The growth and enhanced catalytic performance of Au@Pd core–shell nanodendrites†
Haihua
Wang
,
Zhenhua
Sun
*,
Yi
Yang
and
Dangsheng
Su
*
Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Science, Shenyang 110016, China. E-mail: zhsun@imr.ac.cn; dangsheng@fhi-berlin.mpg.de; Fax: +86 24 83970019; Tel: +86 24 83978238
First published on 23rd October 2012
Abstract
Au@Pd core–shell nanodendrites were synthesized by reducing H2PdCl4 with ascorbic acid onto the surface of Au polyhedra at room temperature. The Au@Pd core–shell nanodendrites consisting of a Au core and nanoporous Pd shell, exhibited plasmonic properties and higher catalytic activity in comparison with Au@Pd core–shell nanocubes.
Bimetallic core–shell nanostructures have received increasing interest due to their different physicochemical properties, such as optical, magnetic, electronic and catalytic properties relative to their monometallic counterparts.1–6 Owing to the synergistic effects between a metal core and the shell of bimetallic core–shell nanostructures, there is large enhancement in activity,7–10 selectivity11 and stablity,12,13 compared with pure metal components. So far, bimetallic core–shell nanostructures with smooth surfaces have been successfully synthesized by different methods.14–21 For example, Yang's group has synthesized Pt@Pd core–shell nanocubes through epitaxial seeded growth.14 Han's group has obtained Au@Pd core–shell nanooctahedrons by a one-step synthesis route.21 However, the surfaces of these core–shell nanocrystals are smooth non-porous structures, hindering their applications in several fields, especially catalysis. On the other hand, the metal shell with a smooth surface will conceal the functionality of the inner metal core. Hence, it is very important to cultivate the growth of the core–shell nanostructures with a rough surface and porous structure for giving rise to superior properties of nanomaterials.22–26
Recently, Xia's group successfully synthesized Pd@Pt bimetallic nanodendrites with high catalytic activity for the oxygen reduction reaction (ORR).22 Wang's group synthesized Pd@Pt nanodendritic nanowires with enhanced electrocatalytic activity.23 In these core–shell nanostructures, the catalytic performance of the metal shells has been improved due to their porous structure. But the advantages of inner metal cores have not yet been revealed. In this work, we have successfully synthesized Au@Pd core–shell nanodendrites with bifunctional plasmonic and catalytic properties. Specifically, Au@Pd core–shell nanodendrites exhibit high catalytic performance for electrocatalysis towards the methanol oxidation reaction and Suzuki coupling reaction between phenylboronic acid and iodobenzene. Meanwhile, the surface plasmon resonance (SPR) peak of the Au core is still observed in the extinction spectra of the Au@Pd core–shell nanodendrites because of the non-compact porous Pd shells. We believe that our work will help to develop bifunctional nanomaterials, and Au@Pd core–shell nanodendrites have potential applications in plasmon-enhanced catalysis.
The Au@Pd core–shell nanodendrites were synthesized by reducing H2PdCl4 with ascorbic acid onto the surface of Au polyhedra (Fig. S1†), employing cetyltrimethylammonium chloride (CTAC) as the stabilizing agent. Experimental details can be seen in the ESI.†Fig. 1a shows the transmission electron microscopy (TEM) image of the as-grown Au@Pd core–shell nanodendrites, which indicates that the nanodendrites are composed of a Au core and Pd shell. The Pd shell is constituted by numerous tiny dendrite-like Pd nanocrystals. The image obtained by high-angle annular dark-field scanning transmission-electron microscopy (HAADF-STEM) (Fig. 1b) further clearly reveals the dendritic nature of the Au@Pd core–shell nanostructures. The cores with bright contrast are Au polyhedra, while the shells with dark contrast are Pd shells. The Pd nanoarms are highly branched, resulting in the formation of nanoporous shell regions. The HAADF-STEM imaging together with energy dispersive X-ray (EDX) elemental mapping and the line profile of a single Au@Pd core–shell nanodendrite (Fig. 1c–f) further reveals that the core and shell are composed of Au and Pd, respectively. Interestingly, the thickness of the Pd nanobranches on the Au core could be easily controlled via changing the amount of Pd precursor (Fig. 2a–f). And the UV-visible absorption intensity of the Pd shell at ∼260 nm gradually increases with increasing molar quantity of added H2PdCl4 (Fig. S2†). Simultaneously, the peaks of the surface plasmon band of Au@Pd nanodendrites exhibit a red shift and get wider with the increasing thickness of the Pd shell (Fig. 2g), owing to an increase in the refractive index of the medium surrounding the Au nanopolyhedra.27–29 Since the shell of Pd can strongly damp out the dipolar plasmon oscillations of Au cores, a thin shell thickness can almost lead to damping the SPR peaks of the core for Au@Pd core–shell nanostructures with a smooth surface.15,21 However, for Au@Pd core–shell nanodendrites, the SPR peak of Au is still observed because of the non-compact porous Pd nanobranches. The interesting characteristics will be important for the use of plasmonic materials in catalytic systems. Thus, the plasmonic and catalytic properties of the Au@Pd core–shell nanodendrites can be easily tuned via changing the synthetic parameters.
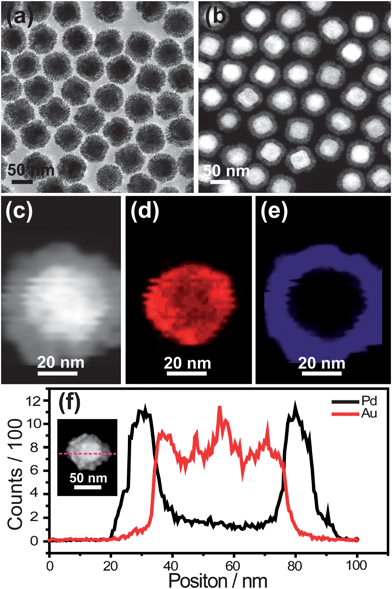 | ||
| Fig. 1 Au@Pd core–shell nanodendrites. (a) TEM image. (b and c) HAADF-STEM images. (d and e) EDX elemental maps of Au and Pd, respectively. (f) Line profiles of Au and Pd recorded along the dashed line shown in the STEM image of the Au@Pd nanostructures, which is given in the inset. | ||
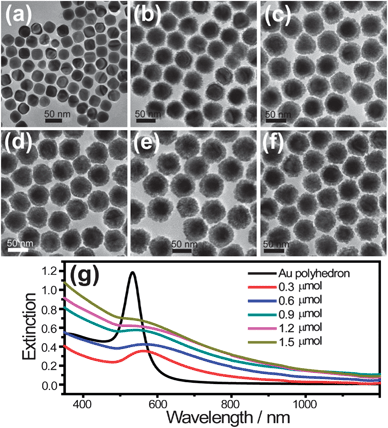 | ||
| Fig. 2 (a) TEM images of original Au polyhedra. (b–f) TEM images of synthesized Au@Pd core–shell nanodendrites at different amounts of H2PdCl4 (molar quantities 0.3, 0.6, 0.9, 1.2 and 1.5 μmol, respectively). (g) The corresponding extinction spectra of the Au nanopolyhedra and Au@Pd core–shell nanodendrites. | ||
The crystalline structure of Au@Pd core–shell nanodendrites were further characterized by high-resolution TEM (HRTEM) and X-ray diffraction (XRD) measurements. The porous structure of the Au@Pd core–shell nanodendrites is clearly observed and is shown in the HRTEM image in Fig. S3a.† The nanodendritic Pd arms with an average diameter of 3–7 nm covered the surface of the Au cores. The rich edges and corner atoms derived from the dendritic Pd nanoporous structures were highly valuable for the enhancement of the Pd catalytic performance. Fig. S3b† shows the Pd lattice planes of (111) and (200) with spacings of 0.22 and 0.20 nm, respectively. These observations are consistent with the XRD patterns (Fig. S3c†), which can be indexed as the face-centered-cubic form of Au (JCPDS 04-0784) and Pd (JCPDS 46-1043), respectively.
It is well-known that surfactant molecules play an important role in the growth process of Au@Pd core–shell nanostructures.30,31 Wang's group have synthesized Au@Pd core–shell nanocubes using cetyltrimethylammonium bromide (CTAB) as a stabilizing agent.15 Herein, we have synthesized Au@Pd core–shell nanodendrites by using CTAC as surfactant molecule. In order to verify the role of the CTAC in the synthesis, a series of control experiments were conducted using different CTAB-to-CTAC molar ratios as the stabilizing agents (Fig. 3). When pure CTAB was utilized instead of CTAC, Au@Pd core–shell nanocubes (Fig. 3a) were obtained, which accorded with the literature.15 No porous structures can be observed in the Pd shell. When mixtures of CTAC and CTAB were used, Au@Pd core–shell nanodendrites could also be obtained (Fig. 3b–c). However, the roughness of the Pd shell increased and the size of the nanodendritic Pd arms became smaller with decreasing CTAB-to-CTAC molar ratios. The above controlled experiments clearly demonstrate that CTAC plays a very important role in the growth of Au@Pd core–shell nanodendrites. This finding is consistent with a previous study,32 where the growth of Pd porous nanocrystals was ascribed to the weaker binding strength of Cl− ions to the Pd surface in comparison with Br− ions. We believe that such a mechanism can be also employed in the growth of Au@Pd core–shell nanodendrites.
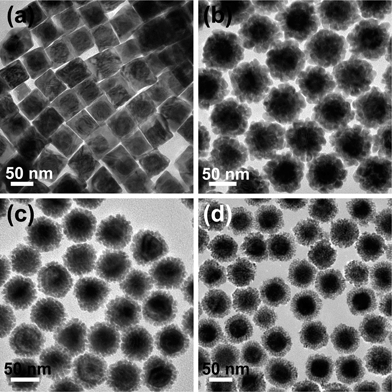 | ||
Fig. 3 TEM images of the Au@Pd core–shell nanostructures obtained from growth at different CTAB-to-CTAC molar ratios. (a) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, (b) 4 0, (b) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 2 1, (c) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and (d) 0 3, and (d) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
Electrochemical properties of the Au@Pd core–shell nanostructures were measured to examine their potential applications in electrocatalysis. Au polyhedra showed no electrocatalytic activity according to the cyclic voltammograms (CVs) without oxidation and reduction peaks (Fig. S4†). Therefore, the electrochemical reaction mainly occurred in the Pd shells. Fig. 4a shows the CVs for Au@Pd core–shell nanostructures, in N2-purged H2SO4 (0.5 M) at a sweep rate of 50 mV s−1. According to the area of the reductive peak, labeled by the dashed box shown in Fig. 4a, the electrochemically active surface area (ECSA) of the Au@Pd core–shell nanostructures is calculated to be 1.4 and 0.06 m2 gPd−1 for the Au@Pd core–shell nanodendrites and nanocubes, respectively. The detailed calculated process of the ECSA can be seen in the ESI.† The ECSA of the Au@Pd core–shell nanodendrites is over 23 times that of Au@Pd nanocubes. Fig. 4b gives the electrocatalytic activity of the two Au@Pd core–shell nanostructures for methanol oxidation. The mass current of the Au@Pd core–shell nanodendrites is about 14 times that of the Au@Pd core–shell nanocubes. The study has proved that onset potential is an important parameter to evaluate the catalytic activity at low potential for electrocatalysts in the methanol oxidation reaction.33,34 For nanodendrites, the onset potential is at about −0.7 V. However, for nanocubes, the onset potential value is about −0.5 V. Au@Pd nanodendrites have a more negative onset potential value compared with nanocubes. These results indicate that the Au@Pd core–shell nanodendrites show much better electrocatalytic activity than nanocubes. Some works have pointed out that the enhanced electrocatalytic activities of dendritic nanostructures should be ascribed to the large surface area and high concentration of coordinatively unsaturated surface Pd atoms.35,36 In addition, the enhancement of electrocatalytic activity was also attributed to the interaction between the Pd shell and Au core because the interaction can prevent the accumulation of poisoning-intermediates and more Pd sites were available for the reactant molecules.37 In our Au@Pd core–shell nanodendrites, Au could act as an electronic promoter for Pd in the same way as it has enhanced the Pt activity in the selective oxidation of primary alcohols.38 Therefore, the enhanced electrocatalytic activity of Au@Pd core–shell nanodendrites can be attributed to the joint effect of the large surface area caused by the porous Pd shell and the interaction between the Au core and Pd shell.
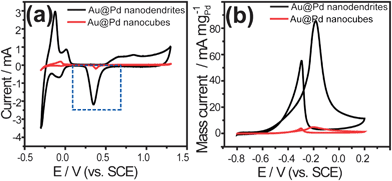 | ||
| Fig. 4 CVs for Au@Pd core–shell nanostructures. (a) CVs recorded in N2-purged H2SO4 (0.5 M). (b) Mass activity of Au@Pd nanostructures for methanol oxidation in N2-purged mixture composed of KOH (0.1 M) and methanol (0.25 M). Scan rate: 50 mV s−1. | ||
The Suzuki coupling reaction, which utilizes Pd as the catalyst, plays a very crucial role in the chemical industry.39 Therefore, the catalytic activity of the Au@Pd core–shell nanostructures for catalyzing the Suzuki coupling reaction between iodobenzene and phenylboronic acid was studied. Fig. 5a shows the reaction equation of the Suzuki coupling reaction between phenylboronic acid and iodobenzene. Yields of biphenyl using different nanostructures as the catalyst and the cycle performance of Au@Pd core–shell nanodendrites are illustrated in Fig. 5b. The yield of biphenyl is 1.3% without any catalysts in the reaction systems. And the yield is not improved when Au polyhedra are used as the catalyst. The yield is increased to 12.9%, when Au@Pd core–shell nanocubes are employed as the catalyst. Interestingly, the yield of biphenyl is significantly increased to 99.9% when an equal amount of Au@Pd nanodendrites as nanocubes is used as the catalyst. In addition, the cycle performance test of the Au@Pd core–shell nanodendrites was carried out. After four cycles, the yield of biphenyl was still above 90% (Fig. 5b) and the dendritic structures of catalyst were also retained (Fig. S5†). The above catalytic performance test indicates that Au@Pd nanodendrites have good catalytic performance and have potential applications in industry catalysis.
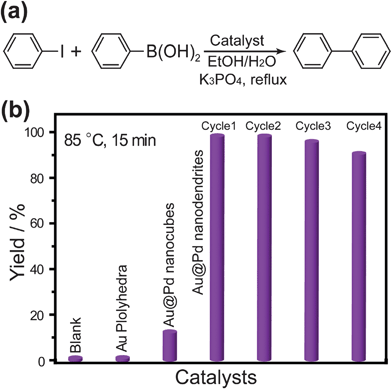 | ||
| Fig. 5 (a) The equation of the Suzuki coupling reaction between phenylboronic acid and iodobenzene. (b) Yields of biphenyl using different nanostructures as the catalyst and cycle performance of Au@Pd core–shell nanodendrites. | ||
In summary, we have presented an effective route for the controllable growth of the uniform Au@Pd core–shell nanodendrites. The porous Au@Pd core–shell nanodendrites display bifunctional plasmonic and catalytic properties. The large surface area and high concentration of coordinatively unsaturated surface Pd atoms should result in enhanced catalytic activity for methanol oxidation and also the Suzuki coupling reaction in comparison with Au@Pd core–shell nanocubes. We believe that the bifunctional plasmonic and catalytic properties of Au@Pd core–shell nanodendrites can have potential applications for plasmon-enhanced catalysis.
This work was supported by the National Natural Science Foundation of China (no. 51001098), the Institute of Metal Research (no. 09NBA211A1) and the National Basic Research Program (973 Program, no. 2011CBA00504).
Notes and references
- M. B. Cortie and A. M. McDonagh, Chem. Rev., 2011, 111, 3713 CrossRef CAS.
- M. Sastry, A. Swami, S. Mandal and PR. Selvakannan, J. Mater. Chem., 2005, 15, 3161 RSC.
- A. Burns, H. Ow and U. Wiesner, Chem. Soc. Rev., 2006, 35, 1028 RSC.
- H. Ataee-Esfahani, L. Wang and Y. Yamauchi, Chem. Commun., 2010, 46, 3684 RSC.
- F. Tao, M. E. Grass, Y. Zhang, D. R. Butcher, J. R. Renzas, Z. Liu, J. Y. Chung, B. S. Mun, M. Salmeron and G. A. Somorjai, Science, 2008, 322, 932 CrossRef CAS.
- K. Zhang, Y. J. Xiang, Z. C. Wu, L. L. Feng, W. W. He, J. B. Liu, W. Y. Zhou and S. S. Xie, Langmuir, 2009, 25, 1162 CrossRef CAS.
- M. Chen, D. Kumar, C.-W. Yi and D. W. Goodman, Science, 2005, 310, 291 CrossRef CAS.
- L. Kuai, S. Z. Wang and B. Y. Geng, Chem. Commun., 2011, 47, 6093 RSC.
- C.-H. Cui, J.-W. Yu, H.-H. Li, M.-R. Gao, H.-W. Liang and S.-H. Yu, ACS Nano, 2011, 5, 4211 CrossRef CAS.
- S. J. Guo, J. Li, S. J. Dong and E. Wang, J. Phys. Chem. C, 2010, 114, 15337 CAS.
- S. Zhou, K. Mcllwrath, G. Jackson and B. Eichhorn, J. Am. Chem. Soc., 2006, 128, 1780 CrossRef CAS.
- A. Cao and G. Veser, Nat. Mater., 2010, 9, 75 CrossRef CAS.
- X. Q. Huang, S. H. Tang, B. J. Liu, B. Ren and N. F. Zheng, Adv. Mater., 2011, 23, 3420 CrossRef CAS.
- S. E. Habas, H. Lee, V. Radmilovic, G. A. Somorjai and P. Yang, Nat. Mater., 2007, 6, 692 CrossRef CAS.
- F. Wang, L.-D. Sun, W. Feng, H. J. Chen, M. H. Yeung, J. F. Wang and C.-H. Yan, Small, 2010, 6, 2566 CrossRef CAS.
- B. Lim, H. Kobayashi, T. Yu, J. Wang, M. J. Kim, Z.-Y. Li, M. Rycenga and Y. Xia, J. Am. Chem. Soc., 2010, 132, 2506 CrossRef CAS.
- Y. J. Xiang, X. C. Wu, D. F. Liu, X. Y. Jiang, W. G. Chu, Z. Y. Li, Y. Ma, W. Y. Zhou and S. S. Xie, Nano Lett., 2006, 6, 2290 CrossRef CAS.
- F.-R. Fan, D.-Y. Liu, Y.-F. Wu, S. Duan, Z.-X. Xie, Z.-Y. Jiang and Z.-Q. Tian, J. Am. Chem. Soc., 2008, 130, 6949 CrossRef CAS.
- B. Lim, J. Wang, P. H. C. Camargo, M. Jiang, M. J. Kim and Y. Xia, Nano Lett., 2008, 8, 2535 CrossRef CAS.
- F. Wang, C. H. Li, L.-D. Sun, H. S. Wu, T. Ming, J. F. Wang, J. C. Yu and C.-H. Yan, J. Am. Chem. Soc., 2011, 133, 1106 CrossRef CAS.
- Y. W. Lee, M. J. Kim, Z. H. Kim and S. W. Han, J. Am. Chem. Soc., 2009, 131, 17036 CrossRef CAS.
- B. Lim, M. Jiang, P. H. C. Camargo, E. C. Cho, J. Tao, X. Lu, Y. Zhu and Y. Xia, Science, 2009, 324, 1302 CrossRef CAS.
- S. J. Guo, S. J. Dong and E. Wang, Chem. Commun., 2010, 46, 1869 RSC.
- Z. M. Peng and H. Yang, J. Am. Chem. Soc., 2009, 131, 7542 CrossRef CAS.
- K. M. Yeo, S. Choi, R. M. Anisur, J. Kim and I. S. Lee, Angew. Chem., Int. Ed., 2011, 50, 745 CrossRef CAS.
- J. G. Xu, A. R. Wilson, A. R. Rathmell, J. Howe, M. F. Chi and B. J. Wiley, ACS Nano, 2011, 5, 6119 CrossRef CAS.
- Z. H. Sun, Z. Yang, J. H. Zhou, M. H. Yeung, W. H. Ni, H. K. Wu and J. F. Wang, Angew. Chem., Int. Ed., 2009, 48, 2881 CrossRef CAS.
- H. H. Wang, Z. H. Sun, Q. H. Lu, F. W. Zeng and D. S. Su, Small, 2012, 8, 1167 CrossRef CAS.
- Z. H. Sun, Z. H. Bao, C. H. Fang and J. F. Wang, Langmuir, 2012, 28, 9082 CrossRef CAS.
- C.-L. Lu, K. S. Prasad, H.-L. Wu, J. A. Ho and M. H. Huang, J. Am. Chem. Soc., 2010, 132, 14546 CrossRef CAS.
- J. W. Hong, Y. W. Lee, M. Kim, S. W. Kang and S. W. Han, Chem. Commun., 2011, 47, 2553 RSC.
- F. Wang, C. H. Li, L.-D. Sun, C.-H. Xu, J. F. Wang, J. C. Yu and C.-H. Yan, Angew. Chem., Int. Ed., 2012, 51, 1 CrossRef.
- C. X. Xu, L. Wang, X. L. Mu and Y. Ding, Langmuir, 2010, 26, 7437 CrossRef CAS.
- P.-P. Fang, S. Duan, X.-D. Lin, J. R. Anema, J.-F. Li, O. Buriez, Y. Ding, F.-R. Fan, D.-Y. Wu, B. Ren, Z. L. Wang, C. Amatore and Z.-Q. Tian, Chem. Sci., 2011, 2, 531 RSC.
- L. Kuai, B. Y. Geng, S. Z. Wang and Y. Sang, Chem.–Eur. J., 2012, 18, 9423 CrossRef CAS.
- L. Kuai, X. Yu, S. Z. Wang, Y. Sang and B. Y. Geng, Langmuir, 2012, 28, 7168 CrossRef CAS.
- W. Zhou and J. Y. Lee, Electrochem. Commun., 2007, 9, 1725 CrossRef CAS.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362 CrossRef CAS.
- B. Lim, M. Jiang, J. Tao, P. H. C. Camargo, Y. Zhu and Y. Xia, Adv. Funct. Mater., 2009, 19, 189 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization and catalytic performance measurement of Au nanopolyhedra and Au@Pd core–shell nanostructures, TEM image of Au nanopolyhedra and Au@Pd core–shell nanodendrites after four cycles of the Suzuki coupling reaction, TEM and high-resolution images of a single Au@Pd core–shell nanodendrite, and XRD pattern of Au@Pd core–shell nanodendrites, UV-vis spectrum of Au@Pd nanodendrites in the range 200–400 nm, references. See DOI: 10.1039/c2nr32849f |
| This journal is © The Royal Society of Chemistry 2013 |
