant interactions between the MWCNTs and SnO2. Physical interactions, including Coulomb interactions and van der Waals forces, play key roles under an increasing reaction time. Raman spectroscopy and thermal analysis proved to be two valid methods for the characterization of the interactions between SnO2 and MWCNTs. The strong interactions result in a low G/D strength ratio, as well as a low thermal stability of the MWCNTs in the SnO2/MWCNT heterojunctions. The binding energy of the MWCNTs and SnO2 was calculated based on differential thermal analysis and Hess's law and the results agreed with the interfacial morphological characteristics. This work presents a facile and low-cost approach to study the interfacial structures of carbon-based nanomaterials and opens a new possibility for investigating structural property relationships.
1. Introduction
In the past few decades, carbon based nanomaterials have attracted great interest due to their unique properties. Among them, carbon nanotubes (CNTs)1 and their aggregations exhibit p-type semiconductor characteristics and have shown excellent sensing properties.2,3 CNT based nanocomposites are also attractive owing to their special properties, which to a great extent result from the synergistic reactions between the component species. Tin oxide (SnO2) is a typical n-type wide band gap semiconductor, and has been a widely utilized gas-sensing material.4–8 For polycrystalline SnO2 nanomaterials, the stability and repeatability of the sensors often suffered degradation owing to the aggregation and growth of the nanoparticles. The formation of SnO2/MWCNTs heterojunctions can avoid aggregation, which will improve the stability and repeatability of the sensors.9 In addition, the Schottky barrier between SnO2 and CNTs is very low,10,11 which is preferable for electron transport between SnO2 and MWCNTs. So, SnO2/MWCNT heterojunctions exhibit good sensing properties,11–15 as well as increased specific mass capacity and extended durability as anodes of lithium-ion batteries .16,17Several strategies have been performed to prepare SnO2/MWCNT heterojunctions, including chemical vapor deposition,18 the microwave-polyol process,19 the hydrothermal method,16 the vapor phase method,10 the solution mixing method,20 and the SnCl2 solution method.21,22 The SnCl2 solution method is the simplest, and the obtained interfacial structures are strongly dependent on the preparation conditions, such as the amount of HCl and the reaction temperature.17,20 For the characterization, some special methods, such as X-ray absorption near-edge structures,23–26 and atomic scanning transmission electron microscopy27 have been utilized for the study of the interfacial structures, especially the interactions between the component species of the carbon based materials. However, it is still a challenge to investigate the interfacial structures using common methods. In addition, the interaction mechanism has never been clearly understood.
In our previous work,28 using SnO2/MWCNT heterojunctions as a precursor, porous SnO2 nanotubes were prepared. The structures of the obtained SnO2 nanotubes were somewhat dependent on the SnO2/MWCNT heterojunctions, which were prepared with different reaction times. Thus indicating that the reaction time has some influence on the interfacial structures of the SnO2/MWCNT heterojunctions. However, few report systemic studies on the influences of the reaction time on their interfacial structures. Herein we report a facile approach to control the interfacial structures of SnO2/MWCNT heterojunctions by controlling the reaction time. In addition, chemical interactions, Coulomb interactions, and van der Waals forces were taken into account to explain the reaction time-controlled interfacial structures. Raman spectra and thermal analysis were employed to characterize the interactions between the SnO2 and MWCNTs. The binding energies of the SnO2/MWCNT heterojunctions were investigated based on Hess's law and differential thermal analysis.
2. Experimental
2.1. Preparation of SnO2/MWCNT heterojunctions
MWCNTs with a 20–30 nm diameter were purchased from Shenzhen Nanotech Port Co. Ltd. The purification processes of the MWCNTs are the same as in our previous report.28 In a typical synthesis of SnO2/MWCNT heterojunctions, 1 g of tin(II) chloride (SnCl2·2H2O, Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) was dissolved in 40 mL of distilled H2O, and then 0.25 mL of HCl (38%, Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) was added. Subsequently, 10 mg of the purified MWCNTs was dispersed in the above solution. The mixture was sonicated for 20 min and then stirred at room temperature for a certain time. The SnO2/MWCNT heterojunctions were then separated by centrifugation and washed with distilled water several times until the pH of the solution was neutral. The obtained SnO2/CNT heterojunctions were denoted as SnO2/CNT-X, where X is the reaction time (hr).2.2. Characterization
The prepared SnO2/MWCNT heterojunctions were characterized by field emission scanning electron microscopy (FE-SEM, Sirion 200, operated at 5 kV), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HR-TEM, JEOL-2010, operated at 120 kV), and Raman spectroscopy (Raman spectrum, LABRAM-HR, He–Ne laser excitation line at 514.5 nm). The weight content of SnO2 and the released heat was measured by thermal gravimetric analysis and differential thermal analysis (TGA and DTA, Pyris 1, heating rate 10 deg min−1 in flow air).3. Results and discussion
3.1. Influence of the reaction time on the interfacial structures
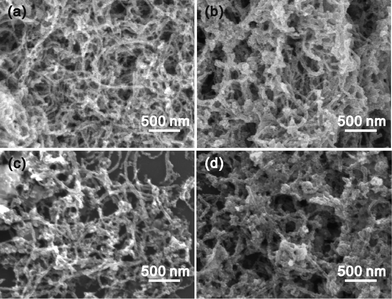
Fig. 1 presents the SEM images of the SnO2/MWCNT heterojunctions synthesized by controlling the reaction time from 1 to 4 h. With the increased reaction time, the coated particles tend to congregate together. TGA curves shown in Fig. 2 present the weight contents of SnO2 in the SnO2/MWCNT-1 and SnO2/MWCNT-2 were 39.0% and 42.3%, respectively. In SnO2/MWCNT-3, the weight content of SnO2 was only slightly increased to 44.1%. Further prolonging the reaction time to 4 h, the weight content decreased to 39.6%. It should be mentioned that all of the SnO2/MWCNT heterojunctions were prepared by using the same methods, except for the reaction time. The results imply that some SnO2 particles desquamated from the MWCNT surface in the fourth hour. However, from Fig. 1, it is clear that the diameters of the obtained heterojunctions increased with the increase in reaction time. The results suggest that SnO2/MWCNT heterojunctions with different structures were obtained. | ||
| Fig. 1 FE-SEM images of SnO2/MWCNT heterojunctions prepared with 1 h (a), 2 h (b), 3 h (c), and 4 h (d) of reaction time. | ||
 | ||
| Fig. 2 TGA curves of the SnO2/MWCNT heterojunctions prepared with different reaction times. | ||
TEM and HRTEM images give more convincing evidence, and the results are shown in Fig. 3 and 4. Fig. 3 present the interfacial structures of SnO2/MWCNT-2. The surface of the MWCNTs was coated with uniform and dense SnO2 nanoparticles, and the average crystallite size of the SnO2 nanoparticles was about 3–4 nm. The thickness of the SnO2 layer was less than 10 nm. Fig. 4 presents different interfacial structures of SnO2/MWCNT-4. The crystallite size was about 4 nm. However, though the weight content of the SnO2 is low, the SnO2 layer is obviously thicker than the ones of SnO2/MWCNT-2. From Fig. 4a–c, is clear that many SnO2 particles congregated together, and did not evenly coat the surface of the MWCNTs. Furthermore, some SnO2 particles left the MWCNTs completely uncovered, as shown in Fig. 4d. The results suggest the interactions between the SnO2 and MWCNTs were weaker than those of SnO2/MWCNT-2. Therefore some SnO2 particles were lost during the centrifugation and wash process.
 | ||
| Fig. 3 TEM (a, b) and HRTEM (c, d) images of SnO2/MWCNT-2. The white dashed line in (c) presents the sidewall of a CNT. | ||
 | ||
| Fig. 4 TEM (a, b, d) and HRTEM (c) images of the SnO2/MWCNT-4. The red circles present the congregated SnO2 particles, and the blue ones present the desquamated parts. The white dashed line in (c) presents the sidewall of a CNT. | ||
In order to test the above conjectures, the MWCNTs in SnO2/MWCNT-2 and SnO2/MWCNT-4 were removed by thermal treatment at 650 °C for 5 min in air.28SEM images of the obtained porous SnO2 nanotubes before and after thermal treatment are shown in Fig. 5a and b, respectively. The diameter of the SnO2 nanotubes obviously increased with the increase in reaction time. In Fig. 5b, some aggregated SnO2 particles were observed. The results are consistent with the morphology of the SnO2/MWCNT heterojunctions shown in Fig. 1. In addition, the above two heterojunctions were dispersed in water, and then treated by vigorous sonication for 1 h. After that, the MWCNTs were removed and the obtained SnO2 nanotubes are shown in Fig. 5c, d, respectively. In Fig. 5c, large numbers of unabridged SnO2 nanotubes and only a few aggregated SnO2 particles were observed. Compared with Fig. 5a, the results confirm the high stability of SnO2/MWCNT-2. However, after vigorous sonication, a great many of the aggregated SnO2 particles and only a few short SnO2 nanotubes were observed in Fig. 5d. The results mean some SnO2 particles were detached from the tubes during the sonication process due to the weak interactions between the SnO2 and MWCNTs.
 | ||
| Fig. 5 FE-SEM images of the SnO2 nanotubes obtained by calcination the SnO2/MWCNT-2 before (a) and after sonication (c). FE–SEM images of the SnO2 nanotubes obtained by calcination the SnO2/MWCNT-4 before (b) and after sonication (d). | ||
3.2. Characterization of the interactions between SnO2 and CNTs
In the SnO2/MWCNT heterojunctions, the interactions between the SnO2 and MWCNTs will change the structures and thermal stabilities of the MWCNTs. The SnO2/MWCNT heterojunctions were characterized by Raman spectrum, and the results are shown in Fig. 6a. The corresponding G/D intensity values are shown in Fig. 6b. The G/D values of the pure MWCNTs, the SnO2/MWCNT-1, and the SnO2/MWCNT-2 are 1.12, 1.10, and 1.07, respectively. With a longer reaction time, the G/D values increased. Though the change in the G/D values was not considerable, the results imply the change of the interactions between them. The D-band of MWCNTs is known to be related to defects, and the increase of the D band is often attributed to the direct sidewall functionalization of the MWCNTs.29,30 In this case, the aromatic carbons were converted from an sp2 to an sp3 hybridization, which resulted in an increase in the average size of the sp3 domains. Before the preparation of SnO2/MWCNT heterojunctions, the MWCNTs were calcined at 350 °C for 2 h in air, and then refluxed in 7.0 mol L−1 of HNO3 at 120 °C for 12 h. In the preparation process of SnO2/MWCNT heterojunctions, no more defects were formed because HCl was a non-oxidizing acid. Therefore , the decrease of the G/D values resulted from the increase in sp3 hybridization. The results also imply that the SnO2 coating promoted the formation of C–O bonds using the lattice oxygen of SnO2,31 which suggests the formation of chemical interactions between the SnO2 and MWCNTs. For SnO2/MWCNT-4, the G/D values were similar to the ones of pure MWCNTs. So, the chemical interactions weakened with the prolonged reaction time. | ||
| Fig. 6 Raman spectra (a) and the G/D values (b) of the SnO2/MWCNT heterojunctions prepared with different reaction times. | ||
Fig. 7a presented the DTG curves of pure MWCNTs and the SnO2/MWCNT heterojunctions. The onset oxidation temperatures (To) and the oxidation peak temperatures (Tp) are shown in Fig. 7b. The To of the pure MWCNTs is 477 °C. The To of the MWCNTs in the heterojunctions from SnO2/MWCNT-1 to SnO2/MWCNT-4 were 421 °C, 383 °C, 422 °C, and 446 °C, respectively. The change in Tp is similar to the change in To. The results indicate that the MWCNTs became unstable in the heterojunctions. Considering the diameter of MWCNTs, the lattice strain primarily determines the thermal stability, overruling the effect of defects of MWCNTs.32 The interactions between the SnO2 and MWCNTs result in a compressive stress along a direction perpendicular to the tube surface.33 So, the lattice strain resulting from the compressive stress determines the thermal stability of MWCNTs. In addition, the low G/D values, resulting from the strong interactions, also imply that the apparent activation energy for the oxidation of MWCNTs was decreased.34 As a result, the strong interactions will favor the oxidation of the MWCNTs by SnO2 particles in a similar manner to the Mars–van Krevelen mechanism.35 In Fig. 7a, b, the To and Tp of MWCNTs in SnO2/MWCNT-2 are the lowest, which further suggests that the interactions in SnO2/MWCNT-2 were the strongest.
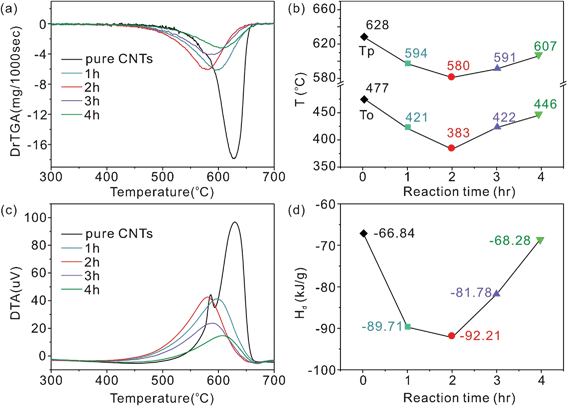 | ||
| Fig. 7 DTG (a) and DTA curves (c) of the SnO2/MWCNT heterojunctions prepared with different reaction times. (b) The relationships between the reaction time and the onset oxidation temperature (To), and the oxidation peak temperature (Tp). (d) The relationships between the reaction time and the released heat (Hd) of the heterojunctions. | ||
The strong interactions also have influence on the released heat by MWCNT decomposition (Hd). Fig. 7c presents the differential thermal analysis (DTA) curves of the SnO2/MWCNT heterojunctions, and the corresponding Hd values are shown in Fig. 7d. The Hd value of pure MWCNTs is −66.84 kJ mol−1. The Hd values of the MWCNTs in SnO2/MWCNT-1 and SnO2/MWCNT-2 increased to −89.71 and −92.21 kJ mol−1, respectively. Further increasing the reaction time to 3 h and 4 h, the Hd values were decreased to −81.78 and −68.28 kJ mol−1, respectively. Based on Hess's law, the different Hd values should be attributed to the different initial state of MWCNTs because the final oxidation states of the MWCNTs were the same. Fig. 8 presents a Bonn Haber cycle designed for the preparation and decomposition of SnO2/MWCNT heterojunctions. The binding energy (Hb) of SnO2 and MWCNTs in the heterojunctions can be calculated from the Hd values. The Hb values from the SnO2/MWCNT-1 to the SnO2/MWCNT-4 were 19.35, 25.37, 15.03, and 1.98 kJ mol−1, respectively, as shown in Fig. 8. The Hb values are much lower than the common chemical bonds, and similar to van der Waals forces. The results suggest that the chemical interactions between the SnO2 and the MWCNTs are very weak, which may be attributed to the inadequate contact between the particles and tubes. The high Hb value means the strong interactions. So, the interactions in SnO2/MWCNT-2 are the strongest, which consistent with the interfacial structural characters and the Raman analysis.
 | ||
| Fig. 8 Bonn Haber cycle presents the relationships between the released heat (Hd) and the binding energy (Hb) of the SnO2/MWCNT heterojunctions. The relationship between the reaction time and Hb of the heterojunctions. | ||
3.3. Influence of the reaction time on the interactions
It is well known that the addition of HCl can suppress the hydrolysis of SnCl2. Under the same experimental conditions, the minimum amount of HCl is 0.13 ml.21 The formation of SnO2 can be ascribed to the reaction between Sn2+ cations and dissolved oxygen in water. In the present work, 0.25 ml HCl was added, which is about 2 times greater than that of the minimum amount. At the initial reaction stage, the oxygen-containing functional groups on the side wall of MWCNTs act as active sites for the SnO2 coating.13,22 So, the chemical interactions between the SnO2 and MWCNTs are the predominant interactions. After 2 h of reaction, more SnO2 particles were formed and the MWCNTs were coated with close-packed multilayer SnO2 particles, as shown in Fig. 3. The outer particles could not form chemical bonds with MWCNTs so the coating of these SnO2 nanoparticles was attributed to physical adsorption via physical interactions.Coulomb interactions and van der Waals forces are two major physical interactions between the SnO2 particles and MWCNTs. In the present work, the strong acidity resulted in the formation of C–OH2+ and Sn–OH2+ on the MWCNT and SnO2 surface, respectively.36,37 As a result, the repulsive electrostatic force between the MWCNTs and the outer SnO2 particles was very strong. In addition, with the oxidation of Sn2+ to Sn4+ by the dissolved oxygen in water, the ionic strength of the solution was increased which resulted in the decrease of the repulsive electrostatic force between the particles.38 At the same time, the repulsive electrostatic force between the SnO2 and MWCNTs were decreased simultaneously. However, the radius vector between the outer SnO2 particles and MWCNTs was larger than for the SnO2 particles. The decreased repulsive electrostatic force between the SnO2 and MWCNTs can not avoid the congregation of the outer SnO2 particles. So, the physically adsorbed SnO2 particles were unstable, and tended to congregate together.
Van der Waals forces have been used to investigate the interactions between CNTs and CNT based nanocomposites.39–41 Herein, van der Waals forces also have great influence on the interactions of between the SnO2 and MWCNTs. Van der Waals forces between CNTs and other nanoscale species are likely to be much more long-range (∼1/r3) than those typically observed between colloidal species (∼1/r6).41 So, with the decrease of the repulsive electrostatic force between the SnO2 particles, the distance between them also decreased. As a result, the attractive van der Waals forces between the SnO2 particles increased rapidly. So, the surface of the SnO2/MWCNT heterojunctions turned appreciably coarser with the prolonged reaction time, which can be attributed to the increased attractions between the outer SnO2 nanoparticles.
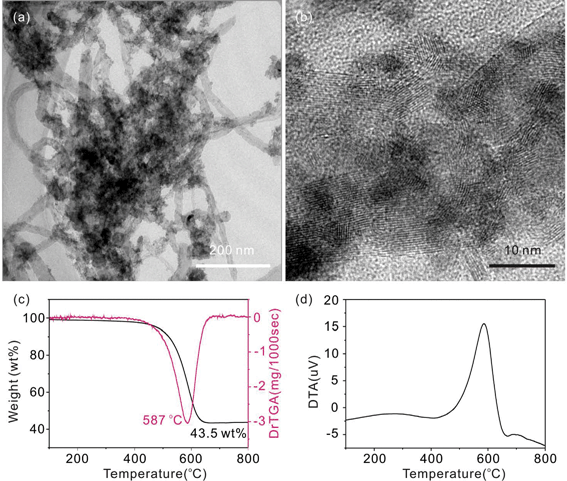
By changing the acidity of the solution, SnOx/MWCNT heterojunctions with different morphology and oxidation state were prepared by Fang et al.21 The strong acidity is disadvantageous to the formation of the SnO2, as well as the attractive physical interactions. In our previous work,42 in the process of SnO2/MWCNT-2 preparation, small amount of iron cations (Fe3+) were added to the SnCl2 solution. Using the Fe3+ doped SnO2/MWCNT heterojunctions (Fe-SnO2/MWCNT-2) as a precursor, SnO2 nanowires were prepared though thermal treatment in an inert atmosphere. Here, the interfacial structures of the Fe–SnO2/MWCNT-2 were investigated in order to test the influence of ionic strength discussed above. TEM and HRTEM images of the Fe–SnO2/MWCNT-2 are shown in Fig. 9a, b. The atomic content of iron in the product was 0.34%. From Fig. 9a, b, it is clear that only a few SnO2 particles coated onto the surface of MWCNTs. More importantly, these SnO2 particles were attached on the MWCNTs surface in a monolayer fashion, which suggests that the interactions between them are chemical interactions. Fig. 9c shows that the weight content of SnO2 in Fe–SnO2/MWCNT-2 was 43.5%, which is similar to the SnO2/MWCNT-2. The results mean that the added Fe3+ can not suppress the formation of SnO2. However, most of the SnO2 particles could not form the multilayer SnO2 coating, which means that the attractive physical interactions between the SnO2 and MWCNTs were very weak. The possible reason may be attributed to the repulsive electrostatic force between the Sn–OH2+ and the Fe3+, which was concentrated on the surface of SnO2 and the MWCNTs, respectively. On the contrary, the increased ionic strength lowers the repulsive electrostatic force between the SnO2 particles, and as a result, more SnO2 particles congregated together.
In addition, the thermal stability of the MWCNTs in Fe–SnO2/MWCNT-2 was also studied, as shown in Fig. 9c, d. The To and the Tp of Fe–SnO2/MWCNT-2 were 423 and 587 °C, respectively. The Hd and the Hb values were −77.98 and 11.14 kJ mol−1, respectively. The results indicate that the lattice strain of the MWCNTs in Fe–SnO2/MWCNT-2 was lower than the ones of SnO2/MWCNT-2, which can be attributed to the weak interactions between the SnO2 and MWCNTs. In combination with the interfacial morphologies, the results further confirm that Raman spectra and thermal analysis are two valid methods to evaluate the interactions between the SnO2 and MWCNTs.
4. Conclusions
SnO2/MWCNT heterojunctions were synthesized using the SnCl2 solution method. The chemical bonds were the predominant interactions between the MWCNTs and the inner layer SnO2 nanoparticles. The physical interactions, including Coulomb interactions and van der Waals forces, between the outer layer SnO2 and MWCNTs were determined by the acidity and the ionic strength of the solution. Raman spectrum and thermal analysis were chosen and proved to be two effect methods to evaluate the interactions between the SnO2 and MWCNTs. The binding energy of the MWCNTs and SnO2 was calculated based on differential thermal analysis and Hess's law.Acknowledgements
This work was supported by the One Hundred Person Project of the Chinese Academy of Sciences, the Natural Science Foundation of Anhui University of Traditional Chinese Medicine (Grant No. 2011zr017B), the China Postdoctoral Science Foundation (Grant No. 2011M501073), the National Key Scientific Program, Nanoscience and Nanotechnology (Grant No. 2011CB933700), and the National Natural Science Foundation of China (Grant Nos. 90923033, 60801021, 61071054, and 20907035).References
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- J. Kong, N. R. Franklin, C.W. Zhou, M. G. Chapline, S. Peng, K. Cho and H. J. Dai, Science, 2000, 287, 622–625 CrossRef CAS.
- P. G. Collins, K. Bradley, M. Ishigami and A. Zettl, Science, 2000, 287, 1801–1804 CrossRef CAS.
- M. Law, H. Kind, B. Messer, F. Kim and P. D. Yang, Angew. Chem., Int. Ed., 2002, 41, 2405–2408 CrossRef CAS.
- Y. J. Chen, L. Nie, X. Y. Xue, Y. G. Wang and T. H. Wang, Appl. Phys. Lett., 2006, 88, 083105 CrossRef.
- N. Pinna, G. Neri, M. Antonietti and M. Niederberger, Angew. Chem., Int. Ed., 2004, 43, 4345–4349 CrossRef CAS.
- F. Song, H. L. Su, J. Han, J. Q. Xu and D. Zhang, Sens. Actuators, B, 2010, 145, 39–45 CrossRef.
- Y. L. Wang, X. C. Jiang and Y. N. Xia, J. Am. Chem. Soc., 2003, 125, 16176–16177 CrossRef CAS.
- Y. J. Chen, C. L. Zhu and T. H. Wang, Nanotechnology, 2006, 17, 3012–3017 CrossRef CAS.
- W. G. Fan, L. Gao and J. Sun, J. Am. Ceram. Soc., 2006, 89, 2671–2673 CrossRef CAS.
- G. H. Lu, L. E. Ocola and J. H. Chen, Adv. Mater., 2009, 21, 2487–2491 CrossRef CAS.
- B.Y. Wei, M. C. Hsu, P. G. Su, H. M. Lin, R. J. Wu and H. J. Lai, Sens. Actuators, B, 2004, 101, 81–89 CrossRef.
- Y. X. Liang, Y. J. Chen and T. H. Wang, Appl. Phys. Lett., 2004, 85, 666–668 CrossRef CAS.
- A. Yang, X. M. Tao, R. X. Wang, S. C. Lee and C. Surya, Appl. Phys. Lett., 2007, 91, 133110 CrossRef.
- F. L. Meng, Y. Jia, J. Y. Liu, M. Q. Li, Y. F. Sun, J. H. Liu and X. J. Huang, Anal. Methods, 2010, 2, 1710–1714 RSC.
- Z. H. Wen, Q. Wang, Q. Zhang and J. H. Li, Adv. Funct. Mater., 2007, 17, 2772–2778 CrossRef CAS.
- C. H. Xu, J. Sun and L. Gao, J. Phys. Chem. C, 2009, 113, 20509–20513 CAS.
- Q. Kuang, S. F. Li, Z. X. Xie, S. C. Lin, X. H. Zhang, S. Y. Xie, R. B. Huang and L. S. Zheng, Carbon, 2006, 44, 1166–1172 CrossRef CAS.
- J. Y. Bai, Z. D. Xu and Y. F. Zheng, Chem. Lett., 2006, 35, 96–97 CrossRef CAS.
- N. V. Hieu, L. T. B. Thuy and N. D. Chien, Sens. Actuators, B, 2008, 129, 888–895 CrossRef.
- W. Q. Han and A. Zettl, Nano Lett., 2003, 3, 681–683 CrossRef CAS.
- H. T. Fang, X. Sun, L. H. Qian, D. W. Wang, F. Li, Y. Chu, F. P. Wang and H. M. Cheng, J. Phys. Chem. C, 2008, 112, 5790–5794 CAS.
- J. G. Zhou, H. T. Fang, Y. F. Hu, T. K. Sham, C. X. Wu, M. Liu and F. Li, J. Phys. Chem. C, 2009, 113, 10747–10750 CAS.
- J. G. Zhou, H. T. Fang, J. M. Maley, J. Y. P. Ko, M. Murphy, Y. Chu, R. Sammynaiken and T. K. Sham, J. Phys. Chem. C, 2009, 113, 6114–6117 CAS.
- J. G. Zhou, X. T. Zhou, X. H. Sun, R. Y. Li, M. Murphy, Z. F. Ding, X. L. Sun and T. K. Sham, Chem. Phys. Lett., 2007, 437, 229–232 CrossRef CAS.
- R. V. Hull, L. Li, Y. C. Xing and C. C. Chusuei, Chem. Mater., 2006, 18, 1780–1788 CrossRef CAS.
- R. Zan, U. Bangert, Q. Ramasse and K. S. Novoselov, Nano Lett., 2011, 11, 1087–1092 CrossRef CAS.
- Y. Jia, L. F. He, Z. Guo, X. Chen, F. L. Meng, T. Luo, M. Q. Li and J. H. Liu, J. Phys. Chem. C, 2009, 113, 9581–9587 CAS.
- Y. Lin, K. A. Watson, M. J. Fallbach, S. Ghose, J. G. Smith, D. M. Delozier, W. Cao, R. E. Crooks and J. W. Connell, ACS nano, 2009, 3, 871–884 CrossRef CAS.
- H. Wang, X. Xiang and F. Li, J. Mater. Chem., 2010, 20, 3944–3952 RSC.
- D. Eder and A. H. Windle, J. Mater. Chem., 2008, 18, 2036–2043 RSC.
- D. K. Singh, P. K. Iyer and P. K. Giri, J. Appl. Phys., 2010, 108, 084313 CrossRef.
- C. T. Zhi, Y. Bando, C. C. Tang and D. Golberg, J. Phys. Chem. B, 2006, 110, 8548–8550 CrossRef CAS.
- M. Lanza, S. Santangelo, G. Messina, S. Galvagno and C. Milone, ChemPhysChem, 2010, 11, 1925–1931 CAS.
- P. Mars and D. W. van Krevelen, Chem. Eng. Sci., 1954, 3, 41–59 CrossRef CAS.
- J. Hu, C. L. Chen, X. X. Zhu and X. K. Wang, J. Hazard. Mater., 2009, 162, 1542–1550 CrossRef CAS.
- R. H. R. Castro, B. B. S. Murad and D. Gouvêa, Ceram. Int., 2004, 30, 2215–2221 CrossRef CAS.
- G. A. Rance and A. N. Khlobystov, Phys. Chem. Chem. Phys., 2010, 12, 10775–10780 RSC.
- Y. Zhang, N. W. Franklin, R. J. Chen and H. J. Dai, Chem. Phys. Lett., 2000, 331, 35–41 CrossRef CAS.
- Z. Zanolli, R. Leghrib, A. Felten, J. J. Pireaux, E. Llobet and J. C. harlier, ACS Nano, 2011, 5, 4592–4599 CrossRef CAS.
- G. A. Rance, D. H. Marsh, S. J. Bourne, T. J. Reade and A. N. Khlobystov, ACS Nano, 2010, 4, 4920–4928 CrossRef CAS.
- Y. Jia, X. Chen, Z. Guo, J. Y. Liu, F. L. Meng, T. Luo, M. Q. Li and J. H. Liu, J. Phys. Chem. C, 2009, 113, 20583–20588 CAS.
| This journal is © The Royal Society of Chemistry 2012 |