Electrospun eggroll-like CaSnO3 nanotubes with high lithium storage performance†
Linlin
Li
ab,
Shengjie
Peng
a,
Yan Ling
Cheah
a,
Jin
Wang
a,
Peifen
Teh
a,
Yahwen
Ko
a,
Chuiling
Wong
a and
Madhavi
Srinivasan
*ab
aSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore. E-mail: madhavi@ntu.edu.sg; Fax: +65 6790 9081; Tel: +65 6790 4606
bTUM-CREATE Center for Electromobility, Nanyang Technological University, 62 Nanyang Drive, 637459, Singapore
First published on 30th October 2012
Abstract
Novel eggroll-like CaSnO3 nanotubes have been prepared by a single spinneret electrospinning method followed by calcination in air for the first time. The electrospun sample as a lithium-ion battery electrode material exhibited improved cycling stability and rate capability by virtue of the high surface area and unique hollow interior structure, compared to nanorod-structured CaSnO3.
One-dimensional (1D) nanostructured materials have attracted much attention due to their unique properties and potential applications in chemical sensors, catalysts, photonic devices, and energy storage devices.1,2 Among several techniques for creating 1D nanostructures, electrospinning is a simple and versatile way to produce well-defined 1D nanostructures such as nanotubes, nanowires, nanofibers, and nanobelts at micro- or nano-scales on a large scale.3,4 1D nanostructured materials with various morphologies, including dense, porous, hollow, and core–shell structures have been successfully obtained by controlling processing parameters and their unique functional features can be correlated with various applications.5
Recently, 1D metal oxides have been investigated as alternative anode materials for lithium-ion batteries (LIBs) owing to their large specific surface areas and facilitating Li+ diffusion in the active materials.6–9 As one of the most important metal oxides, Sn-based binary or ternary oxides enjoy a place of pride because of its high theoretical capacity (782 mA h g−1) and low operating potential versus Li.10–14 However, the large irreversible capacity and a lack of stability during cycling have been the major drawbacks for the Sn-based oxides. To alleviate these problems, various hollow interior nanostructures with large specific surface areas and short lithium diffusion pathways15–22 have been designed to improve the reversible capacity and cyclability of Sn-based oxides, where the void space could effectively accommodate the stress relaxation induced by large volume change and provide a short lithium ion diffusion pathway. Moreover, composite structures composed of an electrochemically inactive/active matrix such as MOx (M = Zn, Sr, Mn, Co, Ca etc.)23–29 and carbon30,31 have been proposed, where the uniformly distributed matrix could not only buffer the volume change during cycling but also prevent the aggregation of active materials, thus leading to improved cycling stability. Furthermore, the lithium storage properties of Sn-based oxides are also significantly influenced by the morphologies, sizes, and structures. By keeping this in mind, CaSnO3 with suitable morphologies and structures should be desirable as anode materials for LIBs. So far, although CaSnO3 with different structures such as core–shell,28 flower-like33 and nanoparticles27 has been prepared and studied as an anode material, the cycling stability still could not meet the requirement for practical use in LIBs. Our previous work reported the good electrochemical performance of electrospun CaSnO3 one-dimensional structures.34 As the electrochemical performances of materials have a strong correlation with their shapes, sizes and structures, novel CaSnO3 nanostructures with improved electrochemical performance based on the simple electrospinning method should be important and need further research.
Herein, we firstly prepare CaSnO3 nanotubes (denoted as CSO-NT) with eggroll-like morphology by a simple electrospinning method followed by calcination in air (Experimental section, ESI†). By rationally tailoring their morphology and structure, CaSnO3 nanotubes manifest remarkably improved lithium storage properties and better cycling performance compared to the nanorod-structured CaSnO3 (denoted as CSO-NR). This is attributed to the large specific surface areas for storing more lithium ions and the unique internal hollow space for buffering the volume changes.
The as-spun nanofibers obtained by using different weight ratios of metallic precursor and polyacrylonitrile (PAN) polymer exhibit uniform nanofibers with smooth surfaces. (Fig. S1, ESI†). To decide the decomposition temperature of the as-spun fibers, thermogravimetric analysis (TGA) analysis was further performed, as shown in Fig. S2 (see ESI†). A large weight loss from 300 °C to 400 °C takes place and remains ∼53 wt% after 600 °C, indicating the thorough removal of PAN and decomposition of the metallic precursor. Hence, a sintering temperature of 600 °C was used for the as-spun nanofibers, expecting the formation of CaSnO3 at this temperature. Meanwhile, FTIR spectra reveal that all the peaks belonging to the PAN disappeared and only Sn–O–Sn vibrations could be observed after sintering at 600 °C for 24 h (Fig. S3, ESI†),11,12 confirming the removal of PAN and the crystallization of CaSnO3.
Fig. 1a displays the XRD pattern of CSO-NT. The well-defined peaks correspond to orthorhombic CaSnO3. The lattice parameters of CaSnO3 evaluated by Rietveld refinement are a = 5.523 Å, b = 5.669 Å, and c = 7.894 Å, which match well with JCPDS data (card no. 77-1797, a = 5.532 Å, b = 5.681 Å, c = 7.906 Å).27 Furthermore, XPS analysis confirms that Ca and Sn possess +2 and +4 oxidation states, respectively, and the atom ratio of Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O is about 19.36%
O is about 19.36%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.04%
20.04%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60.60% (Fig. S4, ESI†). All of these results further demonstrate that CaSnO3 with high phase purity and crystallinity could be easily obtained via sintering in air at 600 °C for 24 h. Fig. 1b shows that the CaSnO3 presents an orthorhombic perovskite structure (space group Pbnm),27,35 which is distorted from cubic symmetry by an octahedral tilting distortion. It is basically constructed from a complex network of distorted SnO6 octahedral, with the Ca2+ ions occupying the octahedral cavities. The distorted SnO6 octahedrons share their corners to build a three dimensional framework of tunnels for Li diffusion.
60.60% (Fig. S4, ESI†). All of these results further demonstrate that CaSnO3 with high phase purity and crystallinity could be easily obtained via sintering in air at 600 °C for 24 h. Fig. 1b shows that the CaSnO3 presents an orthorhombic perovskite structure (space group Pbnm),27,35 which is distorted from cubic symmetry by an octahedral tilting distortion. It is basically constructed from a complex network of distorted SnO6 octahedral, with the Ca2+ ions occupying the octahedral cavities. The distorted SnO6 octahedrons share their corners to build a three dimensional framework of tunnels for Li diffusion.
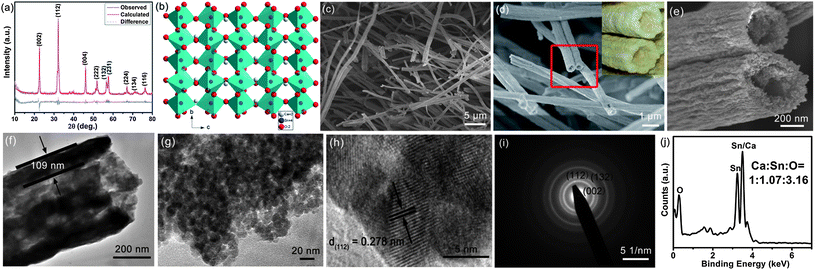 | ||
| Fig. 1 (a) Rietveld refined XRD pattern, (b) crystal structure of CSO-NT. (c and d) FESEM images, (e) corresponding high-magnification FESEM image of CSO-NT, taken from the square area in (d). The inset in d is a photograph of egg-roll. (f and g) TEM images, (h) HRTEM image, and corresponding (i) SAED pattern, (j) EDX image of CSO-NT. | ||
The morphology of CSO-NT is further characterized by FESEM, as shown in Fig. 1c. After sintering at 600 °C for 24 h, CSO-NT perfectly maintains the fibrous morphology. The corresponding magnified FESEM images (Fig. 1d and e) clearly show that CSO-NT contains uniform interior hollow structures with rough surfaces. The average diameter of CSO-NT is ∼560 nm and the length is longer than ∼20 μm (Fig. 1d). Interestingly, CSO-NT looks quite like egg-rolls and possesses not only similar interior space but also the channel-like surface structure (Fig. 1e and the inset in Fig. 1d). To elucidate the unique structural feature, TEM was performed as shown in Fig. 1f–i. Hollow tube-like morphology with a wall thickness of 109 nm is clearly revealed in Fig. 1f, which is in agreement with the FESEM observation. Moreover, the HRTEM images confirm that CSO-NT consists of nanoparticles of ∼12 nm in size (Fig. 1g). The lattice fringe is found to be ∼0.278 nm, corresponding to the d112-spacing in the XRD pattern of CSO-NT (Fig. 1h). SAED pattern demonstrates that CSO-NT is polycrystalline (Fig. 1i). The rings can be well indexed to (002), (112) and (132) planes of CaSnO3. In addition, EDX spectra indicates a Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 1
O ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.07
1.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.16, corroborating the XRD study (Fig. 1j). Therefore, CSO-NT with the egg-roll like morphology was successfully prepared by a simple electrospinning technique.
3.16, corroborating the XRD study (Fig. 1j). Therefore, CSO-NT with the egg-roll like morphology was successfully prepared by a simple electrospinning technique.
It is known that the diameters, size distributions, and morphologies of 1D CaSnO3 nanostructures are critically influenced by many factors, such as metal precursor (denoted as M) concentration, viscosity of precursor solution, electrospinning parameters, and so on.2,3,5 In this work, it is found that M concentration plays a key role in the formation of 1D CaSnO3 nanostructures with different morphologies. In order to elucidate the effect of M concentration on the final products, CSO-NT and CSO-NR were prepared by rationally tuning the weight ratios of M over PAN from 1.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (CSO-NT) to 0.65
1 (CSO-NT) to 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (CSO-NR).
1 (CSO-NR).
As shown in Fig. 2a and b, CSO-NR with an average diameter of 380 nm and lengths up to several micrometers can be obtained. The TEM image of CSO-NR in Fig. 2c shows a dense nanorod structure and the inner is solid due to no obvious difference between the edge and the central part. Furthermore, XRD and EDX patterns of CSO-NR reveal that there is no detectable impurity phase (Fig. 2d and its inset). It demonstrates that the variation of M concentration has no effect on the formation of CaSnO3 single phase. Meanwhile, the lattice spacing of 0.278 nm corresponds to the (112) plane, revealing their orthorhombic structure (inset in Fig. 2c).
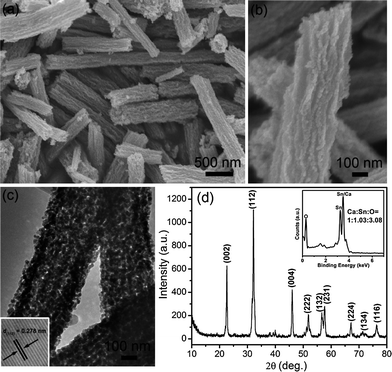 | ||
| Fig. 2 (a and b) FESEM images, (c) TEM image, and (d) XRD pattern of CSO-NR. Insets in (c) and (d) are the corresponding HRTEM image and EDX images of CSO-NR, respectively. | ||
It is worth noting that CSO-NR also possesses a channel-like morphology similar to the CSO-NT. Thus, the surface areas of CSO-NT and CSO-NR are further characterized by BET analysis to investigate their detailed structures, as shown in Fig. 3. Both samples exhibit similar nitrogen adsorption–desorption isotherms, indicating the characteristic of type IV isotherms with a type H3 hysteresis loop, typical for mesoporous structures. The pore size distributions from both samples, calculated using the Barrett–Joyner–Halenda (BJH) method from the desorption branch of the isotherms, revealing a relatively wider size distribution (inset in Fig. 3). These results indicate that CSO-NT possesses a higher BET specific surface area (37.8 m2 g−1) than CSO-NR (14.2 m2 g−1) by virtue of the interior hollow space and unique structural features.
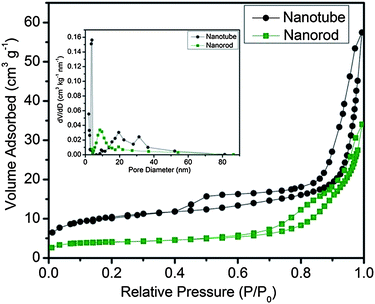 | ||
| Fig. 3 N2 adsorption–desorption isotherms of CSO-NT and CSO-NR. Inset is corresponding pore-size distribution. | ||
The electrochemical properties of both samples as anode materials for LIBs are further investigated. Fig. 4a depicts the comparative cycling performances of both samples and the Coulombic efficiency of CSO-NT with a voltage window of 0.005–3 V at a current rate of 60 mA g−1. Clearly, a reversible capacity of 648 mA h g−1 can be delivered by CSO-NT up to 50 cycles, which is about 1.6 times of that for CSO-NR (410 mA h g−1). Even though the Coulombic efficiency of CSO-NT for the first cycle is relatively low, it quickly increases in the subsequent cycles and retains about 98.1% after 50 cycles. Additionally, the rate capability of CSO-NT is also much better than that of CSO-NR, as shown in Fig. 4b. At a high current density of 2 A g−1, CSO-NT manifests a capacity of 416 mA h g−1, whereas a capacity of only 271 mA h g−1 is delivered by CSO-NR, indicating a magnificent high-rate performance. When the current density is reduced back to be 500 mA g−1, CSO-NT still can deliver a reversible capacity of 513 mA h g−1, implying a stable electrode structure even under the high current rate. Moreover, the significantly reduced charge transfer resistance (40 Ω) for CSO-NT towards CSO-NR (87 Ω) partially accounts for the excellent cycling performance of CSO-NT (Fig. S5, ESI†).
| CaSnO3 + 4Li+ + 4e− → CaO + 2Li2O + Sn | (1) |
| 4.4Li+ + 4.4e− + Sn ↔ Li4.4Sn | (2) |
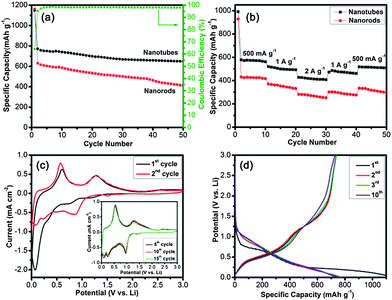 | ||
| Fig. 4 Cycling performance of CSO-NT and CSO-NR (a) at a current density of 60 mA g−1 (b) at different current densities. (c) Cyclic voltammetry (CV) of CSO-NT at a scan rate of 0.1 mV s−1 in the voltage window of 0.005–3.0 V for the first two cycles. The inset is the 5th, 10th, 15th cycles at a scan rate of 0.1 mV s−1. (d) The discharge–charge profiles of CSO-NT. | ||
In order to fully understand the influence of the nanotube and channel structure on the electrochemical properties of the as-prepared samples, CSO-NT is further characterized with cyclic voltammetry (CV), as shown in Fig. 4c. During the first cycle, a broad cathodic peak at ∼1.21 V could be observed and disappears in the following cycling, which can be attributed to the destruction of the structure (amorphization of crystal lattice), accompanied by the formation of electrochemical inactive CaO matrix and active Sn-metal (eqn (1)). The broad cathodic peak centered at ∼0.76 V presumably corresponds to the formation of the solid electrolyte interphase (SEI) film and, simultaneously, to the start of the formation of Li–Sn alloys.31,32 The characteristic pair of peaks centered at cathodic and anodic potentials of ∼0.07 and ∼0.6 V can be ascribed to the alloying and dealloying reaction between Li and Sn (eqn (2)), respectively. It should be noted that the anodic peak of ∼1.28 V is also observed, indicating the corresponding oxidation of Sn metal to Sn oxides. Interestingly, in the second cycle, the cathodic peak of ∼0.07 V splits into a doublet, appearing at ∼0.07 and ∼0.22 V, which implies that the Li–Sn alloying reaction occurs in two stages in further cycling.27 Meanwhile, it can be noticed that a cathodic peak appearing at 0.65–1.10 V increases in intensity and another clear cathodic peak at 1.35–1.55 V appears, further demonstrating that there are partly reversible reactions existing in Sn oxides and Sn metal.8,16,18 Additionally, the intensity of the anodic peak at ∼0.6 V increases significantly in the second cycle, indicating the existence of a possible activation process in the electrode materials. Nevertheless, the cathodic and anodic peaks overlap very well during the 5th, 10th, and 15th cycles, suggesting good reversibility of the electrochemical reactions (inset in Fig. 4c).
Fig. 4d reveals the discharge–charge voltage profiles of CSO-NT at a current density of 60 mA g−1. The voltage profiles are consistent with the previous report.27 The initial discharge–charge capacity of CSO-NT is 1149/738 mA h g−1, leading to a large initial irreversible loss of about 38%. Such irreversible loss is inevitable because of the large irreversible reduction of Sn oxides to Sn metal and the formation of the SEI film.32 In the second cycle, the discharge and charge capacities are found to be 772 and 737 mA h g−1, respectively, corresponding to a much higher Coulombic efficiency of 95.4%. Thus, it is obvious that CSO-NT shows much better cycling stability and lithium storage properties compared to CSO-NR. More significantly, nanofibrous morphology of CSO-NT electrode can be clearly observed even after 50 cycles (Fig. S6, ESI†). This also demonstrates the good electrochemical performance of CSO-NT. The enhanced electrochemical performance can be attributed to the unique interior hollow structures and channel-like morphology of CSO-NT. Specifically, the central void space of CSO-NT can not only provide sufficient space to buffer the volume change but also facilitate fast lithium ion diffusion during lithiation/delithiation. Moreover, CSO-NT with a wall thickness of 109 nm and unique channel-like morphology also benefit in retaining the structural stability and partially accommodating the pulverization of electrode. Furthermore, the relatively large surface area of CSO-NT provides a large amount of reactive sites and thus increases the interfaces between the electrolyte and active materials.
In summary, CSO-NT with the eggroll-like morphology has been prepared via a facile electrospinning method. The unique morphology and structural feature of CaSnO3 are significantly influenced by the reactant concentrations. When evaluated as anode materials for LIBs, CSO-NT exhibits greatly enhanced lithium storage properties compared to CSO-NR. This work is believed to benefit in not only preparing binary or ternary metal oxides nanotubes by the simple electrospinning method, but also finding attractive applications in electrochemical energy storage devices.
Acknowledgements
The authors are thankful for the support by funding from the National Research Foundation, Clean Energy Research Project (Grant number: NRF2009EWT-CERP001-036) and TUM CREATE center for Electromobility.References
- Y. Qin, X. D. Wang and Z. L. Wang, Nature, 2008, 451, 809–813 CrossRef CAS.
- D. Li, Y. L. Wang and Y. N. Xia, Adv. Mater., 2004, 16, 361–366 CrossRef CAS.
- A. Greiner and J. H. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670–5703 CrossRef CAS.
- Z. Y. Liu, D. D. Sun, P. Guo and J. O. Leckie, Nano Lett., 2006, 7, 1081–1085 CrossRef.
- X. F. Lu, C. Wang and Y. Wei, Small, 2009, 5, 2349–2370 CrossRef CAS.
- J. K. Feng, M. O. Lai and L. Lu, Electrochem. Commun., 2011, 13, 287–289 CrossRef CAS.
- W. W. Zhou, Y. Y. Tay, X. T. Jia, D. Y. Yau Wai, J. Jiang, H. H. Hoon and T. Yu, Nanoscale, 2012, 4, 4459–4463 RSC.
- S. J. Ding, J. S. Chen and X. W. Lou, Adv. Funct. Mater., 2011, 21, 4120–4125 CrossRef CAS.
- F. Y. Cheng, H. B. Wang, Z. Q. Zhu, Y. Wang, T. R. Zhang, Z. L. Tao and J. Chen, Energy Environ. Sci., 2011, 4, 3668–3675 CAS.
- Z. P. Guo, G. D. Du, Y. Nuli, M. F. Hassan and H. K. Liu, J. Mater. Chem., 2009, 19, 3253–3257 RSC.
- L. F. Cui, J. A. Shen, F. Y. Cheng, Z. L. Tao and J. Chen, J. Power Sources, 2011, 196, 2195–2201 CrossRef CAS.
- H. M. Xiong, W. Z. Shen, B. K. Guo, S. H. Bo, W. J. Cui, L. Q. Chen, H. Li and Y. Y. Xia, J. Mater. Chem., 2011, 21, 2845–2847 RSC.
- W. J. Cui, F. Wang, J. Wang, C. X. Wang and Y. Y. Xia, Electrochim. Acta, 2011, 56, 4812–4818 CrossRef CAS.
- C. A. Bonino, L. Ji, Z. Lin, O. Toprakci, X. Zhang and S. A. Khan, ACS Appl. Mater. Interfaces, 2011, 3, 2534–2542 CAS.
- W. M. Zhang, J. S. Hu, Y. G. Guo, S. F. Zheng, L. S. Zhong, W. G. Song and L. J. Wan, Adv. Mater., 2008, 20, 1160–1165 CrossRef CAS.
- X. M. Yin, C. C. Li, M. Zhang, Q. Y. Hao, S. Liu, L. B. Chen and T. H. Wang, J. Phys. Chem. C, 2010, 114, 8084–8088 CAS.
- C. F. Zhang, X. Peng, Z. P. Guo, C. B. Cai, Z. X. Chen, D. Wexler, S. Li and H. K. Liu, Carbon, 2012, 50, 1897–1903 CrossRef CAS.
- S. J. Ding and X. W. Lou, Nanoscale, 2011, 3, 3586–3588 RSC.
- R. Q. Liu, N. Li, D. Y. Li, G. F. Xia, Y. M. Zhu, S. Y. Yu and C. Wang, Mater. Lett., 2012, 73, 1–3 CrossRef CAS.
- H. D. Liu, J. M. Huang, X. L. Li, J. Liu and Y. X. Zhang, Ceram. Int., 2012, 38, 5145–5149 CrossRef CAS.
- C. Li, W. Wei, S. M. Fang, H. X. Wang, Y. Zhang, Y. H. Gui and R. F. Chen, J. Power Sources, 2010, 195, 2939–2944 CrossRef CAS.
- C. Wang, G. H. Du, K. Ståhl, H. X. Huang, Y. J. Zhong and J. Z. Jiang, J. Phys. Chem. C, 2012, 116, 4000–4011 CAS.
- A. Rong, X. P. Gao, G. R. Li, T. Y. Yan, H. Y. Zhu, J. Q. Qu and D. Y. Song, J. Phys. Chem. B, 2006, 110, 14754–14760 CrossRef CAS.
- C. Li, Y. Q. Zhu, S. M. Fang, H. X. Wang, Y. H. Gui, L. Bi and R. F. Chen, J. Phys. Chem. Solids, 2011, 72, 869–874 CrossRef CAS.
- S. J. Lei, K. B. Tang, C. H. Chen, Y. Jin and L. Zhou, Mater. Res. Bull., 2009, 44, 393–397 CrossRef CAS.
- G. Wang, X. P. Gao and P. W. Shen, J. Power Sources, 2009, 192, 719–723 CrossRef CAS.
- Y. Sharma, N. Sharma, G. V. S. Rao and B. V. R. Chowdari, Chem. Mater., 2008, 20, 6829–6839 CrossRef CAS.
- X. Y. Hu, T. Xiao, W. Huang, W. Tao, B. J. Heng, X. Q. Chen and Y. W. Tang, Appl. Surf. Sci., 2012, 258, 6177–6183 CrossRef CAS.
- N. Feng, S. L. Peng, X. L. Sun, L. Qiao, X. W. Li, P. Wang, D. K. Hu and D. Y. He, Mater. Lett., 2012, 76, 66–68 CrossRef CAS.
- H. D. Liu, J. M. Huang, X. L. Li, J. Liu, Y. X. Zhang and K. Du, Appl. Surf. Sci., 2012, 258, 4917–4921 CrossRef CAS.
- L. Y. Jiang, X. L. Wu, Y. G. Guo and L. J. Wan, J. Phys. Chem. C, 2009, 113, 14213–14219 CAS.
- J. Y. Lee, R. F. Zhang and Z. L. Liu, J. Power Sources, 2000, 90, 70–75 CrossRef CAS.
- S. Zhao, Y. Bai and W. F. Zhang, Electrochim. Acta, 2010, 55, 3891–3896 CrossRef CAS.
- L. L. Li, S. J. Peng, J. Wang, Y. L. Cheah, P. F. Teh, Y. W. Ko, C. L. Wong and M. Srinivasan, ACS Appl. Mater. Interfaces, 2012 DOI:10.1021/am301664e.
- H. Mizoguchi, H. W. Eng and P. M. Woodward, Inorg. Chem., 2004, 43, 1667–1680 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, SEM images of as-spun nanofibers, TGA curve, FTIR spectra, XPS spectra, and respective Nyquist plots of CSO-NT and CSO-NR. See DOI: 10.1039/c2nr32766j |
| This journal is © The Royal Society of Chemistry 2013 |
