Synthesis of nucleoside mono- and triphosphates bearing oligopyridine ligands, their incorporation into DNA and complexation with transition metals†
Lubica
Kalachova
,
Radek
Pohl
and
Michal
Hocek
*
Institute of Organic Chemistry and Biochemistry, Academy of Science of the Czech Republic, Gilead & IOCB Research Center, Flemingovo nam. 2, CZ-16610, Prague 6, Czech Republic. E-mail: hocek@uochb.cas.cz; Fax: +420 220183559; Tel: +420 220183324
First published on 27th September 2011
Abstract
Modified nucleoside mono- (dARMPs and dCRMPs) and triphosphates (dARTPs and dCRTPs) bearing bipyridine or terpyridine ligands attached viaacetylene linker were prepared by single-step aqueous-phase Sonogashira cross-coupling of 7-iodo-7-deaza-dAMP or -dATP, and 5-iodo-dCMP or -dCTP with the corresponding bipyridine- or terpyridine-linked acetylenes. The modified dNRTPs were successfully incorporated into the oligonucleotides by primer extension experiment (PEX) using different DNA polymerases and the PEX products were used for post-synthetic complexation with Fe2+.
Introduction
Nucleic acids bearing diverse functional groups are attracting growing interest due to their potential applications in chemical biology, bioanalysis, or nanotechnology.1 Transition metal complexes within (metal-base-pairs2 or intercalators3) and outside (metal complex covalently attached to a nucleobase, sugar or phosphate)4–7 the DNA duplex have been extensively studied. Diverse oligopyridine-metal complexes attached to DNA have been prepared and applied in stabilization of duplexes,4redox5 and photoredox6labeling or for self assembly of 2D and 3D nanostructures.7 We8,9 and others10 have previously developed efficient syntheses of bipyridine- (bpy) and terpyridine- (tpy) modified nucleoside building blocks. However, despite the apparent application potential, chemical synthesis of oligopyridine-nucleobase linked oligonucleotides (ONs) and their post-synthetic complexations have been reported very scarcely.11 In parallel with our project, the Wagenknecht group has developed12 a chemical synthesis of tpy-linked ONs and their complexation with Ni2+ and Zn2+.Apart from chemical synthesis,13 base-modified DNA can be also prepared enzymatically14,15 by polymerase incorporation of base- modified 2′-deoxyribonucleoside triphosphates (dNTPs) bearing substituents at position 5 of pyrimidines or at position 7 of 7-deazapurines. Combination of a direct cross-coupling modification of dNTPs with enzymatic incorporation resulted in efficient and general two-step synthesis of base-modified DNA.16 Here we report the synthesis of oligopyridine-linked nucleotides and dNTPs and enzymatic synthesis and complexation of oligopyridine-linked DNA.
Result and discussion
Synthesis of modified nucleotides and dNTPs
In order to prepare modified oligonucleotidesvia enzymatic incorporation, oligopyridine ligands had to be attached to the nucleobase in dNTPs via suitable linker (in our case rigid, linear and electron-conjugate acetylene tether). To develop the synthetic methodology and complexation of ONs, the chemistry was first performed on model nucleosides monophosphates (dNMPs). The first target compounds of our choice were 7-substituted 7-deaza-2′-deoxyadenosine (dARMPs) and 5-substituted 2′-deoxycytidine 5′-monophosphates (dCRMPs). They were prepared by single-step aqueous-phase Sonogashira cross-coupling reaction of halogenated nucleoside monophosphates dAIMP and dCIMP with terminal acetylenes 1a–c18 (Scheme 1, Table 1). The reactions were generally performed in the presence of Pd(OAc)2, water soluble tris(3-sulfonatophenyl)phosphane (TPPTS) ligand, CuI and Hünnig base in the mixture water/acetonitrile (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C for 1.5 h. The desired corresponding products dA6bpyMP, dA5bpyMP, dAtpyMP and dC6bpyMP, dC5bpyMP, dCtpyMP were isolated after the purification on reverse-phase HPLC in good yields (from 47% to 89%, Table 1).
1) at 80 °C for 1.5 h. The desired corresponding products dA6bpyMP, dA5bpyMP, dAtpyMP and dC6bpyMP, dC5bpyMP, dCtpyMP were isolated after the purification on reverse-phase HPLC in good yields (from 47% to 89%, Table 1).
| Entry | Monophosphate | Alkyne | Product | Yield |
|---|---|---|---|---|
| 1 | dC I MP | 1a | dC 6bpy MP | 89% |
| 2 | dC I MP | 1b | dC 5bpy MP | 89% |
| 3 | dC I MP | 1c | dC tpy MP | 47% |
| 4 | dA I MP | 1a | dA 6bpy MP | 52% |
| 5 | dA I MP | 1b | dA 5bpy MP | 70% |
| 6 | dA I MP | 1c | dA tpy MP | 57% |
 | ||
Scheme 1 Synthesis of modified dNRMPs and dNRTPs. Reagents and conditions: i) Pd(OAc)2 (5 mol%), TPPTS (5 equiv. to Pd), CuI (10 mol%), Et(i-Pr)2N (10 equiv.), H2O/CH3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C. 1), 80 °C. | ||
Having developed the methodology for modification of nucleotides, we have proceeded with the direct functionalization of dNTPs by Sonogashira cross-coupling, in analogy to our previously developed procedures5,16 (Scheme 1, Table 2). To avoid the hydrolysis of the starting and final triphosphates, the reaction mixture was heated at 80 °C only for 1 h.5,16 The corresponding products dA6bpyTP, dA5bpyTP, and dAtpyTP, as well as dC6bpyTP, dC5bpyTP, and dCtpyTP were isolated after the purification on reverse-phase HPLC in good yields (40–48% for dARTPs and 59–69% for dCRTPs, Table 2).
| Entry | Triphosphate | Alkyne | Product | Yield |
|---|---|---|---|---|
| 1 | dC I TP | 1a | dC 6bpy TP | 67% |
| 2 | dC I TP | 1b | dC 5bpy TP | 59% |
| 3 | dC I TP | 1c | dC tpy TP | 69% |
| 4 | dA I TP | 1a | dA 6bpy TP | 42% |
| 5 | dA I TP | 1b | dA 5bpy TP | 48% |
| 6 | dA I TP | 1c | dA tpy TP | 40% |
Incorporation of modified dNTPs by DNA polymerases
All the functionalized dARTPs or dCRTPs were tested as substrates for several thermostable DNA polymerases in primer extension experiment (PEX). Each PEX experiment, analyzed by denaturing polyacrylamide gel electrophoresis (PAGE), was compared with positive (all four natural dNTPs) and negative control experiments (absence of one natural dNTPs) in order to exclude any mis-incorporations. Single and multiple incorporation of oligopyridine-functionalized triphosphate were tested (for sequences of primer and templates see Table 3).| a In the template (temp) ONs segments that form a duplex with primer are printed in italics, the replicated segments are printed in bold. For magnetic separation of the extended primer strands, the templates 5′-end biotinylated. The acronyms used in the text for primer products are analogs to those introduced for templates (e.g. the PEX product pexrnd16 was synthesized on temprnd16 template. | |
|---|---|
| primrnd | 5′-CATGGGCGGCATGGG-3′ |
| temprnd16 | 5′-CTAGCATGAGCTCAGTCCCATGCCGCCCATG-3′ |
| tempC | 5′-CCCGCCCATGCCGCCCATG-3′ |
| tempA | 5′-CCCTCCCATGCCGCCCATG-3′ |
| tempA1 | 5′-TCCCATGCCGCCCATG-3′ |
Single-nucleotide extension experiments were tested separately with each of the three dARTPs and dCRTPs by using 19-mer templates tempA and tempC (Fig. 1). Experiments using Pwo polymerase were mostly successful to give fully extended products for all of the dNTPs except dCtpyTP (lane 11), which gave the mixture of fully and partially extended ONs. Therefore the reaction condition for incorporation of dCRTPs were further optimized and the use of DyNAzyme II enzyme leads to fully extended products. The lack of extension in negative control (A- or C-) proves that no miss-incorporation occurred. Each spot of a PEX-product (even in the positive and negative control experiments) is accompanied by a weak band of one nucleotide shorter product due to 3′-5′ exonuclease activity of the enzyme.
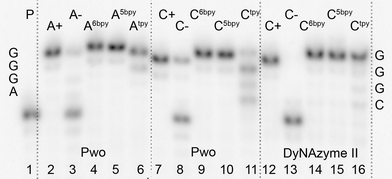 | ||
| Fig. 1 Denaturing PAGE analysis of PEX experiment synthetized on tempA (lines 2–6) and tempC (lines 7–16) with Pwo or DyNAzyme II polymerases. 5′-32P-end labelled primer-template was incubated with different combinations of natural and functionalized dNTPs. P: Primer; A+: natural dATP, dGTP; A-: dGTP; A6bpy: dA6bpyTP, dGTP; A5bpy: dA5bpyTP, dGTP; Atpy: dAtpyTP, dGTP; C+: natural dCTP, dGTP; C-: dGTP; C6bpy: dC6bpyTP, dGTP; C5bpy: dC5bpyTP, dGTP; Ctpy: dCtpyTP, dGTP. | ||
In order to compare the efficiency in incorporation of the oligopyridine-modified dNTPs in comparison with the natural ones, we have performed a simple kinetics study in single-nucleotide PEX reaction. The experiments with unmodified and modified dATPs were performed using Pwo polymerase and two modified dARTPs in comparison with the natural dATP (Fig. 2). The PEX with the natural dATP was finished within 1 min whereas the PEX with dA5bpyTP took 2 min and, with the more bulky dAtpyTP, even 5 min to complete. This shows that the modified dARTPs are worse substrates for the polymerase. Therefore, the reaction time for multiple incorporations must be prolonged to 30 min to ensure full length product formation.
 | ||
| Fig. 2 Comparison of the rate of the single-nucleotide PEX with natural A+ (dATP) and modified (dA5bpyTP, dAtpyTP) nucleotides using Pwo polymerase with tempA1 without natural dGTP. The reaction mixtures were incubated for time intervals indicated (in minutes), followed by stopping the reaction by addition of PAGE loading buffer and immediate heating. | ||
Multiple incorporations were tested on 31-mer template temprnd16 containing four copies of each of the four bases and requiring incorporation of four modified dNRTPs in separate positions. Several polymerases were tested: Pwo, DyNAzyme II, Vent (exo−), Deep Vent, Deep Vent (exo−), Phusion, KOD XL and Therminator. Incorporation of dARTPs using Pwo or Deep Vent leads to fully extended ONs (Fig. 3, lanes 5–7), while incorporation of dC6bpyTP and dCtpyTP (lanes 8 and 10) gave a mixture of ONs of different length (for incorporation using Deep Vent and other polymerasess see ESI†). Even after the optimization of condition (testing different DNA polymerases, higher concentration of enzyme and dCRTPs) the incorporation of dCtpyTP was less feasible and resulted in early termination of PEX (lane 15). Five time higher concentration of DyNAzyme II DNA polymerase in combination with double concentration of dCRTP was the most efficient in the incorporation of dCRTP. The products of pexrnd16 slightly differ in electrophoretic mobilities visible on gel partly (combination of the effects of the higher molecular weight and possible secondary structure formation). Therefore, the successful incorporation and full-length products were verified by measurement of MALDI mass spectra of PEX products. Single stranded DNA was prepared by PEX with biotinylated templates and then isolated by magnetoseparation.16b The correct masses were confirmed for the fully-extended products (see ESI†).
 | ||
| Fig. 3 Denaturing PAGE analysis of PEX experiment synthesized on temprnd16 with Pwo and DyNAzyme II polymerases. 5′-32P-end labelled primer-template was incubated with different combinations of natural and functionalized dNTPs. P: Primer; +: natural dNTPs; A-: dTTP, dCTP, dGTP; C-:dATP, dTTP, dGTP; A6bpy: dA6bpyTP, dTTP, dCTP, dGTP; A5bpy: dA5bpyTP, dTTP, dCTP, dGTP; Atpy: dAtpyTP, dTTP, dCTP, dGTP; C6bpy: dC6bpyTP, dATP, dTTP, dGTP; C5bpy: dC5bpyTP, dATP, dTTP, dGTP; Ctpy: dCtpyTP, dATP, dTTP, dGTP. | ||
Complexation studies
All six nucleoside monophosphates (dNRMPs) were tested as model compounds for further complexation studies on oligopyridine modified oligonucleotides. Aqueous solutions of dNRMPs were mixed with 0.5 equiv. of divalent metal such as Cu2+, Zn2+, Ni2+ and Fe2+. After incubation for 10 min. at room temperature, the complex formation was detected by UV/Vis spectroscopy. The spectra were recorded for the non-metalated and metalated monophospate. While MLCT bands of bpy- or tpy-modified monophosphates with Cu2+, Zn2+, Ni2+ are partially overlapping with dominating absorbance at 350 nm due to bpy and tpy ligands (see ESI†), the complex formed by mixingdNtpyMP with Fe2+ can be easily detected due to characteristic absorbance at 580 nm17 (Fig. 4, magenta line). | ||
| Fig. 4 UV/Vis spectra of: A) dAtpyMP, B) dCtpyMP with divalent metals. | ||
After successful complexation of nucleoside monophosphates, we proceeded with complexation of tpy-modified oligonucleotides with Fe2+, due to this easy detectable MLCT band. For the complexation studies detected by UV/Vis spectroscopy, we have chosen ON prepared by PEX on larger scale using Deep Vent polymerase and template temprnd16, due to the higher concentration of tpy-modification in product. Natural DNA was prepared by incorporaion of dCTP, dGTP, dTTP and dATP, while the modified ON was prepared by using dAtpyTP as surrogates of natural dATP. Oligonucleotides had to be purified to remove unincorporated dAtpyTP. After addition of 0.5 equiv. of Fe(BF4)2·6H2O per each modification and incubation for several hours at room temperature, the UV/Vis spectra were recorded for non-metalated and metalated DNA duplexes, either natural or modified one (Fig. 5). The dominant absorbance at ca. 260 nm originates from the absorbance of natural nucleotides (black line), small absorbance band at ca. 350 nm is due to the presence of tpy-modification (green line) while the band at ca. 590 is the MLCT band7c of complex formed by tpy-modified ON with Fe2+ (blue line). Similar MLCT band is not observed for the natural DNA mixed with Fe2+ (red line).
 | ||
| Fig. 5 UV/Vis spectra of natural and modified DNA duplexes with Fe(BF4)2·6H2O. | ||
The complex formation was also detected by native polyacrylamide gel electrophoresis. For the first experiments (monoincorporations), ONs were prepared by the PEX experiment using tempA and dATP (natural DNA) or dAtpyTP, or tempC and dCTPs (natural DNA) or dCtpyTP. The PEX products were directly, without previous purification, mixed with 1 eqiv. of Fe(BF4)2·6H2O or FeCl2 (calculated to the amount of modified dNtpyTP in PEX experiment) and incubated at room temperature for 3 h. Successful complex formation of pexA and pexC, containing one tpy-modification with Fe2+ ions is clearly shown by bands with slower mobility (Fig. 6, lanes 4 and 8), while no change in mobility was not observed for natural DNA mixed with Fe2+ ions (lanes 2 and 6).
 | ||
| Fig. 6 Non-denaturing gel electrophoresis (8% SB_PAGE) of DNA duplexes in the absence and in the presence of Fe2+ for pexA (A) or pexC (B). 5′-32P-end labelled primer-template was incubated with different combinations of natural and functionalized dNTPs: A+: unmodified DNA (dATP, dGTP); A+/Fe2+: unmodified DNA (dATP, dGTP) mixed with Fe2+; Atpy: tpy-modified DNA (dAtpyTP, dGTP); Atpy/Fe2+: tpy-modified DNA (dAtpyTP, dGTP) mixed with Fe2+; C+: unmodified DNA (dCTP, dGTP); C+/Fe2+: unmodified DNA (dCTP, dGTP) mixed with Fe2+; Ctpy: tpy-modified DNA (dCtpyTP, dGTP); Ctpy/Fe2+: tpy-modified DNA (dCtpyTP, dGTP) mixed with Fe2+. | ||
Similar results were shown also for pexrnd16 containing four tpy-modifications (Fig. 7). Since the dCtpyTP was not a good substrate in multiple incorporations, this experiment was only performed with Atpy. Here the bands of the complexes are more smeared since mixtures of possible products are formed.
 | ||
| Fig. 7 Non-denaturing gel electrophoresis (8% SB_PAGE) of DNA duplexes in the absence and in the presence of Fe2+ pexrnd16. 5′-32P-end labelled primer-template was incubated with different combinations of natural and functionalized dNTPs: A+: unmodified DNA (dATP, dTTP, dCTP, dGTP); A+/Fe2+: unmodified DNA (dATP, dTTP, dCTP, dGTP) mixed with Fe2+; Atpy: tpy-modified DNA (dAtpyTP, dTTP, dCTP, dGTP); Atpy/Fe2+: tpy-modified DNA (dAtpyTP, dTTP, dCTP, dGTP) mixed with Fe2+. | ||
Conclusions
Novel dNRMPs and dNRTPs bearing oligopyridine ligands attached viaacetylene linker were prepared in single-step aqueous-phase Sonogashira cross-coupling reaction of iodinated dNIMPs or dNITPs with corresponding terminal acetylene. Functionalized dNRTPs were shown to be good substrates for DNA polymerases and were incorporated into the DNA by primer extension. Pwo polymerase, which was successfully used for incorporation of modified dARTPs, did not incorporate dCRTPs with the same efficiency. For incorporation of modified dCRTPs, DyNAzyme II was identified as the most suitable enzyme, although the multiple incorporation of dCtpyTP was still less successful, resulting early termination of PEX experiment. Oligopyridine functionalized ONs, containing either one or four modifications, were successfully used for post-synthetic complexation with Fe2+ metals ions. Therefore, the Atpy-containing DNA has potential for self-assembly studies.Experimental
Sonogashira cross-coupling reaction were performed under argon atmosphere. Oligopyridinyl acetylenes,18 halogenated monophosphates16g,19 and halogenated triphosphates16a,c were prepared according to the literature procedures. Other chemicals were purchased from commercial suppliers and were used as received. Preparative HPLC separations were performed on column packed with 10 μm C18 reversed phase (Phenomenex, Luna C18(2)). NMR spectra were measured on a Bruker 500 or Bruker 600 (500 or 600 MHz for 1H, 125.7 or 150.9 MHz for 13C and 202.3 for 31P) in D2O (referenced to dioxane as internal standard, δH = 3.75 ppm, δC = 69.3 ppm, standard for 31P NMR was external H3PO4) or in CD3OD (referenced to TMS as an internal standard). Chemical shifts are given in ppm (δ scale), coupling constants (J) in Hz. Complete assignment of all NMR signals was achieved by use of a combination of H,H-COSY, H,C-HSQC, and H,C-HMBC experiments. NMR spectra of dNTPs were measured in phosphate buffer at pH 7.1. Mass spectra were measured on LCQ classic (Thermo-Finnigan) spectrometer using ESI or Q-Tof Micro (Waters, ESI source, internal calibration with lockspray). Mass spectra of functionalized DNA were measured by Maldi-TOF, Reflex IV (Bruker) with nitrogen laser. UV/Vis spectra were measured on Varian CARY 100 Bio spectrophotometer at room temperature.General procedure for Sonogashira cross-coupling – synthesis of modified dNRMPs
Mixture CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (1.5 ml) and Et(i-Pr)2N (10 equiv.) were added to an argon-purged flask containing halogenated nucleoside monophosphatedCIMP or dAIMP (60 mg), an alkyne 1a–c (1.5 equiv.) and CuI (10 mol%). In a separate flask, Pd(OAc)2 (5 mol%) and TPPTS (5 equiv. to Pd) were combined, evacuated and purged with argon followed by addition of CH3CN/H2O (1
2) (1.5 ml) and Et(i-Pr)2N (10 equiv.) were added to an argon-purged flask containing halogenated nucleoside monophosphatedCIMP or dAIMP (60 mg), an alkyne 1a–c (1.5 equiv.) and CuI (10 mol%). In a separate flask, Pd(OAc)2 (5 mol%) and TPPTS (5 equiv. to Pd) were combined, evacuated and purged with argon followed by addition of CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (0.5 ml). The mixture of catalyst was then injected into the reaction mixture and the reaction mixture was stirred at 80 °C for 1.5 h. The solvent was evaporated in vacuo. Products were purified by semi-preparative HPLC on C18 column using linear gradient of 0.1 M TEAB (triethylamonium bicarbonate) in H2O to 0.1 M TEAB in H2O/MeOH (1
2) (0.5 ml). The mixture of catalyst was then injected into the reaction mixture and the reaction mixture was stirred at 80 °C for 1.5 h. The solvent was evaporated in vacuo. Products were purified by semi-preparative HPLC on C18 column using linear gradient of 0.1 M TEAB (triethylamonium bicarbonate) in H2O to 0.1 M TEAB in H2O/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent. Several co-distillations with water and conversion to sodium salt form (Dowex 50 in Na+ cycle) followed by freeze-drying from water, gave the products as brownish or yellowish powder.
1) as an eluent. Several co-distillations with water and conversion to sodium salt form (Dowex 50 in Na+ cycle) followed by freeze-drying from water, gave the products as brownish or yellowish powder.
5-[(2′′,2′′′-bipyridin-6′′-yl)ethynyl]-2′-deoxycytidine 5′-O-monophosphate (dC6bpyMP)
This compound was prepared according to the general procedure from 5-iodo-2′-deoxycytidine monophosphate dCIMP and 1a in the yield 89%.
1H NMR (600.1 MHz, CD3OD): 2.26 (ddd, 1H, Jgem = 13.7, J2′b,1′ = 7.1, J2′b,3′ = 6.2, H-2′b); 2.41 (ddd, 1H, Jgem = 13.7, J2′a,1′ = 6.0, J2′a,3′ = 3.6, H-2′a); 4.05 (dt, 1H, Jgem = 10.4, JH,P = J5′b,4′ = 4.4, H-5′b); 4.10 (m, 1H, H-4′); 4.12 (ddd, 1H, Jgem = 10.4, JH,P = 6.0, J5′a,4′ = 3.6, H-5′a); 4.56 (dt, 1H, J3′,2′ = 6.2, 3.6, J3′,4′ = 3.6, H-3′); 6.27 (dd, 1H, J1′,2′ = 7.1, 6.0, H-1′); 7.49 (dd, 1H, J5′′′,4′′′ = 7.3, J5′′′,6′′′ = 4.8, H-5′′′); 7.88 (d, 1H, J5′′,4′′ = 7.7, H-5′′); 7.99 (m, 2H, H-4′′,4′′′); 8.23 (d, 1H, J3′′,4′′ = 7.9, H-3′′); 8.27 (d, 1H, J3′′′,4′′′ = 7.9, H-3′′′); 8.45 (s, 1H, H-6); 8.70 (d, 1H, J6′′′,5′′′ = 4.8, H-6′′′); 13C NMR (150.9 MHz, CD3OD): 41.66 (CH2-2′); 65.10 (d, JC,P = 4.5, CH2-5′); 72.41 (CH-3′); 82.78 (bpy-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C); 87.88 (CH-1′); 88.36 (d, JC,P = 8.4, CH-4′); 91.71 (C-5); 94.66 (bpy-C
C); 87.88 (CH-1′); 88.36 (d, JC,P = 8.4, CH-4′); 91.71 (C-5); 94.66 (bpy-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C); 122.26 (CH-3′′); 122.90 (CH-3′′′); 125.64 (CH-5′′′); 128.81 (CH-5′′); 138.95 (CH-4′′′); 139.44 (CH-4′′); 143.86 (C-6′′); 147.22 (CH-6); 150.57 (CH-6′′′); 156.50 (C-2′′′); 156.68 (C-2); 157.58 (C-2′′); 166.26 (C-4); 31P NMR (202.3 MHz, CD3OD): 5.06; MS (ES−): found m/z: 484.2 (M), 485.2 (M+H), 486.2 (M+2H); HRMS (ES−): m/z calcd for C21H19O7N5P: 484.1028; found: 484.1028.
C); 122.26 (CH-3′′); 122.90 (CH-3′′′); 125.64 (CH-5′′′); 128.81 (CH-5′′); 138.95 (CH-4′′′); 139.44 (CH-4′′); 143.86 (C-6′′); 147.22 (CH-6); 150.57 (CH-6′′′); 156.50 (C-2′′′); 156.68 (C-2); 157.58 (C-2′′); 166.26 (C-4); 31P NMR (202.3 MHz, CD3OD): 5.06; MS (ES−): found m/z: 484.2 (M), 485.2 (M+H), 486.2 (M+2H); HRMS (ES−): m/z calcd for C21H19O7N5P: 484.1028; found: 484.1028.
General procedure for Sonogashira cross-coupling – synthesis of modified dNRTPs
Mixture CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (1.5 ml) and Et(i-Pr)2N (10 equiv.) were added to an argon-purged flask containing halogenated nucleoside triphosphatedCITP or dAITP (60 mg), an alkyne 1a–c (1.5 equiv. for dCITP and 2 equiv. for dAITP) and CuI (10 mol%). In a separate flask, Pd(OAc)2 (5 mol%) and TPPTS (5 equiv. to Pd) were combined, evacuated and purged with argon followed by addition of CH3CN/H2O (1
2) (1.5 ml) and Et(i-Pr)2N (10 equiv.) were added to an argon-purged flask containing halogenated nucleoside triphosphatedCITP or dAITP (60 mg), an alkyne 1a–c (1.5 equiv. for dCITP and 2 equiv. for dAITP) and CuI (10 mol%). In a separate flask, Pd(OAc)2 (5 mol%) and TPPTS (5 equiv. to Pd) were combined, evacuated and purged with argon followed by addition of CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (0.5 ml). The mixture of catalyst was then injected into the reaction mixture and the reaction mixture was stirred at 80 °C for 1 h. The solvent was evaporated in vacuo. Products were purified by semi-preparative HPLC on C18 column using linear gradient of 0.1 M TEAB (triethylamonium bicarbonate) in H2O to 0.1 M TEAB in H2O/MeOH (1
2) (0.5 ml). The mixture of catalyst was then injected into the reaction mixture and the reaction mixture was stirred at 80 °C for 1 h. The solvent was evaporated in vacuo. Products were purified by semi-preparative HPLC on C18 column using linear gradient of 0.1 M TEAB (triethylamonium bicarbonate) in H2O to 0.1 M TEAB in H2O/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent. Several co-distillations with water and conversion to sodium salt form (Dowex 50 in Na+ cycle) followed by freeze-drying from water, gave the products as white or yellow powder.
1) as an eluent. Several co-distillations with water and conversion to sodium salt form (Dowex 50 in Na+ cycle) followed by freeze-drying from water, gave the products as white or yellow powder.
5-[(2′′,2′′′-bipyridin-6′′-yl)ethynyl]-2′-deoxycytidine 5′-O-triphosphate (dC6bpyTP)
This compound was prepared according to the general procedure from 5-iodo-2′-deoxycytidine monophosphate dCITP and 1a in the yield 67%.
1H NMR (499.8 MHz, D2O, refdioxane = 3.75 ppm, pD = 7.1, phosphate buffer): 2.22 (dt, 1H, Jgem = 14.1, J2′b,1′ = J2′b,3′ = 6.8, H-2′b); 2.42 (ddd, 1H, Jgem = 14.1, J2′a,1′ = 6.3, J2′a,3′ = 4.1, H-2′a); 4.23 (m, 3H, H-4′,5′); 4.57 (dt, 1H, J3′,2′ = 6.8, 4.1, J3′,4′ = 4.1, H-3′); 6.11 (dd, 1H, J1′,2′ = 6.8, 6.3, H-1′); 7.45 (bdd, 1H, J5′′′,4′′′ = 7.8, J5′′′,6′′′ = 4.2, H-5′′′); 7.57 (bd, 1H, J5′′,4′′ = 7.8, H-5′′); 7.92 (bt, 1H, J4′′,3′′ = J4′′,5′′ = 7.8, H-4′′); 7.95 (m, 2H, H-3′′, H-4′′′); 8.02 (bd, 1H, J3′′′,4′′′ = 7.8, H-3′′′); 8.03 (s, 1H, H-6); 8.54 (bd, 1H, J6′′′,5′′′ = 4.2, H-6′′′); 13C NMR (125.7 MHz, D2O, refdioxane = 69.3 ppm, pD = 7.1, phosphate buffer): 41.93 (CH2-2′); 67.92 (d, JC,P = 5.6, CH2-5′); 72.90 (CH-3′); 83.58 (bpy-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C); 88.16 (d, JC,P = 8.7, CH-4′); 89.11 (CH-1′); 94.06 (C-5); 96.48 (bpy-C
C); 88.16 (d, JC,P = 8.7, CH-4′); 89.11 (CH-1′); 94.06 (C-5); 96.48 (bpy-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C); 124.25 (CH-3′′); 124.88 (CH-3′′′); 127.45 (CH-5′′′); 130.46 (CH-5′′); 141.35, 141.49 (CH-4′′, CH-4′′′); 144.23 (C-6′′); 148.11 (CH-6); 151.53 (CH-6′′′); 156.84 (C-2′′′); 158.03 (C-2′′); 158.21 (C-2); 167.00 (C-4); 31P NMR (202.3 MHz, D2O, refphosphate buffer = 2.35 ppm, pD = 7.1): −21.06 (t, J = 19.7, Pβ); −10.10 (d, J = 19.6, Pα); −6.74 (bd, J = 19.6, Pγ); MS (ES−): found m/z: 644.0 (M-1), 564.1 (M–PO3H2–1); HRMS (ES−): m/z calcd for C21H21O13N5P3: 644.0354; found: 644.0348.
C); 124.25 (CH-3′′); 124.88 (CH-3′′′); 127.45 (CH-5′′′); 130.46 (CH-5′′); 141.35, 141.49 (CH-4′′, CH-4′′′); 144.23 (C-6′′); 148.11 (CH-6); 151.53 (CH-6′′′); 156.84 (C-2′′′); 158.03 (C-2′′); 158.21 (C-2); 167.00 (C-4); 31P NMR (202.3 MHz, D2O, refphosphate buffer = 2.35 ppm, pD = 7.1): −21.06 (t, J = 19.7, Pβ); −10.10 (d, J = 19.6, Pα); −6.74 (bd, J = 19.6, Pγ); MS (ES−): found m/z: 644.0 (M-1), 564.1 (M–PO3H2–1); HRMS (ES−): m/z calcd for C21H21O13N5P3: 644.0354; found: 644.0348.
Primer extension, purification and analysis of the PEX products
Synthetic ONs were purchased from Sigma Aldrich (USA). Primer: 5′-CAT GGG CGG CAT GGG-3′; templates: 5′-CTA GCA TGA GCT CAG TCC CAT GCC GCC CAT G-3′(temprnd16), 5′-CCC GCC CAT GCC GCC CAT G-3′ (tempC), 5′-CCCTCC CAT GCC GCC CAT G-3′ (tempA), TCC CAT GCC GCC CAT G-3′ (tempA1) (segments forming duplex with the primer are in italics, the replicated segments are in bold). Templates used in experiment involving the DBstv magnetoseparation procedure were biotinylated at their 5′ ends. Streptavidine magnetic beads were obtained from Sigma Aldrich (MagSelect, USA) or Novagen (MagPrep, USA), Pwo DNA polymerase from PeqLab (Germany), DyNAzyme II and Phusion DNA polymerases from Finnzymes (Finland), KOD XL DNA polymerase from Novagen, Vent (exo−), Deep Vent, Deep Vent (exo−) and Therminator DNA polymerases as well as T4 polynukleotide kinase and natural nucleoside triphosphate (dATP, dCTP, dGTP and dCTP) from New England Biolabs (Great Britain) and γ-32P-ATP from Izotop, Institute of isotopes Co, Ltd. (Hungary).For magnetoseparation unlabelled primers and biotinylated templates were used.
General procedure for complexation
Acknowledgements
This work is a part of the research projects Z4 055 0506 supported by the Academy of Sciences of the Czech Republic. It was specifically supported by the Czech Science Foundation (203/09/0317), by the Ministry of Education, Youth and Sports (LC512) and by Gilead Sciences, Inc. (Foster City, CA, U. S. A.).Notes and references
- (a) R. R. Breaker, Curr. Opin. Chem. Biol., 1997, 1, 26 CrossRef CAS; (b) M. Famulok, G. Mayer and M. Blind, Acc. Chem. Res., 2000, 33, 591 CrossRef CAS; (c) M. Famulok, Curr. Opin. Mol. Ther., 2005, 7, 137 CAS; (d) K. V. Gothelf and T. H. LaBean, Org. Biomol. Chem., 2005, 3, 4023 RSC; (e) F. A. Aldaye and H. F. Sleiman, Pure Appl. Chem., 2009, 81, 2157 CrossRef CAS.
- (a) G. H. Clever, C. Kaul and T. Carell, Angew. Chem., Int. Ed., 2001, 46, 6226 CrossRef; (b) C. Switzer, S. Sinha, P. H. Kim and B. D. Heuberger, Angew. Chem., Int. Ed., 2005, 44, 1529 CrossRef CAS; (c) G. H. Clever, Y. Söltl, H. Burks, W. Spahl and T. Carell, Chem.–Eur. J., 2006, 12, 8708 CrossRef CAS; (d) K. Tanaka, A. Tengeiji, T. Kato, N. Toyama, M. Shiro and M. Shionoya, J. Am. Chem. Soc., 2002, 124, 12494 CrossRef CAS; (e) N. Zimmermann, E. Meggers and P. G. Schultz, J. Am. Chem. Soc., 2002, 124, 13684 CrossRef CAS; (f) K. Tanaka and M. Shionoya, J. Org. Chem., 1999, 64, 5002 CrossRef CAS; (g) Ch. Switzer and D. Shin, Chem. Commun., 2005, 1342 RSC; (h) H. Weizman and Y. Tor, J. Am. Chem. Soc., 2001, 123, 3375 CrossRef CAS.
- (a) A. A. Gorodetsky and J. K. Barton, Langmuir, 2006, 22, 7917 CrossRef CAS; (b) P. K. Bhattacharya, H. J. Lawson and J. K. Barton, Inorg. Chem., 2003, 42, 8811 CrossRef CAS; (c) C. Stinner, M. D. Wightman, S. O. Kelley, M. G. Hill and J. K. Barton, Inorg. Chem., 2001, 40, 5245 CrossRef CAS; (d) J. L. Kisko and J. K. Barton, Inorg. Chem., 2000, 39, 4942 CrossRef CAS; (e) R. E. Holmlin, J. A. Yao and J. K. Barton, Inorg. Chem., 1999, 38, 174 CrossRef CAS; (f) S. J. Franklin, C. R. Treadway and J. K. Barton, Inorg. Chem., 1998, 37, 5198 CrossRef CAS; (g) A. J. Boersma, R. P. Megens, B. L. Feringa and G. Roelfes, Chem. Soc. Rev., 2010, 39, 2083 RSC; (h) G. Roelfes and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 3230 CrossRef CAS; (i) G. Roelfes, A. J. Boersma and B. L. Feringa, Chem. Commun., 2006, 635 RSC.
- (a) M. Kalek, A. S. Madsen and J. Wengel, J. Am. Chem. Soc., 2007, 129, 9392 CrossRef CAS; (b) M. M. Rodriguez-Ramos and J. J. Wilker, JBIC, J. Biol. Inorg. Chem., 2010, 15, 629 CrossRef CAS; (c) S. Ghosh, I. Pignot-Paintrand, P. Dummy and E. Defrancq, Org. Biomol. Chem., 2009, 7, 2729 RSC; (d) G. Bianké, V Chaurin, M. Egorov, J. Lebreton, E. C. Constable, C. E. Housecroft and R. Häner, Bioconjugate Chem., 2006, 17, 1441 CrossRef; (e) K. Wiederholt and L. W. McLaughlin, Nucleic Acids Res., 1999, 27, 2487 CrossRef CAS.
- M. Vrábel, P. Horáková, H. Pivoňková, L. Kalachova, H. Černocká, H. Cahová, R. Pohl, P. Šebest, L. Havran, M. Hocek and M. Fojta, Chem.–Eur. J., 2009, 15, 1144 CrossRef.
- (a) D. J. Hurley and Y. Tor, J. Am. Chem. Soc., 1998, 120, 2194 CrossRef CAS; (b) D. J. Hurley and Y. Tor, J. Am. Chem. Soc., 2002, 124, 3749 CrossRef CAS; (c) D. J. Hurley and Y. Tor, J. Am. Chem. Soc., 2002, 124, 13231 CrossRef CAS; (d) H. Weizman and Y. Tor, J. Am. Chem. Soc., 2002, 124, 1568 CrossRef CAS; (e) D. J. Hurley, S. E. Seeman, J. C. Mazura and Y. Tor, Org. Lett., 2002, 4, 2305 CrossRef CAS; (f) S. I. Khan, A. E. Beilstein and M. W. Grinstaff, Inorg. Chem., 1999, 38, 418 CrossRef CAS; (g) S. I. Khan, A. E. Beilstein, G. D. Smith, M. Sykora and M. W. Grinstaff, Inorg. Chem., 1999, 38, 2411 CrossRef CAS; (h) J. Telser, K. A. Cruickshank, K. S. Schanze and T. L. Netzel, J. Am. Chem. Soc., 1989, 111, 7221 CrossRef CAS.
- (a) D. Mitra, N. Di Cesare and H. F. Sleiman, Angew. Chem., Int. Ed., 2004, 43, 5804 CrossRef CAS; (b) H. Yang and H. F. Sleiman, Angew. Chem., Int. Ed., 2008, 47, 2443 CrossRef CAS; (c) H. Yang, A. Z. Rys, C. K. McLaughlin and H. F. Sleiman, Angew. Chem., Int. Ed., 2009, 48, 9919 CrossRef CAS; (d) H. Yang, C. K. McLaughlin, F. A. Adlaye, G. D. Hamblin, A. Z. Rys, I. Rouiller and H. F. Sleiman, Nat. Chem., 2009, 1, 390 CrossRef CAS; (e) J. S. Choi, C. W. Kang, K. Jung, J. W. Yang, Y.-G. Kim and H. Han, J. Am. Chem. Soc., 2004, 126, 8606 CrossRef CAS; (f) K. M. Stewart and L. W. McLaughlin, J. Am. Chem. Soc., 2004, 126, 2050 CrossRef CAS; (g) K. W. Gothelf, A. Thomsen, M. Nielsen, E. Clóand and R. S. Brown, J. Am. Chem. Soc., 2004, 126, 1044 CrossRef CAS. Review: (h) K. Tanaka and M. Shionoya, Coord. Chem. Rev., 2007, 251, 2732 CrossRef CAS. Review: (i) H. Yang, K. L. Metera and H. F. Sleiman, Coord. Chem. Rev., 2010, 254, 2403 CrossRef CAS. Review: (j) T. J. Bandy, A. Brewer, J. R. Burns, G. Marth, T. Nguyen and E. Stulz, Chem. Soc. Rev., 2011, 40, 138 RSC.
- L. Kalachova, R. Pohl and M. Hocek, Synthesis, 2009, 105 CAS.
- M. Vrábel, R. Pohl, I. Votruba, M. Sajadi, S. A. Kovalenko, N. P. Ernsting and M. Hocek, Org. Biomol. Chem., 2008, 6, 2852 Search PubMed.
- S. T. Gaballah, C. E. Kerr, B. E. Eaton and T. L. Netzel, Nucleosides, Nucleotides Nucleic Acids, 2002, 21, 547 CAS.
- (a) T. Ruhl and E. Stulz, Supramol. Chem., 2010, 22, 103 CrossRef CAS; (b) J. R. Burns, J. Zekonyte, G. Siligardi, R. Hussain and E. Stulz, Molecules, 2011, 16, 4912 CrossRef CAS.
- T. Ehrenschwender, A. Barth, H. Puchta and H.-A. Wagenknecht, Org. Biomol. Chem. 10.1039/c1ob06421e.
- (a) H. Hashimoto, M. G. Nelson and C. Schwitzer, J. Am. Chem. Soc., 1993, 115, 7128 CrossRef CAS; (b) H. Rosemeyer, N. Ramzaeva, E.-M. Becker, E. Feiling and F. Seela, Bioconjugate Chem., 2002, 13, 1274 CrossRef CAS; (c) J. A. Brazier, T. Shibata, J. Townsley, B. F. Taylor, E. Frary, N. H. Williams and D. M. Williams, Nucleic Acids Res., 2005, 33, 1362 CrossRef CAS.
- Reviews: (a) S. H. Weisbrod and A. Marx, Chem. Commun., 2008, 5675 RSC; (b) M. Hocek and M. Fojta, Org. Biomol. Chem., 2008, 6, 2233 RSC.
- (a) S. Jäger, G. Rasched, H. Kornreich-Leshem, M. Engesser, O. Thum and M. Famulok, J. Am. Chem. Soc., 2005, 127, 15071 CrossRef; (b) C. Lam, C. Hipolito and D. M. Perrin, Eur. J. Org. Chem., 2008, 4915 CrossRef CAS; (c) T. Gourlain, A. Sidorov, N. Mignet, S. J. Thorpe, S. E. Lee, J. A. Grasby and D. M. Williams, Nucleic Acids Res., 2001, 29, 1898 CrossRef CAS; (d) L. H. Thoresen, G.-S. Jiao, W. C. Haaland, M. L. Metzker and K. Burgess, Chem.–Eur. J., 2003, 9, 4603 CrossRef CAS; (e) M. Kuwahara, J. Nagashima, M. Hasegawa, T. Tamura, R. Kitagata, K. Hanawa, S. Hososhima, T. Kasamatsu, H. Ozaki and H. Sawai, Nucleic Acids Res., 2006, 34, 5383 CrossRef CAS; (f) A. Shoji, T. Hasegawa, M. Kuwahara, H. Ozaki and H. Sawai, Bioorg. Med. Chem. Lett., 2007, 17, 776 CrossRef CAS; (g) S. Obeid, M. Yulikow, G. Jeschke and A. Marx, Angew. Chem., Int. Ed., 2008, 47, 6782 CrossRef CAS; (h) B. Holzberger and A. Marx, Bioorg. Med. Chem., 2009, 17, 3653 CrossRef CAS; (i) V. Borsenberger and S. Howorka, Nucleic Acids Res., 2009, 37, 1477 CrossRef CAS; (j) V. Borsenberger, M. Kukwikila and S. Howorka, Org. Biomol. Chem., 2009, 7, 3826 RSC; (k) C. T. Wirges, J. Timper, M. Fischler, A. S. Sologubenko, J. Mayer, U. Simon and T. Carell, Angew. Chem., Int. Ed., 2009, 48, 219 CrossRef CAS; (l) K. Gutsmiedl, D. Fazio and T. Carell, Chem.–Eur. J., 2010, 16, 6877 CrossRef CAS; (m) A. Baccaro and A. Marx, Chem.–Eur. J., 2010, 16, 218 CrossRef CAS; (n) N. Ramsay, A.-S. Jemth, A. Brown, N. Crampton, P. Dear and P. Holliger, J. Am. Chem. Soc., 2010, 132, 5096 CrossRef CAS.
- (a) P. Čapek, H. Cahová, R. Pohl, M. Hocek, C. Gloekner and A. Marx, Chem.–Eur. J., 2007, 13, 6196 CrossRef; (b) P. Brázdilová, M. Vrábel, R. Pohl, H. Pivoňková, L. Havran, M. Hocek and M. Fojta, Chem.–Eur. J., 2007, 13, 9527 CrossRef; (c) H. Cahová, L. Havran, P. Brázdilová, H. Pivoňková, R. Pohl, M. Fojta and M. Hocek, Angew. Chem., Int. Ed., 2008, 47, 2059 CrossRef; (d) H. Cahová, R. Pohl, L. Bednárová, K. Nováková, J. Cvačka and M. Hocek, Org. Biomol. Chem., 2008, 6, 3657 RSC; (e) H. Macíčková-Cahová and M. Hocek, Nucleic Acids Res., 2009, 37, 7612 CrossRef; (f) J. Riedl, P. Horáková, P. Šebest, R. Pohl, L. Havran, M. Fojta and M. Hocek, Eur. J. Org. Chem., 2009, 3519 CrossRef CAS; (g) V. Raindlová, R. Pohl, M. Šanda and M. Hocek, Angew. Chem., Int. Ed., 2010, 49, 1064 CrossRef; (h) S. Ikonen, H. Macíčková-Cahová, R. Pohl, M. Šanda and M. Hocek, Org. Biomol. Chem., 2010, 8, 1194 RSC; (i) H. Macíčková-Cahová, R. Pohl and M. Hocek, ChemBioChem, 2011, 12, 431 CrossRef; (j) P. Kielkowski, R. Pohl and M. Hocek, J. Org. Chem., 2011, 76, 3457 CrossRef CAS; (k) H. Macíčková-Cahová, R. Pohl, P. Horáková, L. Havran, J. Špaček, M. Fojta and M. Hocek, Chem.–Eur. J., 2011, 17, 5833 CrossRef.
- P. S. Braterman, J. I. Song and R. D. Peacock, Inorg. Chem., 1992, 31, 555 CrossRef CAS.
- V. Grosshenny, F. M. Romero and R. Ziessel, J. Org. Chem., 1997, 62, 1491 CrossRef CAS.
- P. Kielkowski, H. Macíčková-Cahová, R. Pohl and M. Hocek, Angew. Chem., Int. Ed., 2011, 50, 8727 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental part and spectral data, additional PAGEs, MALDI and additional UV/vis spectra. See DOI: 10.1039/c1ob06359f |
| This journal is © The Royal Society of Chemistry 2012 |
