UVA, UVB and incidence of cutaneous malignant melanoma in Norway and Sweden†‡
Johan
Moan
ab,
Zivile
Baturaite
a,
Alina Carmen
Porojnicu
a,
Arne
Dahlback
b and
Asta
Juzeniene
*a
aDepartment of Radiation Biology, Institute for Cancer Research, the Norwegian Radium Hospital, Oslo University Hospital, Montebello, N-0310, Oslo, Norway. E-mail: asta.juzeniene@rr-research.no; Fax: +47-22781207; Tel: +47-22934260
bInstitute of Physics, University of Oslo, Blindern, 0316, Oslo, Norway
First published on 11th October 2011
Abstract
Latitudinal dependencies of UVA and UVB were studied together with relevant epidemiological data for squamous cell carcinoma (SCC) and cutaneous malignant melanoma (CMM) in Norway and Sweden. Our data support the hypothesis that solar UVA radiation may play a role for CMM induction. The etiologies of SCC and CMM are different according to a latitudinal dependency and differences in age curves. Sun exposure patterns, age-related decay rates of repair of UV damage and sex hormones may play different roles for the two skin cancers. Also, UVB induction of vitamin D may be involved. CMM incidence rates among young people have decreased or been constant since about 1990 in Norway and Sweden. All reasons for UVA contributing to CMM will be discussed.
Introduction
Solar radiation is believed to be the main risk factor for the major forms of skin cancer: squamous cell carcinoma (SCC), basal cell carcinoma (BCC) and cutaneous malignant melanoma (CMM).1,2 The former two are classified as non-melanoma skin cancers and have much lower death risks per case of incidence than CMM: less than one percent versus 20–30 percent in most countries.1,2 CMM, SCC and BCC have different etiologies.3,4 The action spectra for erythema and SCC (Fig. 1) are likely to be strongly UVB weighted, and there seems to be a relationship with the total UV exposure, notably in the case of SCC.5 | ||
| Fig. 1 Action spectra for erythema in human skin according to CIE,6 for SCC in mouse skin according SCUP5 and for CMM in fish according Setlow et al.7 | ||
For CMM, the influence of UV radiation has been debated for decades.1,2,8–14 Some authors even argue that UV plays no major role.15 The main arguments for and against a relationship between UV and CMM incidence are listed in Tables 1 and 2.
| Main arguments for a relationship between UV and CMM | References | |
|---|---|---|
| 1. | Sunburn episodes are risk factors for CMM. | 13,16–19 |
| 2. | CMMs used to occur mainly on sun-exposed skin. | 14,16,20 |
| 3. | CMM can be induced in some animals by UV (examples: Angora goats, Sinclair swine, Monodelphis domestica (an opossum) and Xiphophorus (a small swordfish). | 21 |
| 4. | Patients with CMM have increased risk of BCC and SCC. | 22 |
| 5. | In Europe, CMM is more frequent in the north than in the south. The whole area is mainly populated by Caucasians. | 23,24 |
| 6. | Some CMMs contain mutations pointing towards UVB damage (cyclobutane pyrimidine dimers, pyrimidine (6–4) pyrimidone photoproducts). | 12,14,20,25,26 |
| 7. | Lentigo maligna melanoma is clearly related to UV exposure. | 27 |
| 8. | CMMs often arise in the borders of pigmented nevi. | 17,28 |
| 9. | CMM risk decreases with increasing pigmentation. Skin pigments absorb UV. | 29–32 |
| 10. | CMM risk increases upon migration to more sunny countries. | 18,33 |
| 11. | CMM patients often have low DNA repair capacity and low minimum erythema doses (MEDs). | 8,34–37 |
| 12. | Patients with xeroderma pigmentosum (abnormal DNA repair) have at least 1000 times increased CMM risks. | 8,36,38 |
| 13. | Some reports indicate increased CMM risk for persons frequently using sunbeds. | 39–42 |
| Main arguments against a relationship between UV and CMM | References | |
|---|---|---|
| 1. | CMM is more frequent among people with indoor occupations giving low accumulated UV exposures, (white-collar workers), than among people with large accumulated UV exposures (farmers, fishermen, etc.). | 43–45 |
| 2. | The localization pattern of CMM on the body is different from that of SCC, which is clearly UV related. | 1 |
| 3. | CMM is uncommon among albino Africans; opposite to what is found for BCC and SCC. | 46 |
| 4. | The incidence rate of CMM in sunny Australia is only two times higher than in the high-latitude country Norway, while the incidence rates of BCC and SCC are 10 to 20 times higher. | 47 |
| 5. | In Europe, CMM is more frequent in the north than in the south. The whole area is mainly populated by Caucasians. | 24,47 |
| 6. | Around CMM lesions little solar elastosis is found48 Solar elastosis is related to accumulated UV exposure. | 48,49 |
| 7. | Sun and artificial sources of UVB are efficient generators of vitamin D which seems to reduce carcinogenesis and tumor progression. It has been demonstrated that higher 25-hydroxyvitamin D3 levels, at CMM diagnosis, were associated with both thinner tumours and better survival from melanoma, independent of Breslow thickness. | 48,50–52 |
| 8. | CMM may be a disease related to affluence, since the incidence rates increases with increasing gross domestic product (GDP) | 13 |
| 9. | A number of chemicals seem to be causing CMM. There is a relationship between polycyclic aromatic hydrocarbons (PAH), benzene, and/or polychlorinated biphenyls (PCB) exposure of workers in the petroleum and automobile industry and an increased risk for CMM. | 53 |
The discussion of whether UVA can generate CMM is of interest from the viewpoints of photobiology and physics, and also health politics. The main argument for and against a causation are listed in Tables 3 and 4.
| Main arguments for a relationship of UVA and CMM. | References | |
|---|---|---|
| 1. | In populations with similar skin type there is a clear latitudinal gradient, which is larger for BCC and SCC than for CMM. The UVB gradient is also larger than the UVA gradient. The incidence rate of CMM in sunny Australia is only two times higher than in the high-latitude country Norway, while the incidence rates of BCC and SCC are 20 to 40 times higher. | 24 |
| 2. | CMM risk decreases with increasing skin pigmentation. Melanin absorbs UVA. | 32 |
| 3. | CMMs contain some DNA damage pointing towards UVA damage (8-oxo-7,8-dihydroguanine). | 8–10,26,54 |
| 4. | Some reports indicate increased CMM risk for persons frequently using sunbeds. Most sunbeds emit relatively more UVA than the sun does. | 39–42,55,56 |
| 5. | Albino Africans lacking melanin have very high rates of BCC and SCC but low rates of CMM. This seems to suggest that melanin, which absorbs UVA, is a chromophore for CMM. | 46 |
| 6. | There seems to be little CMM protective effect of only UVB absorbing sunscreens. However, a broad-spectrum (UVB and UVA) sunscreen reduces risk of CMM. | 57–60 |
| 7. | Around CMM lesions little solar elastosis is found, indicating low UVB exposure. Solar elastosis is related to accumulated UVB exposure. | 48,49,61 |
| Main arguments against a relationship of UVA and CMM | References | |
|---|---|---|
| 1. | UVA induces CMM neither in Monodelphis domestica nor in transgenic mice. | 62–65 |
| 2. | Recently, the original Xiphophorus experiments of Setlow et al. were attempted to be reproduced, but did not show any CMM-generating effect of UVA. | 7,66 |
The arguments for melanomagenic effects of UV, notably of UVA, are, in our opinion, stronger than the arguments against, and it seems that intermittent exposures, resulting in sunburns, are particularly dangerous as judged from the pattern of body distribution of SCC and CMM.1 The distribution of SCC and BCC on different parts of the body is different and changing with time.1,67 The density of SCC and BCC per unit skin area is, and has always been, largest on the face, as might be expected if the total sun exposure is important.1,68,69 The same was true for CMM until the 1980s when the density became larger on the trunk in some populations (Norway, Canada, Switzerland) for young persons (<50 years).70–72 This was attributed to intermittent sun exposure and sunburns.
The relative role of UVB and UVA in the etiology of CMM is of crucial importance for health authorities formulating rules for legal sunbeds. At present sunbeds emit similar UVB fluence rates as those in Mediterranean noon sun but six to ten times higher UVA fluence rates.55,56
We will try to elucidate the relative role of UVA and UVB for CMM and SCC by studying the north–south gradient of CMM and SCC in Norway and Sweden. As earlier stated, the north–south gradient is larger for the annual fluences of UVB than for that of UVA.1,67
Materials and methods
Incidence rates of CMM and SCC in Norway and Sweden
We have analyzed epidemiological data from the Cancer Registries of Norway and Sweden for overall CMMs and SCCs in different counties.The age-standardized incidence rates for the world standard population (ASIR (W)) per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons for CMM by age (0–49 and older than 50 years of age) in Sweden and in Norway are retrieved from the online data base of International Agency for Research on Cancer (IARC).73–77
000 persons for CMM by age (0–49 and older than 50 years of age) in Sweden and in Norway are retrieved from the online data base of International Agency for Research on Cancer (IARC).73–77
Ultraviolet doses in Norwegian and Swedish counties
Relevant UVA doses are determined under the assumption that the action spectrum (Fig. 1) of CMM is similar to the action spectrum for melanoma in Xiphophorus fish78 and that UVB doses are represented by CIE action spectrum for erythema.6 Calculations are based on daily satellite measurements (Total Ozone Mapping Spectrometer (TOMS) on Nimbus-7 satellite). The calculations include daily cloud cover and total ozone. The annual doses are averages over a ten-year period (1980–1990). A cylinder representation of the human skin surface was used as described and argued for earlier.79 Further arguments for this model are based on latitudinal dependencies of CMM rates on different body localizations and will be presented later (manuscript in preparation). The conclusions arrived in the present work would be essentially the same if a planar, horizontal surface were used, although the north–south gradients would be different.ASIR was used to compare time trends between CMM at different age groups (0–49 and older than 50 years of age) for the north, mid/west and south regions of Norway. Assignment of the Norwegian counties into three regions, north, mid/west and south, was based on ambient annual UV doses, calculated and measured as earlier described.80,81 The south region of Norway (mean latitude 60°) has high annual ambient UV exposure (37 × 104 J m−2). The mid/west region (mean latitude 64°) has middle annual ambient UV exposure (30.5 × 104 J m−2) and the north region (mean latitude 70°) has low annual ambient UV exposure (26 × 104 J m−2).80,81
The data were analyzed using SigmaPlot 11.0 software from Systat Software, Inc. (Richmond, CA, USA). Significant P-value was considered < 0.05.
Results
The incidence rates of CMM and SCC for both sexes are increasing in Norway and Sweden (Fig. 2). The slopes are very similar for the two countries and are significantly (P < 0.05) larger for SCC than for CMM (Fig. 2). The slopes for CMM are similar for men and women (Fig. 2A, B). For SCC, the overall incidence rates in Sweden are about two times larger for men than for women (Fig. 2D). Unfortunately, we do not have separate incidence rates of SCC in Norway for men and women. | ||
Fig. 2 The age-standardized incidence rates (according to the world standard population (ASIR (W)) per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 women and men for CMM (A, B) and SCC (C, D) in Norway (A, C) and in Sweden (B, D). 000 women and men for CMM (A, B) and SCC (C, D) in Norway (A, C) and in Sweden (B, D). | ||
We have calculated, on the basis of measurements as described in materials and methods, the UVA and the UVB annual fluences for the different regions of Norway and Sweden (Fig. 3A). The UVA/UVB ratio decreases with decreasing latitude (Fig. 3B), and so do the ratios of CMM/SCC incidence rates (Fig. 3C). These ratios are smaller for men than for women (Fig. 3C), in agreement with Fig. 2B and 2D.
 | ||
| Fig. 3 UVA and UVB annual fluences at different latitudes (A), ratios of UVA/UVB annual fluences at different latitudes (B), and ratios of incidence rates of CMM and SCC for men and women (C). | ||
SCC comes later in life than CMM (Fig. 4). For CMM the age curves (in both countries) are different for men and women: For persons younger than 50 years the rates are highest for women, while for persons older than 50 years the rates are highest for men (Fig. 4A). Thus, the overall ASIR of CMM are similar for the two sexes (Fig. 2A, B).
 | ||
Fig. 4 Age distributions of the age-standardized incidence rates (according to the world standard population (ASIR (W)) per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of CMM (A) and SCC (B) for men and women in Norway and Sweden. For SCC we have data for men and women only for Sweden. 000 of CMM (A) and SCC (B) for men and women in Norway and Sweden. For SCC we have data for men and women only for Sweden. | ||
The incidence rates of CMM for both sexes increase with decreasing latitude (Fig. 5A). The increase is larger for the oldest persons (Fig. 5). Thus, the ratio of rates for >50 and <50 years is largest for old people living in the south parts of Norway (Fig. 5B).
 | ||
Fig. 5 Latitudinal dependencies of the age-standardized incidence rates of CMM in Norway (according to the world standard population (ASIR (W)) per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (A) for both sexes younger and older than 50 years of age, and for the corresponding ratios (B). 000 (A) for both sexes younger and older than 50 years of age, and for the corresponding ratios (B). | ||
The incidence rates for the younger generations (<50 years) have decreased or stayed constant after about 1990, notably in the south region of Norway (Fig. 6A–C). In view of this and of the age curves shown in Fig. 4, we calculated the time gradients (on the basis of Fig. 6) for the periods before and after 1990 and for younger (<50 years age) and older (>50 years age) persons (Fig. 7). Except for younger men (<50 years age) in the north region (for whom the data are scattered due to few cases) all gradients before 1990 are positive and almost similar (Fig. 7). All gradients are smaller after 1990 than before 1990. For younger men (<50 years age) they are even negative, showing decreasing incidence rates. The same is true for younger women (<50 years age) living in the south region (Fig. 6 and 7).
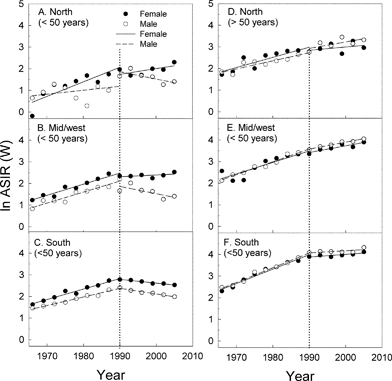 | ||
Fig. 6 Time development of the age-standardized incidence rates of CMM (according to the world standard population (ASIR (W)) per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for persons at two age groups (0–49 (A–C) and >50 years (E–F)) in the north (A,D), the mid/west (B, E) and the south (C, F) regions of Norway. 000 for persons at two age groups (0–49 (A–C) and >50 years (E–F)) in the north (A,D), the mid/west (B, E) and the south (C, F) regions of Norway. | ||
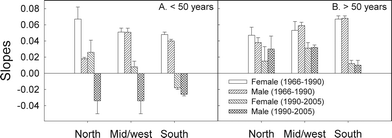 | ||
| Fig. 7 Time gradients (slopes from Fig. 6) of the CMM incidence rates in Norway for two age groups (younger and older than 50 years). The values are given for the periods 1966–1990 and 1990–2005 using approximate regression lines. | ||
Discussion
The present data (Fig. 2 and 3) are more detailed than our earlier findings,24 but agree with these where we compared the CMM, SCC and BCC rates in Scandinavia with those in Australia, and suggest that UVA might be melanomagenic. In the period 1983–1987 the SCC and BCC rates were 20–40 times higher in Australia than in Norway, but the CMM rates were only a factor of 2–3 larger.24 We also found that the annual fluences of UVA were around 1.3 times larger in Australia than in Scandinavia, and those of UVB were around 2 times larger.24 We conclude that all our epidemiological data for latitudinal dependencies of CMM and SCC rates indicate that UVA may play a role for human CMM carcinogenesis.24 If this is true and can be confirmed in future studies, the findings suggest to reduce the legal UVA fluence rates in sunbeds and not to recommend sunscreens that absorb mainly UVB and transmit UVA. However, sun-like sunbeds, with less UVA than those used now, would act as summer sun and might, carefully used, improve the winter levels of vitamin D. Keeping a constant vitamin D level throughout the year might be optimal. This has been argued for from a physiological and from a biochemical point of view.82 Most of the human evolution occurred in Africa, where vitamin D is produced in skin with a constant rate throughout the year. The relationship between vitamin D and CMM has recently been reviewed.51,52,83 A preventive role of vitamin D cannot be ruled out. Thus, it was recently found that weekend and holiday sun exposure (which are likely to be of intermittent character) were strongly associated with increased vitamin D level but did not increase the CMM risk.84BCC and SCC, like most internal cancers, increase in incidence with age, as shown here for SCC (Fig. 4). This is also true for CMM incidence on the face in Norway, but not for CMM on the trunk, where CMM is most frequent among middle-aged people.70 The incidence rates of CMM increased with doubling times of the order of 12–15 years over many decades until about 1990.85 The rate of increase in that early period was almost the same in all regions and for young and old persons (Fig. 6 and 7), i.e. 12–15 years. In Australia, in the United States, Sweden and Canada the CMM rates have stabilized or decreased after 1990, notably for young people.74–77 The decrease is statistically significant (P < 0.05) for the southern part of Norway (Fig. 6). In all cases the gradient of the incidence rate has decreased with time (Fig. 6 and 7). This decrease has taken place in a period with a steep increase in use of sunbeds and the relationship needs further studies.
Recent epidemiological investigations indicate that those who use sunbeds have increased CMM risks.41,42,86–88 This may be related to the high fluence rate of UVA in sunbeds.55,56 Some older investigations show no increased risk, which might be related to different UVA/UVB ratios at earlier times.39 However, even a very recent study showed no increased CMM risk associated with sunbed use.89
Sun exposure may have a dual role in melanomagenesis. Thus, in time periods of increasing rates of CMM on sun exposed body localizations (face, trunk, etc.), the rates of CMM on non-UVB exposed localizations (the uveal part of the eye, the vulva and the perianal region) seem to decrease and vice versa.90,91 Sometimes there is even an opposite latitudinal gradient for exposed and non-exposed localizations.91 Also, the prognosis of CMM is best for CMMs arising on skin with elastosis and signs of high UVB exposure.48,49 All these observations indicate that UVB-induced vitamin D may play a protective role.
Can the age distribution of CMM (Fig. 4) give any information about the role of UVA? Age-related differences in exposure patterns (more intermittent exposures and more use of UVB absorbing sunscreens among young persons) may explain the early onset of CMM compared with that of SCC. However, even before extensive use of UVB-absorbing sunscreens there was a difference between the age of onset for CMM and SCC in Norway.92 Most likely, the differences between the age curves for CMM and SCC (Fig. 4) indicate that the etiologies of these two cancer forms are different. Recent studies demonstrate that melanoma cells contain not only mutations related to past exposure to UV radiation, but also mutations unrelated to UV exposure.93,94 The difference between the curves for CMM in men and those in women may suggest that estrogens play a role for the high incidence among younger women (<50 years age) (Fig. 4), as indicated in other investigations.95–97
The latitudinal dependencies of the age standardized CMM incidence rates for persons younger and older than 50 years of age (Fig. 5) and of the corresponding ratios (Fig. 5) are not easy to explain. Why should relatively more old people get CMM in the south than in the north (Fig. 5B)? It would be speculative to relate this to any role of UVA. Possibly, sunburning among old persons is more frequent in the south than in the north? Or, maybe, accumulation of solar damage is more prominent in the south than in the north, as related to higher life-time exposures? These hypothesis should be tested in the future.
In conclusion the present work supports the hypothesis that UVA radiation from the sun may play a role for CMM induction. The etiologies of SCC and CMM are different as indicated by differences in age curves and latitudinal gradients. Sun exposure patterns, age related repair of different types of UV damage, and sex hormones may play different roles for the two cancers. CMM incidence rates among young people have decreased or been constant since about 1990, while use of sunbeds has increased strongly over the same period. UVB-generation of vitamin D may contribute to the differences in latitudinal gradients of SCC and CMM rates, and should be paid attention to in future investigations and discussions of the etiologies of SCC and CMM.
Acknowledgements
The present work was supported by the Norwegian Cancer Society (Kreftforeningen). We thank the NASA/GSFC TOMS Ozone Processing Team for the use of their Numbus 7 TOMS data.References
- U. Leiter and C. Garbe, Epidemiology of melanoma and nonmelanoma skin cancer–the role of sunlight, Adv. Exp. Med. Biol., 2008, 624, 89–103.
- R. M. Mackie, Long-term health risk to the skin of ultraviolet radiation, Prog. Biophys. Mol. Biol., 2006, 92, 92–96 CrossRef.
- P. Boukamp, UV-induced skin cancer: similarities–variations, J. Dtsch. Dermatol. Ges., 2005, 3, 493–503 Search PubMed.
- K. Y. Tsai and H. Tsao, The genetics of skin cancer, Am. J. Med. Genet., 2004, 131C, 82–92 Search PubMed.
- F. R. de Gruijl, H. J. Sterenborg, P. D. Forbes, R. E. Davies, C. Cole, G. Kelfkens, H. van Weelden, H. Slaper and J. C. van der Leun, Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice, Cancer Res., 1993, 53, 53–60 CAS.
- International Organization for Standardization (ISO), International Commission on Illumination (CIE): International Standard ISO 17166:1999(E)-CIE 007/E:1998, Erythema Reference Action Spectrum and Standard Erythema Dose, International Organization for Standardization (ISO), Geneva, Switzerland, 1999 Search PubMed.
- R. B. Setlow, E. Grist, K. Thompson and A. D. Woodhead, Wavelengths effective in induction of malignant melanoma, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 6666–6670 CAS.
- T. M. Runger, Role of UVA in the pathogenesis of melanoma and non-melanoma skin cancer. A short review, Photodermatol., Photoimmunol. Photomed., 1999, 15, 212–216 CrossRef CAS.
- S. Q. Wang, R. Setlow, M. Berwick, D. Polsky, A. A. Marghoob, A. W. Kopf and R. S. Bart, Ultraviolet A and melanoma: a review, J. Am. Acad. Dermatol., 2001, 44, 837–846 CrossRef CAS.
- L. P. Lund and G. S. Timmins, Melanoma, long wavelength ultraviolet and sunscreens: controversies and potential resolutions, Pharmacol. Ther., 2007, 114, 198–207 CrossRef CAS.
- J. Moan, A. C. Porojnicu and A. Dahlback, Ultraviolet radiation and malignant melanoma, Adv. Exp. Med. Biol., 2008, 624, 104–116.
- K. Rass and J. Reichrath, UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer, Adv. Exp. Med. Biol., 2008, 624, 162–178 Search PubMed.
- D. S. Rigel, Epidemiology of melanoma, Semin. Cutaneous Med. Surg., 2010, 29, 204–209 Search PubMed.
- M. S. Tang, Ultraviolet a light: potential underlying causes of melanoma, Future Oncol., 2010, 6, 1523–1526 Search PubMed.
- O. Hallberg and O. Johansson, Malignant melanoma of the skin - not a sunshine story!, Med. Sci. Monit., 2004, 10, CR336–CR340 Search PubMed.
- S. Gandini, F. Sera, M. S. Cattaruzza, P. Pasquini, O. Picconi, P. Boyle and C. F. Melchi, Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure, Eur. J. Cancer, 2005, 41, 45–60 CrossRef.
- E. L. Rager, E. P. Bridgeford and D. W. Ollila, Cutaneous melanoma: update on prevention, screening, diagnosis, and treatment, Am. Fam. Physician, 2005, 72, 269–276 Search PubMed.
- S. A. Oliveria, M. Saraiya, A. C. Geller, M. K. Heneghan and C. Jorgensen, Sun exposure and risk of melanoma, Arch. Dis. Child., 2005, 91, 131–138 CrossRef.
- Y. M. Chang, J. H. Barrett and D. T. Bishop et al., Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls, Int. J. Epidemiol., 2009, 38, 814–830 Search PubMed.
- E. Erdei and S. M. Torres, A new understanding in the epidemiology of melanoma, Expert Rev. Anti-Infect. Ther., 2010, 10, 1811–1823 Search PubMed.
- R. D. Ley, Animal models of ultraviolet radiation (UVR)-induced cutaneous melanoma, Front. Biosci., 2002, 7, d1531–d1534 Search PubMed.
- G. Kroumpouzos, M. M. Konstadoulakis, H. Cabral and C. P. Karakousis, Risk of basal cell and squamous cell carcinoma in persons with prior cutaneous melanoma, Dermatol. Surg., 2000, 26, 547–550 Search PubMed.
- I. K. Crombie, Variation of melanoma incidence with latitude in North America and Europe, Br. J. Cancer, 1979, 40, 774–781 Search PubMed.
- J. Moan, A. Dahlback and R. B. Setlow, Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation, Photochem. Photobiol., 1999, 70, 243–247 CrossRef CAS.
- N. K. Hayward, Mutation spectrum of the first melanoma genome points finger firmly at ultraviolet light as the primary carcinogen, Pigm. Cell Melanoma Res., 2010, 23, 153–154 Search PubMed.
- H. Ikehata and T. Ono, The Mechanisms of UV Mutagenesis, J. Radiat. Res., 2011, 52, 115–125 Search PubMed.
- M. Situm, Z. Bolanca and M. Buljan, Lentigo maligna melanoma–the review, Coll. Antropol., 2010, 34(Suppl 2), 299–301 Search PubMed.
- R. Hofmann-Wellenhof, H. P. Soyer, I. H. Wolf, J. Smolle, S. Reischle, E. Rieger, R. O. Kenet, P. Wolf and H. Kerl, Ultraviolet radiation of melanocytic nevi: a dermoscopic study, Arch. Dermatol., 1998, 134, 845–850 Search PubMed.
- S. Gandini, F. Sera, M. S. Cattaruzza, P. Pasquini, R. Zanetti, C. Masini, P. Boyle and C. F. Melchi, Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors, Eur. J. Cancer, 2005, 41, 2040–2059 Search PubMed.
- C. M. Olsen, H. J. Carroll and D. C. Whiteman, Estimating the attributable fraction for melanoma: a meta-analysis of pigmentary characteristics and freckling, Int. J. Cancer, 2010, 127, 2430–2445 Search PubMed.
- N. Maddodi and V. Setaluri, Role of UV in cutaneous melanoma, Photochem. Photobiol., 2008, 84, 528–536 CrossRef CAS.
- K. Byrd-Miles, E. L. Toombs and G. L. Peck, Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians, J. Drugs Dermatol., 2007, 6, 10–16 Search PubMed.
- P. Autier, J. F. Dore, O. Gefeller, J. P. Cesarini, F. Lejeune, K. F. Koelmel, D. Lienard and U. R. Kleeberg, Melanoma risk and residence in sunny areas. EORTC Melanoma Co-operative Group. European Organization for Research and Treatment of Cancer, Br. J. Cancer, 1997, 76, 1521–1524 CAS.
- A. L. Kadekaro, K. Wakamatsu, S. Ito and Z. A. Abdel-Malek, Cutaneous photoprotection and melanoma susceptibility: reaching beyond melanin content to the frontiers of DNA repair, Front. Biosci., 2006, 11, 2157–2173 Search PubMed.
- A. Sarasin and P. Dessen, DNA repair pathways and human metastatic malignant melanoma, Curr. Mol. Med., 2010, 10, 413–418 Search PubMed.
- G. M. Kraehn, M. Schartl and R. U. Peter, Human malignant melanoma. A genetic disease?, Cancer, 1995, 75, 1228–1237 Search PubMed.
- J. Di Lucca, M. Guedj, V. Descamps, A. Bourillon, P. Dieude, P. Saiag, P. Wolkenstein, N. Dupin, C. Lebbe, N. Basset-Seguin, B. Grandchamp and N. Soufir, Interactions between ultraviolet light exposure and DNA repair gene polymorphisms may increase melanoma risk, Br. J. Dermatol., 2009, 162, 891–893 Search PubMed.
- K. H. Kraemer, Xeroderma Pigmentosum, University of Washington, Seattle, Seattle (WA), 1993 Search PubMed.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer, The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review, Int. J. Cancer, 2007, 120, 1116–1122.
- W. Ting, K. Schultz, N. N. Cac, M. Peterson and H. W. Walling, Tanning bed exposure increases the risk of malignant melanoma, Int. J. Dermatol., 2007, 46, 1253–1257 CrossRef.
- D. Lazovich, R. I. Vogel, M. Berwick, M. A. Weinstock, K. E. Anderson and E. M. Warshaw, Indoor tanning and risk of melanoma: a case-control study in a highly exposed population, Cancer Epidemiol., Biomarkers Prev., 2010, 19, 1557–1568 Search PubMed.
- M. B. Veierod, H. O. Adami, E. Lund, B. K. Armstrong and E. Weiderpass, Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi, Cancer Epidemiol., Biomarkers Prev., 2010, 19, 111–120 Search PubMed.
- K. R. Cooke, D. C. Skegg and J. Fraser, Socio-economic status, indoor and outdoor work, and malignant melanoma, Int. J. Cancer, 1984, 34, 57–62 Search PubMed.
- F. C. Garland, M. R. White, C. F. Garland, E. Shaw and E. D. Gorham, Occupational sunlight exposure and melanoma in the U.S. Navy, Arch. Environ. Health, 1990, 45, 261–267 CrossRef CAS.
- E. Pukkala, J. I. Martinsen, E. Lynge, H. K. Gunnarsdottir, P. Sparen, L. Tryggvadottir, E. Weiderpass and K. Kjaerheim, Occupation and cancer - follow-up of 15 million people in five Nordic countries, Acta Oncol., 2009, 48, 646–790 Search PubMed.
- A. Yakubu and O. A. Mabogunje, Skin cancer in African albinos, Acta Oncol., 1993, 32, 621–622 Search PubMed.
- J. Moan, A. C. Porojnicu, A. Dahlback and R. B. Setlow, Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 668–673 Search PubMed.
- M. Berwick, B. K. Armstrong, L. Ben-Porat, J. Fine, A. Kricker, C. Eberle and R. Barnhill, Sun exposure and mortality from melanoma, J. Natl. Cancer Inst., 2005, 97, 195–199 CrossRef.
- R. T. Vollmer, Solar elastosis in cutaneous melanoma, Am. J. Clin. Pathol., 2007, 128, 260–264 Search PubMed.
- E. Giovannucci, The epidemiology of vitamin D and cancer incidence and mortality: a review (United States), Cancer, Causes Control, 2005, 16, 83–95 CrossRef.
- S. Field and J. A. Newton-Bishop, Melanoma and vitamin D, Mol. Oncol., 2011, 5, 197–214 Search PubMed.
- D. E. Godar, R. J. Landry and A. D. Lucas, Increased UVA exposures and decreased cutaneous Vitamin D(3) levels may be responsible for the increasing incidence of melanoma, Med. Hypotheses, 2009, 72, 434–443 CrossRef CAS.
- C. Fortes and E. de Vries, Nonsolar occupational risk factors for cutaneous melanoma, Int. J. Dermatol., 2008, 47, 319–328 Search PubMed.
- J. Cadet and T. Douki, Oxidatively generated damage to DNA by UVA radiation in cells and human skin, J. Invest. Dermatol., 2011, 131, 1005–1007 CrossRef CAS.
- L. T. Nilsen, T. N. Aalerud, M. Hannevik and M. B. Veierod, UVB and UVA irradiances from indoor tanning devices, Photochem. Photobiol. Sci., 2011, 10, 1129–1136 RSC.
- B. Gerber, P. Mathys, M. Moser, D. Bressoud and C. Braun-Fahrlander, Ultraviolet emission spectra of sunbeds, Photochem. Photobiol., 2002, 76, 664–668 CrossRef CAS.
- B. L. Diffey, Sunscreens as a preventative measure in melanoma: an evidence-based approach or the precautionary principle?, Br. J. Dermatol., 2009, 161(Suppl 3), 25–27 CrossRef.
- B. Diffey, Sunscreens: expectation and realization, Photodermatol., Photoimmunol. Photomed., 2009, 25, 233–236 Search PubMed.
- E. D. Gorham, S. B. Mohr, C. F. Garland, G. Chaplin and F. C. Garland, Do sunscreens increase risk of melanoma in populations residing at higher latitudes?, Ann. Epidemiol., 2007, 17, 956–963 Search PubMed.
- R. Neale, G. Williams and A. Green, Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial, Arch. Dermatol., 2002, 138, 1319–1325 Search PubMed.
- L. H. Kligman and R. M. Sayre, An action spectrum for ultraviolet induced elastosis in hairless mice: quantification of elastosis by image analysis, Photochem. Photobiol., 1991, 53, 237–242 CrossRef CAS.
- R. D. Ley, Ultraviolet radiation A-induced precursors of cutaneous melanoma in Monodelphis domestica, Cancer Res., 1997, 57, 3682–3684 CAS.
- E. C. De Fabo, F. P. Noonan, T. Fears and G. Merlino, Ultraviolet B but not ultraviolet A radiation initiates melanoma, Cancer Res., 2004, 64, 6372–6376 CAS.
- E. C. De Fabo, Initial studies on an in vivo action spectrum for melanoma induction, Prog. Biophys. Mol. Biol., 2006, 92, 97–104 Search PubMed.
- R. D. Ley, Dose response for ultraviolet radiation A-induced focal melanocytic hyperplasia and nonmelanoma skin tumors in Monodelphis domestica, Photochem. Photobiol., 2001, 73, 20–23 CrossRef CAS.
- D. L. Mitchell, A. A. Fernandez, R. S. Nairn, R. Garcia, L. Paniker, D. Trono, H. D. Thames and I. Gimenez-Conti, Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9329–9334 CrossRef CAS.
- C. Garbe and U. Leiter, Melanoma epidemiology and trends, Clin. Dermatol., 2009, 27, 3–9 CrossRef.
- M. P. Staples, M. Elwood, R. C. Burton, J. L. Williams, R. Marks and G. G. Giles, Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985, Med J. Aust., 2006, 184, 6–10 Search PubMed.
- P. H. Youl, M. Janda, J. F. Aitken, C. B. Del Mar, D. C. Whiteman and P. D. Baade, Body-site distribution of skin cancer, pre-malignant and common benign pigmented lesions excised in general practice, Br. J. Dermatol., 2011, 165, 35–43 Search PubMed.
- J. Moan, A. Dahlback, Z. Lagunova, E. Cicarma and A. C. Porojnicu, Solar radiation, vitamin D and cancer incidence and mortality in Norway, Anticancer Res., 2009, 29, 3501–3509 Search PubMed.
- J. M. Elwood and R. P. Gallagher, Body site distribution of cutaneous malignant melanoma in relationship to patterns of sun exposure, Int. J. Cancer, 1998, 78, 276–280 Search PubMed.
- J. L. Bulliard, D. De Weck, T. Fisch, A. Bordoni and F. Levi, Detailed site distribution of melanoma and sunlight exposure: aetiological patterns from a Swiss series, Ann. Oncol., 2006, 18, 789–794 Search PubMed.
- E. de Vries, F. I. Bray, J. W. Coebergh and D. M. Parkin, Changing epidemiology of malignant cutaneous melanoma in Europe 1953-1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia, Int. J. Cancer, 2003, 107, 119–126 CrossRef CAS.
- A. Jemal, S. S. Devesa, P. Hartge and M. A. Tucker, Recent trends in cutaneous melanoma incidence among whites in the United States, J. Natl. Cancer Inst., 2001, 93, 678–683 Search PubMed.
- D. C. Whiteman, C. A. Bray, V. Siskind, A. C. Green, D. J. Hole and R. M. Mackie, Changes in the incidence of cutaneous melanoma in the west of Scotland and Queensland, Australia: hope for health promotion?, Eur. J. Cancer Prev., 2008, 17, 243–250 CrossRef.
- P. M. Karlsson and M. Fredrikson, Cutaneous malignant melanoma in children and adolescents in Sweden,1993–2002: the increasing trend is broken, Int. J. Cancer, 2007, 121, 323–328 CrossRef CAS.
- D. K. Pruthi, R. Guilfoyle, Z. Nugent, M. C. Wiseman and A. A. Demers, Incidence and anatomic presentation of cutaneous malignant melanoma in central Canada during a 50-year period: 1956 to 2005, J. Am. Acad. Dermatol., 2009, 61, 44–50 CrossRef.
- J. Moan, Z. Lagunova, E. Cicarma, L. Aksnes, A. Dahlback, W. B. Grant and A. C. Porojnicu, Sunbeds as vitamin D sources, Photochem. Photobiol., 2009, 85, 1474–1479 CrossRef CAS.
- A. Dahlback and J. Moan, Annual exposures to carcinogenic radiation from the sun at different latitudes and amplification factors related to ozone depletion. The use of different geometrical representations of the skin surface receiving the ultraviolet radiation, Photochem. Photobiol., 1990, 52, 1025–1028 Search PubMed.
- A. C. Porojnicu, Z. Lagunova, T. E. Robsahm, J. P. Berg, A. Dahlback and J. Moan, Changes in risk of death from breast cancer with season and latitude: sun exposure and breast cancer survival in Norway, Breast Cancer Res. Treat., 2006, 102, 323–328 Search PubMed.
- J. Moan, A. Porojnicu, Z. Lagunova, J. P. Berg and A. Dahlback, Colon cancer: prognosis for different latitudes, age groups and seasons in Norway, J. Photochem. Photobiol., B, 2007, 89, 148–155 CrossRef CAS.
- R. Vieth, How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology, Anticancer Res., 2009, 29, 3675–3684 Search PubMed.
- K. M. Egan, Vitamin D and melanoma, Ann. Epidemiol., 2009, 19, 455–461 Search PubMed.
- J. A. Newton-Bishop, Y. M. Chang, F. Elliott, M. Chan, S. Leake, B. Karpavicius, S. Haynes, E. Fitzgibbon, K. Kukalizch, J. Randerson-Moor, D. E. Elder, D. T. Bishop and J. H. Barrett, Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate, Eur. J. Cancer, 2011, 47, 732–741 Search PubMed.
- E. Cicarma, A. Juzeniene, A. C. Porojnicu, O. S. Bruland, J. Moan and Latitude, gradient for melanoma incidence by anatomic site and gender in Norway 1966–2007, J. Photochem. Photobiol., B, 2010, 101, 174–178 Search PubMed.
- A. E. Cust, B. K. Armstrong, C. Goumas, M. A. Jenkins, H. Schmid, J. L. Hopper, R. F. Kefford, G. G. Giles, J. F. Aitken and G. J. Mann, Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma, Int. J. Cancer, 2011, 128, 2425–2435 CrossRef CAS.
- P. Autier, J. F. Dore, A. M. Eggermont and J. W. Coebergh, Epidemiological evidence that UVA radiation is involved in the genesis of cutaneous melanoma, Curr. Opin. Oncol., 2011, 23, 189–196 CrossRef.
- S. G. Coelho and V. J. Hearing, UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women, Pigm. Cell Melanoma Res., 2010, 23, 57–63 Search PubMed.
- F. Elliott, M. Suppa, M. Chan, S. Leake, B. Karpavicius, S. Haynes, J. H. Barrett, D. T. Bishop and J. A. Newton-Bishop, Relationship between sunbed use and melanoma risk in a large case-control study in the United Kingdom, Int. J. Cancer, 2011 DOI:10.1002/ijc.26347.
- J. Moan, E. Cicarma, R. Setlow, A. C. Porojnicu, W. B. Grant and A. Juzeniene, Time trends and latitude dependence of uveal and cutaneous malignant melanoma induced by solar radiation, Derm.-Endocrinol., 2010, 2, 3–8 Search PubMed.
- J. Moan, A. C. Porojnicu, A. Dahlback, W. B. Grant and A. Juzeniene, Where the sun does not shine: is sunshine protective against melanoma of the vulva?, J. Photochem. Photobiol., B, 2010, 101, 179–183 Search PubMed.
- K. Magnus, The Nordic profile of skin cancer incidence. A comparative epidemiological study of the three main types of skin cancer, Int. J. Cancer, 1991, 47, 12–19 Search PubMed.
- C. Greenman, P. Stephens and R. Smith et al., Patterns of somatic mutation in human cancer genomes, Nature, 2007, 446, 153–158 CrossRef CAS.
- E. D. Pleasance, R. K. Cheetham and P. J. Stephens et al., A comprehensive catalogue of somatic mutations from a human cancer genome, Nature, 2009, 463, 191–196.
- L. Sadoff, J. Winkley and S. Tyson, Is malignant melanoma an endocrine-dependent tumor? The possible adverse effect of estrogen, Oncology, 1973, 27, 244–257 Search PubMed.
- E. R. Koomen, A. Joosse, R. M. Herings, M. K. Casparie, H. J. Guchelaar and T. Nijsten, Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: a population-based case-control study, Ann. Oncol., 2008, 20, 358–364 Search PubMed.
- M. Kvaskoff, A. Bijon, S. Mesrine, M. C. Boutron-Ruault and F. Clavel-Chapelon, Cutaneous Melanoma and Endogenous Hormonal Factors: A Large French Prospective Study, Am. J. Epidemiol., 2011, 173, 1192–1202 Search PubMed.
Footnotes |
| † This article is dedicated to the memory of Dr Egil Kvam for his important work in photobiology and for his contributions to our scientific communities through scientific discussions and through friendship. Egil Kvam pioneered investigations on the relationship between UVA and skin cancer. |
| ‡ Contribution to the themed issue on the biology of UVA. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2012 |
