Human erythema and matrix metalloproteinase-1 mRNA induction, in vivo, share an action spectrum which suggests common chromophores†
Angela
Tewari‡
a,
Christine
Lahmann‡§
b,
Robert
Sarkany
a,
Jörg
Bergemann¶
b and
Antony R.
Young
a
aKing's College London (KCL), King's College London School of Medicine, Division of Genetics and Molecular Medicine, St John's Institute of Dermatology, London, UK. E-mail: antony.r.young@kcl.ac.uk
bBeiersdorf AG, Paul Gerson Unna Skin Research Centre, Hamburg, Germany
First published on 14th November 2011
Abstract
Matrix metalloproteinase 1 (MMP-1) is widely regarded as a biomarker of photoageing. We tested the hypothesis that MMP-1 mRNA expression and erythema share a common action spectrum by comparing the effects of erythemally equivalent doses of UVB, UVA1 and solar simulated radiation (SSR) on acute MMP-1 mRNA expression in whole human skin in vivo. Our results show comparable MMP-1 expression with all three spectra, which supports our hypothesis. The sharing of an action spectrum implies common chromophores, one of which is likely to be DNA. We have previously shown that all spectra that we used readily induce cyclobutane thymine dimers (T<>T) in human epidermis in vivo but we lack quantitative data on damage to dermal DNA. This is important because we do not know if dermal MMP-1 induction occurs via direct damage to the dermis, or indirectly via damage to the epidermis. Our results show that UVB induces about 3 times more T<>T compared with erythemally equivalent doses of UVA1, which is similar to our published epidermal data. This supports previously published work that also implicates an unknown UVA1 chromophore for erythema and MMP-1 induction. However, the distribution of the dermal DNA damage varies considerably with spectrum. In the case of UVB it is primarily in the upper dermis, but with UVA1 it is evenly distributed. Thus, irrespective of chromophores, MMP-1 induction by direct dermal damage by both spectra is possible. The practical conclusions of our data are that the small (<5%) UVB content of solar UVR is likely to be the main cause of photoageing, at least in terms of MMP-1 expression. Furthermore, prevention of erythema by sunscreen use is likely to result in reduced MMP-1 expression.
Introduction
Photoageing and skin cancer are the normal long term consequences of exposure to solar ultraviolet radiation (UVR: ∼295–400 nm), especially in sun sensitive skin types I and II. Skin cancer is a major health problem and has been subject to extensive epidemiological and basic research. There has been relatively limited research into photoageing, even though this is often associated with skin cancer, and mouse studies have shown that neutrophil elastase deficient hairless mice are resistant to photoageing and UVR-induced squamous cell carcinoma (SCC), suggesting a mechanistic link.1The vast majority (>95%) of solar UVR is UVA (320–400 nm), of which UVA1 (340–400 nm) is the major (∼75%) component, but action spectroscopy has shown that the much smaller (<5%) UVB (∼295–320 nm) component is responsible for the majority of DNA photodamage (thymine dimers; T<>T) in human skin in vivo and sunburn;2 this has led to the conclusion that DNA is an important chromophore for erythema. It is also widely accepted that UVB is the main cause of skin cancer,3 though some have advocated an important role for UVA, especially for malignant melanoma.4,5UVA1 is increasingly used in high dose phototherapy for skin disorders6 and is typically the major spectral component of tanning devices.7
Most research on the effects of UVR on skin in vivo has focused on the epidermis because it is the target tissue for photocarcinogenesis. The dermis is also an important target, especially in the context of inflammation and photoageing.8 The structural integrity and function of the dermis are dependent on its extracellular matrix (ECM), which is mainly composed of type I and type II collagen.9Collagen turnover is mediated by matrix metalloproteinases (MMP)10 and their inhibitors (TIMP). MMP-1 is highly induced by UVR in resident keratinocytes and fibroblasts, as well as MMP-3 and MMP-9 in vivo.10–12 Photoageing is thought to be caused by UVR-induced hydrolysis of the dermal ECM that is initiated by MMP-1; a blocking antibody to MMP-1 removed 95 ± 4% of collagenolytic activity from UVR-exposed human skin, strongly implicating MMP-1 as a major enzyme responsible for collagen degradation in photoageing.13
It is often stated without much evidence that UVA is more important than UVB for photoageing as it penetrates deep into the dermis, unlike UVB. Such statements ignore the possibility that epidermally derived factors may influence dermal ECM, and indeed there is evidence that cytokines/TNFα, readily induced by UVR,14,15 can induce MMP synthesis.16,17 Furthermore, there have been very few studies that have investigated UVR penetration of the skin, and studies that have done this have used physical, rather than biological techniques, often on disrupted isolated skin.18,19
There are no human data on the action spectrum for photoageing. Mouse studies20 have suggested that the action spectrum for elastosis, a hallmark of photoageing, is similar to that for erythema, in which UVB is 3–4 orders of magnitude more effective than UVA. Chronic UVA exposure in hairless mice is capable of inducing photodamage like UVB21 and, given the high quantity of UVA in sunlight, there is reason to believe that UVA could make a significant contribution to photoageing.22 One study evaluated skin cancer and elastosis in mice exposed to narrow band UVB and UVA;20UVB induced SCC and elastosis whereas UVA induced elastosis only but was less effective than UVB.
The aim of this study was to test the hypothesis that UVR-induced erythema and MMP-1 mRNA induction share a common action spectrum. We approached this by giving exposures from 3 UVR sources that resulted in equal erythemal responses observed at 24 h after irradiation. The sources were UVB (300 nm), UVA1 and solar simulated radiation (SSR). If our hypothesis were correct, we would predict comparable MMP-1 mRNA expression with comparably effective erythemal exposures. In this context we also assessed the expression of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1).
A common action spectrum for erythema and MMP-1 mRNA induction would suggest common chromophores. There is indirect human in vivo evidence that epidermal DNA is an important chromophore for erythema,2 and in vitro and in vivo evidence that DNA is a chromophore for the induction of MMP-1 mRNA and protein.23 UVR-induced MMP-1 is expressed in the epidermis and dermis at the mRNA and protein level.11,12 It is not known whether the dermal mRNA induction is due to factors released by the epidermal cells, or the result of direct UVR effects on dermal cells, such as fibroblasts. Little is known about dermal DNA photodamage so we also investigated the effects of UVA1 and UVB on the induction and location of dermal T<>T and its repair.
Results
UVA1, UVB and SSR induce comparable expression of MMP-1 mRNA
Fig. 1 shows the induction of mRNA MMP-1 expression by (1a) UVA1 and (1b) UVB. A linear regression model showed substantial dose-response effects for UVB and UVA1 and a substantial subject level effect as can seen by the large inter-person variation in the figures. Overall, the increase is 80.6 times higher (compared to baseline) with UVA1 and 34.3 times higher with UVB, giving a ratio of 2.35 (95% CI 0.44 to 12.6, p = 0.32) which is not significantly different from 1. A non-linear model that we used also allowed for arbitrary non-linear effects of dose and this indicated a threshold of 1 MED for a significant effect for both spectra. The level of SSR-induced mRNA expression is comparable in the two groups of volunteers (p = 0.1) and comparisons of 2 MED SSR, UVB and UVA1 showed no differences (p > 0.1).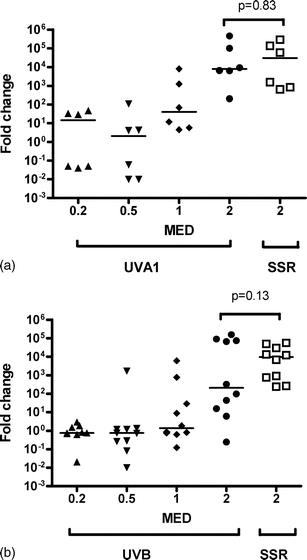 | ||
| Fig. 1 Effect of (a) UVA1 and (b) UVB on MMP-1 mRNA expression in comparison with 2MED SSR. The horizontal bars represent median values. A linear regression model showed a highly significant effect of UVA1 and UVB dose, but that these dose-response curves were not significantly different from each other. | ||
UVA1 induces more TIMP-1 mRNA expression than UVB
Fig. 2 shows the induction of mRNA TIMP-1 expression by (2a) UVA1 and (2b) UVB. The induction of TIMP-1 is much lower than for MMP-1, as we have previously reported for SSR.12 The linear model showed a significant effect of dose for UVB and UVA1 but there was no subject level effect. The overall increase for UVA1 was 2.2 and 1.5 for UVB from baseline, giving a ratio of 1.48 (95% CI 1.09–2.01, p = 0.01), which means that UVA1 is significantly more effective than UVB at inducing TIMP-1 expression. The level of SSR-induced mRNA expression is comparable in the two groups of volunteers (p = 0.08). There is no difference between 2MED UVA1 and 2 MED SSR (p = 0.59), but significantly more TIMP-1 was induced with 2MED SSR compared with 2 MED UVB (p = 0.03). The dose-response results and those from the 2 MED comparisons are consistent given that UVA1 is the major component of SSR.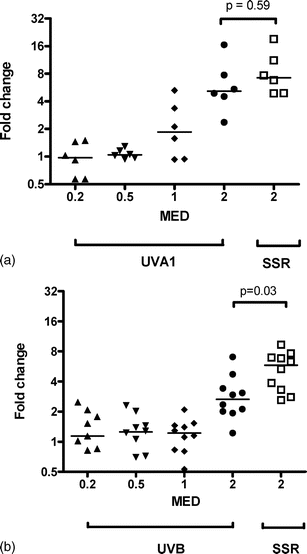 | ||
| Fig. 2 Effect of (a) UVA1 and (b) UVB on TIMP-1 mRNA expression in comparison with 2 MED SSR. The horizontal bars represent median values. A linear regression model showed a highly significant effect of UVA1 and UVB dose, with UVA1 being more effective than UVB. | ||
The effect of dermal depth on UVA1 and UVB-induced T<>T staining
Examples of epidermal and dermal T<>T staining for UVA1 and UVB are shown in Fig. 3a and 3b, respectively. Fig. 4a and 4b show the relationship between T<>T staining and depth from the basal epidermis for UVA1 and UVB, respectively. The data show the degree of T<>T is independent of depth (p = 0.53 for slope) for UVA1, whereas there is a highly significant (p = 0.0001 for slope) loss of stain with depth with UVB.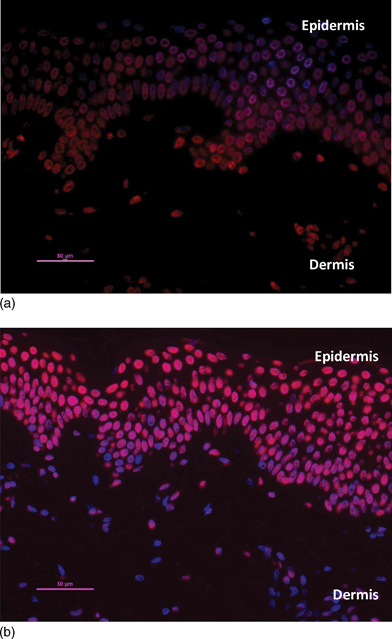 | ||
| Fig. 3 Staining of dermal nuclei for T<>T, 3 notional MED (a) UVA1 (b) UVB. The red stain is indicative of T<>T. Biopsies were taken immediately after exposure. See Tewari et al.24 for quantification of epidermal data that allows comparisons with Fig. 4, 5 and 6. | ||
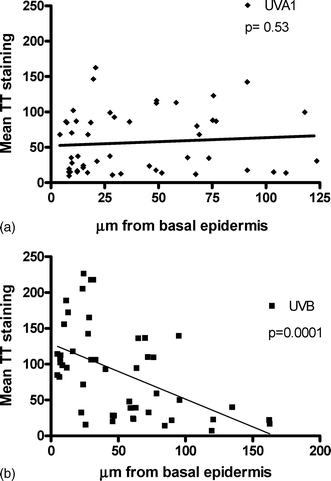 | ||
| Fig. 4 Influence of dermal depth on T<>T staining after 3 notional MED (a) UVA1 and (b) UVB. A total 50 nuclei for each spectrum were examined from 12 volunteers. Biopsies taken immediately after exposure and the data have been analyzed by linear regression and p values refer to slopes of regression lines. The mean T<>T staining (vertical axis) represents the “red intensity” of each nucleus. This is calculated from the average intensity of each pixel within a nucleus and corresponds to the amount of T<>T present. | ||
Dose response and repair kinetics for dermal T<>T with UVA1 and UVB
Fig. 5 shows a dose-dependent increase in T<>T for UVA1 (slope p = 0.009) and UVB (slope p < 0.0001). The maximal damage with UVB is about 3 fold greater that UVA1, which is similar to what we have reported in the epidermis.24Fig. 6 shows that repair of dermal T<>T is slow. The slope for UVA1 is significant (p = 0.03) but that for UVB is not significant (p = 0.62).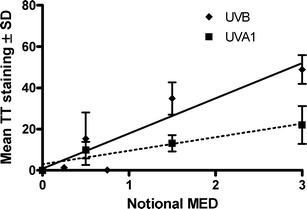 | ||
| Fig. 5 Dose response for dermal T<>T induced by UVA1 and UVB. Both slopes are significant (p < 0.01). The mean T<>T staining (vertical axis) represents the average “red intensity” of nuclei of a given volunteer at a given dose. | ||
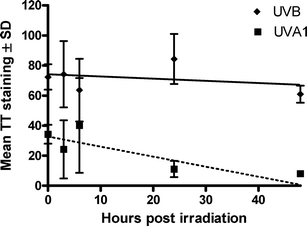 | ||
| Fig. 6 Repair of dermal T<>T after exposure to 3 notional MED UVA1 and UVB. The slope (p 0.03) of UVA1 is significant, but this is not the case for UVB (p = 0.62). The mean T<>T staining (vertical axis) represents the average “red intensity” of the nuclei of a given volunteer at a given post-irradiation time. | ||
Discussion
We have previously shown that SSR induced MMP-1 mRNA in a dose dependent way with maximal expression at 24 h;12 with high levels following 2 MED. In contrast, the effect on TIMP-1 was very modest. Comparable levels of mRNA expression of MMP-1 were seen in the epidermis and dermis with SSR, whereas TIMP-1 was only seen in the dermis.12In the current study, our results are for mRNA only, but other groups have shown that UVB also induces MMP-1 protein and its activity in the dermis and epidermis.10,11 We used 2MED SSR as a positive control in UVB and UVA1 dose-response studies with comparable erythemal exposure. Our data show considerable inter-personal variation in MMP-1 mRNA expression, which has been reported by us12 and others,23 one reason for which may be different single-nucleotide polymorphisms (SNPs) that may influence gene transcription.25 However, the dose-response curves (as MED fractions) for UVB and UVA1-induced MMP-1 were not significantly different, and there was no statistical difference between MMP-1 mRNA expression levels with 2MED by three different spectra. This provides evidence that the action spectra for erythema and MMP-1 mRNA induction are the same or very similar. However, it should be noted that the confidence limits on which this conclusion is based are high, due to large inter-person variability.
This similarity of action spectra suggests common chromophores. Two main photochemical events have been proposed for the induction of MMP-1: i) the formation of cyclobutane pyrimidine dimers (CPD)23 and ii) UVR induced reactive oxygen species (ROS), primarily by UVAviachromophores that have yet to be identified.26 Until recently, DNA was primarily regarded as a UVB chromophore, but studies show that UVA1 induces more CPD, especially T<>T, than expected.27,28 To date, the focus on UVA has primarily been on its ability to generate ROS. This yields oxidized bases, such as 8-oxo-7,8-dihydro-guanine (8-oxoGua), and DNA strand breaks.29DNA, resulting in the formation of CPD, has been proposed as an important chromophore for erythema2 and TNFα15 (which may initiate MMP-1 induction). Laser action spectroscopy for human erythema has identified a minor independent UVA1 absorbing chromophore.30 UVB-induced erythema is oxygen independent but UVA1 induced erythema requires oxygen.31 Overall, these data suggest a role for ROS in UVA1-induced erythema. Thus, our MMP-1 data are consistent with the hypothesis that DNA and an unknown UVA1 absorbing molecule, that probably generates ROS, are shared chromophores for erythema and MMP-1 induction. Support for an important role for DNA comes from human skin in vivo studies in which skin was exposed to 1 MED UVB. Post-irradiation treatment with an activated CPD photolyase reduced MMP-1 mRNA expression as well as epidermal and dermal MMP-1 protein visualized by immunohistology.23
Interpreting action spectroscopy in the skin is difficult because UVR penetration is wavelength dependent and the location of the chromophore and the biological outcome of its activation may be different. Erythema and collagen degradation by MMP-1 are both dermal events that are possibly initiated by photochemical activity in the epidermis; for example, the transfer of media from UVB-irradiated human keratinocytes induces MMP-1 mRNA and protein expression in unirradiated human fibroblasts.23 The dose-range that we used in the MMP-1 studies readily induces dermal T<>T, especially with UVB as shown in Fig. 5, but Fig. 3 and 4 show that the location of the damage is spectrally dependent. UVB-induced lesions are focused in the upper dermis whereas UVA1 induced lesions, although fewer in number (characterised by less red intensity), are evenly spread throughout the dermis. Thus, our data suggest that direct dermal UVR-induced expression of MMP-1 mRNA is possible. Enhanced repair of CPD in skin cellsin vitro and in vivo has been shown to inhibit MMP-1 mRNA and protein.23 We have previously shown that epidermal repair of SSR and UVA1-induced T<>T is slow 24,32,33 and Fig. 6 shows that this is also the case in the dermis; indeed there was no evidence for the repair of UVB-induced lesions. Thus persistent lesions may well initiate dermal MMP-1 expression.
As with SSR, the effects of UVB and UVA1 on TIMP-1, which is only expressed in the dermis, are relatively modest.12 However, there was a significant dose-response with UVB and UVA1 but the effects of the latter were significantly greater than the former. In addition, 2 MED SSR was significantly more effective than 2 MED UVB, whereas the effects of 2 MED UVA1 and 2 MED SSR (mostly UVA1) were the same. One reason for the greater efficacy of UVA1 may be its better penetration into the dermis. The relationship between UVR-induced MMP-1 and TIMP-1 mRNA and protein/activity is not known.
It is often stated that UVA is the main cause of photoageing. However, irrespective of chromophores, if the SSR spectrum that we used is weighted with the CIE action spectrum for erythema,34 we can state that 87% of MMP-1 induction is due to UVB (defined as 280–320 nm). A comparable weighting for London (51°N) noon summer sun would result in 80% of MMP-1 induction being due to UVB. Even assuming that our hypothesis is false, and that UVA1 is 2.35 times more effective than UVB at comparable erythemal exposures, we would still conclude that solar UVB is the main cause of photoageing. These conclusions are based on acute exposures and do not account for any photoadaptation, which has been reported after repeat exposure studies for some endpoints.35 An action spectrum for solar elastosis in hairless mice was also similar to that for human erythema,36 which would also implicate UVB as the most important spectral region in practice. Most action spectra for photoageing markers in mice have shown UVB to be more important than UVA apart from skin “sagging”.22
Sunscreens inhibit erythema, as measured by their sun protection factors (SPF). Comparable SPFs can be obtained with different ratios of UVB:UVA protection, which can predict protection from SSR-induced epidermal T<>T.37 Our results suggest that the inhibition of erythema by sunscreen use of a given labelled SPF should comparably inhibit MMP-1 expression. However, we have also reported that SPF in practice will decrease with lower solar UVB content (dependent on latitude, time of day, etc) with a primarily UVB sunscreen.38 This is not the case with broad spectrum sunscreens in which actual SPF is independent of solar UVB content. Overall, it is better to have a robust index of protection, and we therefore advocate the use of broad spectrum protection with comparable levels of UVB and UVA protection.
Conclusions
Although we have not done a conventional action spectrum study with dose-response curves at different monochromatic wavelengths, our analyses suggests that the action spectra for erythema and acute MMP-1 mRNA expression are the same. This suggests common chromophores and we propose DNA and an unknown UVA1 absorber that may mediate its effects by ROS. In contrast, the action spectra for erythema and TIMP-1 mRNA expression are different, with UVA1 being relatively more effective than UVB. This could mean that the chromophores for MMP-1 and TIMP-1 are different, but it could also reflect the different location of the chromophores within the skin.Maximal ambient exposure during a clear sky summer's day in the UK is about 45 standard erythema doses (SED), of which the UVA1 contribution is approximately 7%; equivalent to 3 SED or ∼1 MED in a skin type I/II person.39 Thus, assuming that the action spectra for erythema and MMP-1 induction are similar, the UVA1 contribution to photoageing in practice is likely to be minor. We can conclude that solar UVB is the main cause of photoageing.
Experimental
Volunteers
The studies were approved by the St Thomas' Hospital, London, UK Ethics Committee (Ref: EC00/006 and Ref: 09/H0802/98) and done in accordance with the declaration of Helsinki. Normal healthy skin type I–II young adults gave written informed consent before taking part. The demographic details of the 16 volunteers for the MMP-1/TIMP-1 studies and the 12 volunteers for the T<>T studies are similar and are shown in Tables 1 and 2, respectively.| Study | Skin type | Sex | Age | SSR MED (J cm−2) | UVA1 MED (J cm−2) |
|---|---|---|---|---|---|
| UVA1 and SSR | I | F | 19 | 5.1 | 100 |
| I | F | 33 | 2.9 | 25 | |
| I | M | 36 | 4.9 | 70.7 | |
| II | F | 33 | 5.8 | 50 | |
| I | M | 35 | 4.9 | 35.4 | |
| II | F | 19 | 5.1 | 100 | |
| Totals and mean ± SD | 4I + 2II | 2M + 4F | 29.3 ± 7.7 | 4.8 ± 0.97 | 63.5 ± 32.2 |
| Study | Skin type | Sex | Age | SSR MED (J cm −2) | UVB MED ( mJ cm −2) |
| UVB and SSR | II | M | 26 | 6.1 | 14 |
| II | M | 22 | 6.1 | 20 | |
| II | F | 22 | 6.1 | 28 | |
| I/II | M | 22 | 3.9 | 28 | |
| II | M | 27 | 4.9 | 40 | |
| I/II | M | 24 | 6.1 | 14 | |
| II | M | 31 | 5.3 | 40 | |
| II | F | 29 | 3.9 | 28 | |
| II | F | 21 | 4.9 | 20 | |
| II | M | 20 | 3.9 | 14 | |
| Totals and mean ± SD | 2I/II + 8II | 7M + 3F | 24.4 ± 3.7 | 5.1 ± 0.98 | 24.6 ± 9.9 |
| Study | Skin type | Sex | Age | UVA1 MED (J cm−2) | UVB MED (mJ cm−2) |
|---|---|---|---|---|---|
| Dose-response | I | F | 28 | 48.8 | 30.0 |
| I | F | 25 | 48.8 | 30.0 | |
| II | M | 20 | 48.8 | 30.0 | |
| II | F | 21 | 53.0 | 30.0 | |
| I | F | 22 | 48.8 | 23.0 | |
| I | M | 34 | 61.1 | 19.0 | |
| Totals and mean ± SD | 4I + 2II | 2M + 4F | 25 ± 5.3 | 51.6 ± 4.9 | 27.0 ± 4.8 |
| Time-course | I | F | 28 | 31.3 | 19 |
| I | F | 28 | 31.3 | 30 | |
| I | M | 22 | 48.8 | 23 | |
| II | F | 24 | 31.3 | 30 | |
| I | M | 23 | 48.8 | 23 | |
| II | F | 23 | 48.8 | 23 | |
| Totals and mean ± SD | 4I + 2II | 2M + 4F | 24.7 ± 2.7 | 40.1 ± 9.6 | 24.7 ± 4.4 |
| Combined | 8I + 4II | 4M + 8F | 24.8 ± 4.0 | 45.8 ± 9.4 | 25.8 ± 4.6 |
UVR sources and dosimetry
The emission spectra of the UVR sources are shown in Fig. 7 (UVB1 source for MMP-1/TIMP-1 data, UVB2 for T<>T data). These were determined with a DM150BC double-monochromator spectroradiometer (Bentham Instruments, Reading, UK) using an integration sphere and gratings blazed at 250 nm. The spectroradiometer was calibrated against national UK standards.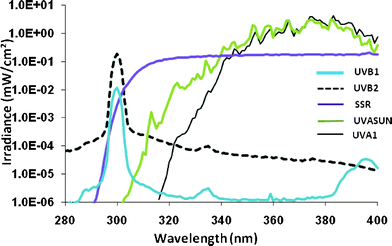 | ||
| Fig. 7 Emission spectra of the sources used. UVB1 corresponds to the spectrum for Fig. 1–2 and UVB2 corresponds to the spectrum for Fig. 3–6. | ||
SSR was produced by an Oriel solar simulator (Model 81292, L.O.T Oriel, Leatherhead, UK) with a 1-kW xenon arc lamp with two dichroic mirrors, a collimator, and a 1-mm WG320 filter. Irradiance was routinely measured by a wide-band thermopile radiometer (Medical Physics, Dryburn Hospital, Durham, UK) that had been calibrated against the spectroradiometric measurements. This was typically 15 mW cm−2 at skin surface (11 cm from source).
Two UVA1 sources were used. For the MMP-1/TIMP-1 studies, this was a UVASun 2000 (Mutzhas, Munich, Germany). Routine dosimetry was done with a radiometer (Model IL442A (with a UVA sensor), International Light technologies, Massachusetts, USA), after calibration against the spectroradiometric measurements. Irradiance was 70–75 mW cm−2 at the skin surface (11 cm from the source). For the T<>T studies, this was a Sellamed 3000 Dr Sellmeier (Sellas, Gevelsberg, Germany) phototherapy device. Irradiance was routinely measured with a radiometer (Model IL1400A, International Light technologies, Massachusetts, USA), after calibration against the spectroradiometric measurements, and was typically ∼74 mW cm−2 at skin surface (24.5 cm from source).
Narrowband UVB (300 nm) was produced by a monochromator (L.O.T. Oriel, Newport, USA: 1-kW xenon arc; grating blazed at 250 nm; slits set for 3 nm full width at half maximum (FWHM) bandwidth) and 2.5 nm FWHM UVB was delivered with a liquid light guide (Oriel), exit diameter 5 mm, in direct contact with the skin. In the studies described in Table 1, irradiance (typically 0.3mW cm−2) was measured with a wide-band thermopile radiometer (Medical Physics, Dryburn Hospital, Durham, UK). In the case of studies in Table 2, irradiance (typically ∼0.5 mW cm−2) was measured with an SEL623 thermopile (International Light Technologies, Massachusetts, USA) attached to an IL1400A photometer calibrated by the United Kingdom Accreditation Service-accredited Guy's and St Thomas' Hospitals Trust UVR laboratory.
Irradiation protocol
A geometric (×√2 for the MMP-1/TIMP-1 study and ×1.25, for the T<>T study) series of doses was given over 1 cm2 areas of previously unexposed buttock skin or over the 5 mm exit diameter of the liquid light guide in the case of 300 nm. A visual assessment of the exposed sites was made 24 h after exposure and the ‘just perceptible MED’ was determined as it is a more reliable threshold endpoint than ‘erythema with a definite border’.40Previously unirradiated buttock skin of the MMP-1/TIMP-1 study volunteers (Table 1) was given 0.2, 0.5, 1 and 2 MED of UVB or UVA and 2 MED of SSR, based on their individual MED and 4 mm punch biopsies were taken 24 h after exposure. This time was based on our previous time-course studies with SSR for MMP-1 and TIMP-1.12
The dose protocols in the T<>T studies were based on “notional MED”. These were derived from the MED from 3 individuals: mean values 48.8 J (SD ± 0) cm−2 for UVA1 and 30.0 (SD ± 0) mJ cm−2 for UVB and the mean MEDs given in Table 2 were within 1–2 SD of these values. Table 2 shows the demographics of the volunteers in the dose response and repair studies. In the former, 6 volunteers were given 0.5, 1.5 and 3 notional MED of UVB and UVA1 on previously unexposed buttock skin and biopsies were taken immediately afterwards. In the latter, 6 volunteers were given 3 notional MED of UVA1 and UVB and biopsies taken at 3 time-points from: immediate, 3, 6, 24 and 48 h (times varied from person to person because of ethical restrictions: all had biopsies at 0 and 24 h, except for 2 UVB sites on 2 volunteers. A non-irradiated control biopsy was taken from all 12 volunteers).
Immunostaining and image analysis
Full details are given in our previous studies.24 Briefly 5 μm paraffin sections were stained with the monoclonal antibody incubation: CPD TDM-2 (CosmoBio, Tokyo, Japan) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 (antibody specificity to UVR induced T<>T 41) and isotype control IgG2a (DAKO, Cambridge, UK) for 90 min at room temperature. Fluorescent imaging was done for AlexaFluor555 (red) and DAPI (nuclear DNA stain; blue) with a Zeiss Axiophot microscope (Harpenden, UK), Nikon DS-U2 camera (Kingston-upon-Thames, UK) with ×20 magnification (and ×1.25 eyepiece objective). Using NIS elements BR v3 software package, images acquired in 2560 × 1920 format and exposed to 20S (gain 1.40× for AlexaFluor555 with 1.5 s exposure, gain 1.40× for DAPI). The contrast setting was the same for all images. DAPI stained nuclei were gated and mean red intensity (Alexa Fluor 555) assessed within each of the nuclei in the dermis. Mean background intensity from the unirradiated control was subtracted from the irradiated samples to control for nonspecific nuclear staining (typically for T<>:T: 10.0 ± 6.4(SD) as previously reported33). Settings for nuclei capture (circularity and diameter of nuclei) were kept constant, and sections subjectively assessed. It was not possible to stain and assess all slides in a single batch. To assess the reproducibility of the techniques, the same positive controls (3 MED UVB and UVA1) were processed with each staining and image analysis run, as well as a non-irradiated control and an isotype control in each case.
2000 (antibody specificity to UVR induced T<>T 41) and isotype control IgG2a (DAKO, Cambridge, UK) for 90 min at room temperature. Fluorescent imaging was done for AlexaFluor555 (red) and DAPI (nuclear DNA stain; blue) with a Zeiss Axiophot microscope (Harpenden, UK), Nikon DS-U2 camera (Kingston-upon-Thames, UK) with ×20 magnification (and ×1.25 eyepiece objective). Using NIS elements BR v3 software package, images acquired in 2560 × 1920 format and exposed to 20S (gain 1.40× for AlexaFluor555 with 1.5 s exposure, gain 1.40× for DAPI). The contrast setting was the same for all images. DAPI stained nuclei were gated and mean red intensity (Alexa Fluor 555) assessed within each of the nuclei in the dermis. Mean background intensity from the unirradiated control was subtracted from the irradiated samples to control for nonspecific nuclear staining (typically for T<>:T: 10.0 ± 6.4(SD) as previously reported33). Settings for nuclei capture (circularity and diameter of nuclei) were kept constant, and sections subjectively assessed. It was not possible to stain and assess all slides in a single batch. To assess the reproducibility of the techniques, the same positive controls (3 MED UVB and UVA1) were processed with each staining and image analysis run, as well as a non-irradiated control and an isotype control in each case.
RT-PCR
We used the TaqMan™ method, with probes for MMP-1, TIMP-1 and GAPDH (housekeeping), as described in detail in our previous studies.12RNA was extracted from whole skin 4 mm punch biopsies. The raw CT data were converted using the comparative ΔΔCT method.Statistics
Statistical analyses were done using Stata (StataCorp LP, College Station, Texas) using a mixed-effects random effects maximum likelihood model (REML) allowing for grouping by subject. Log10gene expression data were analysed using a simple linear regression dose response to UVB and UVA1, as well as a non-linear model. The latter allowed for possible arbitrary non-linear effects of dose by fitting dummy variables to account for the four-dose (MED fraction) levels. The two models gave very similar results, so unless otherwise stated the results are based on the linear model. Graphpad Prism v4 was used for two-tailed paired and unpaired (as appropriate) T tests to compare volunteers' ages, MED and MMP-1 and TIMP-1 mRNA expression. In the case of data from Fig. 1, 2 MED UVB and 2 MED UVA were compared by using the individual volunteer SSR Δ values. Graphpad Prism v4 was also used for linear regression analysis of the T<>T data.Acknowledgements
The DNA work was supported by clinical training fellowships (AT) from the UK Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust, and the UK Medical Research Council/British Skin Foundation/British Association for Dermatology. The MMP-1/TIMP-1 work was supported by Beiersdorf AG, Paul Gerson Unna Skin Research Centre, Hamburg, Germany, and done by CL. We thank Ms Katarzyna Grys and Ms Rosie Gulliver for excellent technical assistance and Mr Graham Harrison for his overall assistance, especially in relation to ethical, logistical and dosimetry aspects. We thank Professor Brian Diffey for helpful discussion and Paul Seed, Senior Lecturer in Medical Statistics, King's College London for the Stata analyses of the gene expression data. We thank Professor Christopher Griffiths, University of Manchester, for the image of the photoaged neck in the graphical abstract.References
- B. Starcher, P. O'Neal, R. D. Granstein and S. Beissert, Inhibition of neutrophil elastase suppresses the development of skin tumors in hairless mice, J. Invest. Dermatol., 1996, 107, 159–163 CAS.
- A. R. Young, C. A. Chadwick, G. I. Harrison, O. Nikaido, J. Ramsden and C. S. Potten, The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema, J. Invest. Dermatol., 1998, 111, 982–988 CrossRef CAS.
- F. R. de Gruijl, H. J. Sterenborg, P. D. Forbes, R. E. Davies, C. Cole, G. Kelfkens, H. van Weelden, H. Slaper and J. C. van der Leun, Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice, Cancer Res., 1993, 53, 53–60 CAS.
- R. D. Ley, Ultraviolet radiation A-induced precursors of cutaneous melanoma in Monodelphis domestica, Cancer Res., 1997, 57, 3682–3684 CAS.
- R. B. Setlow, E. Grist, K. Thompson and A. D. Woodhead, Wavelengths effective in induction of malignant melanoma, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 6666–6670 CrossRef CAS.
- N. R. York and H. T. Jacobe, UVA1 phototherapy: a review of mechanism and therapeutic application, Int. J. Dermatol., 2010, 49, 623–630 CrossRef.
- B. L. Diffey, Use of UV-A sunbeds for cosmetic tanning, Br. J. Dermatol., 1986, 115, 67–76 CrossRef CAS.
- J. H. Rabe, A. J. Mamelak, P. J. McElgunn, W. L. Morison and D. N. Sauder, Photoaging: mechanisms and repair, J. Am. Acad. Dermatol., 2006, 55, 1–19 CrossRef.
- H. S. Talwar, C. E. Griffiths, G. J. Fisher, T. A. Hamilton and J. J. Voorhees, Reduced type I and type III procollagens in photodamaged adult human skin, J. Invest. Dermatol., 1995, 105, 285–290 CAS.
- G. J. Fisher, Z. Q. Wang, S. C. Datta, J. Varani, S. Kang and J. J. Voorhees, Pathophysiology of premature skin aging induced by ultraviolet light, N. Engl. J. Med., 1997, 337, 1419–1428 CrossRef CAS.
- G. J. Fisher, S. C. Datta, H. S. Talwar, Z. Q. Wang, J. Varani, S. Kang and J. J. Voorhees, Molecular basis of sun-induced premature skin ageing and retinoid antagonism, Nature, 1996, 379, 335–339 CrossRef CAS.
- C. Lahmann, A. R. Young, K. P. Wittern and J. Bergemann, Induction of mRNA for matrix metalloproteinase 1 and tissue inhibitor of metalloproteinases 1 in human skin in vivo by solar simulated radiation, Photochem. Photobiol., 2001, 73, 657–663 CrossRef CAS.
- M. Brennan, H. Bhatti, K. C. Nerusu, N. Bhagavathula, S. Kang, G. J. Fisher, J. Varani and J. J. Voorhees, Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin, Photochem. Photobiol., 2003, 78, 43–48 CrossRef CAS.
- R. M. Barr, S. L. Walker, W. Tsang, G. I. Harrison, P. Ettehadi, M. W. Greaves and A. R. Young, Suppressed alloantigen presentation, increased TNF-alpha, IL-1, IL-1Ra, IL-10, and modulation of TNF-R in UV-irradiated human skin, J. Invest. Dermatol., 1999, 112, 692–698 CrossRef CAS.
- S. L. Walker and A. R. Young, An action spectrum (290–320 nm) for TNFalpha protein in human skin in vivo suggests that basal-layer epidermal DNA is the chromophore, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 19051–19054 CrossRef CAS.
- C. Oner, F. Schatz, G. Kizilay, W. Murk, L. F. Buchwalder, U. A. Kayisli, A. Arici and C. J. Lockwood, Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and -3 expression in term decidual cells: implications for treatment of chorioamnionitis-induced preterm delivery, J. Clin. Endocrinol. Metab., 2008, 93, 252–259 CrossRef CAS.
- C. J. Lockwood, C. Oner, Y. H. Uz, U. A. Kayisli, S. J. Huang, L. F. Buchwalder, W. Murk, E. F. Funai and F. Schatz, Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells, Biol. Reprod., 2008, 78, 1064–1072 CrossRef CAS.
- M. J. van Gemert, S. L. Jacques, H. J. Sterenborg and W. M. Star, Skin optics, IEEE Trans. Biomed. Eng., 1989, 36, 1146–1154 CrossRef CAS.
- M. Meinhardt, R. Krebs, A. Anders, U. Heinrich and H. Tronnier, Absorption spectra of human skin in vivo in the ultraviolet wavelength range measured by optoacoustics, Photochem. Photobiol., 2009, 85, 70–77 CrossRef CAS.
- H. C. Wulf, T. Poulsen, R. E. Davies and F. Urbach, Narrow-band UV radiation and induction of dermal elastosis and skin cancer, Photodermatology, 1989, 6, 44–51 CAS.
- L. H. Kligman, F. J. Akin and A. M. Kligman, The contributions of UVA and UVB to connective tissue damage in hairless mice, J. Invest. Dermatol., 1985, 84, 272–276 CrossRef CAS.
- D. L. Bissett, D. P. Hannon and T. V. Orr, Wavelength dependence of histological, physical, and visible changes in chronically UV-irradiated hairless mouse skin, Photochem. Photobiol., 1989, 50, 763–769 CrossRef CAS.
- K. K. Dong, N. Damaghi, S. D. Picart, N. G. Markova, K. Obayashi, Y. Okano, H. Masaki, S. Grether-Beck, J. Krutmann, K. A. Smiles and D. B. Yarosh, UV-induced DNA damage initiates release of MMP-1 in human skin, Exp. Dermatol., 2008, 17, 1037–1044 CrossRef CAS.
- A. Tewari, R. Sarkany and A. R. Young, UVA1 induces cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo, J. Invest. Dermatol., 2011 DOI:10.1038/jid.2011.283.
- P. A. Arakaki, M. R. Marques and M. C. Santos, MMP-1 polymorphism and its relationship to pathological processes, J. Biosci., 2009, 34, 313–320 CrossRef CAS.
- K. Scharffetter-Kochanek, M. Wlaschek, K. Briviba and H. Sies, Singlet oxygen induces collagenase expression in human skin fibroblasts, FEBS Lett., 1993, 331, 304–306 CrossRef CAS.
- T. Douki, A. Reynaud-Angelin, J. Cadet and E. Sage, Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation, Biochemistry, 2003, 42, 9221–9226 CrossRef CAS.
- S. Mouret, C. Baudouin, M. Charveron, A. Favier, J. Cadet and T. Douki, Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13765–13770 CrossRef CAS.
- J. Cadet, T. Douki, J. L. Ravanat and P. Di Mascio, Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation, Photochem. Photobiol. Sci., 2009, 8, 903–911 CAS.
- A. Anders, H. J. Altheide, M. Knalmann and H. Tronnier, Action spectrum for erythema in humans investigated with dye lasers, Photochem. Photobiol., 1995, 61, 200–205 CrossRef CAS.
- M. Auletta, R. W. Gange, O. T. Tan and E. Matzinger, Effect of cutaneous hypoxia upon erythema and pigment responses to UVA, UVB, and PUVA (8-MOP + UVA) in human skin, J. Invest. Dermatol., 1986, 86, 649–652 CAS.
- V. J. Bykov, J. M. Sheehan, K. Hemminki and A. R. Young, In situ repair of cyclobutane pyrimidine dimers and 6-4 photoproducts in human skin exposed to solar simulating radiation, J. Invest. Dermatol., 1999, 112, 326–331 CrossRef CAS.
- A. R. Young, C. A. Chadwick, G. I. Harrison, J. L. Hawk, O. Nikaido and C. S. Potten, The in situ repair kinetics of epidermal thymine dimers and 6-4 photoproducts in human skin types I and II, J. Invest. Dermatol., 1996, 106, 1307–1313 CAS.
- I. C. o. Illumination(CIE), Erythema reference action spectrum and standard erythema dose, ISO 17166:1999/CIE S007-1998.
- M. Norval, P. McLoone, A. Lesiak and J. Narbutt, The effect of chronic ultraviolet radiation on the human immune system, Photochem. Photobiol., 2008, 84, 19–28 CrossRef CAS.
- L. H. Kligman and R. M. Sayre, An action spectrum for ultraviolet induced elastosis in hairless mice: quantification of elastosis by image analysis, Photochem. Photobiol., 1991, 53, 237–242 CrossRef CAS.
- A. R. Young, J. M. Sheehan, C. A. Chadwick and C. S. Potten, Protection by ultraviolet A and B sunscreens against in situ dipyrimidine photolesions in human epidermis is comparable to protection against sunburn, J. Invest. Dermatol., 2000, 115, 37–41 CrossRef CAS.
- A. R. Young, J. Boles, B. Herzog, U. Osterwalder and W. Baschong, A sunscreen's labeled sun protection factor may overestimate protection at temperate latitudes: a human in vivo study, J. Invest. Dermatol., 2010, 130, 2457–2462 CrossRef CAS.
- G. I. Harrison and A. R. Young, Ultraviolet radiation-induced erythema in human skin, Methods, 2002, 28, 14–19 CrossRef CAS.
- A. Quinn, B. L. Diffey, P. Craig and F. PM, Definition of the minimal erythemal dose used for diagnostic phototesting, Br. J. Dermatol., 1994, 131, 56 Search PubMed.
- T. Mori, M. Nakane, T. Hattori, T. Matsunaga, M. Ihara and O. Nikaido, Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA, Photochem. Photobiol., 1991, 54, 225–232 CrossRef CAS.
Footnotes |
| † Contribution to the themed issue on the biology of UVA. |
| ‡ Equal first authors. |
| § PhD student with Beiersdorf when work was done. |
| ¶ Current address: Department of Biomedical Engineering, Albstadt-Sigmaringen University of Applied Sciences, Sigmaringen, Germany. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2012 |
