One-step engineering of silver nanoclusters–aptamer assemblies as luminescent labels to target tumor cells†
Jinjin
Yin
,
Xiaoxiao
He‡
,
Kemin
Wang
*,
Zhihe
Qing
,
Xu
Wu
,
Hui
Shi
and
Xiaohai
Yang
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, College of Biology, Key Laboratory for Bio-Nanotechnology and Molecular Engineering of Hunan Province, Hunan University, Changsha, 410082, P. R. China. E-mail: kmwang@hnu.cn; Fax: +86 731 88821566; Tel: + 86 731 88821566
First published on 11th November 2011
Abstract
We reported one-step engineering of intrinsically fluorescent silver nanoclusters–aptamer assemblies that would allow the development of facile and specific luminescent labels for target tumor cell recognition and analysis.
Few-atom gold and silver nanoclusters, exhibiting strong, robust, and tunable fluorescence emission, have been developed as a new class of fluorophores for chemical sensing and biomedical imaging.1 Especially, oligonucletide-templated silver nanoclusters (AgNCs) have attracted special attention. The fluorescent AgNCs are stabilized by single-stranded DNA or oligonucleotides and exhibit outstanding spectral flourescence property and biocompatibility. Therefore it is promising to prepare the AgNCs using functional DNA or other oligonucletide-based recognition ligands as a template, and hence providing the possibility for engineering a specific, conjugation free and facile one-step method for bio-recognition and analysis. Recently, the one-step fluorescent recognition ligands for highly selective detection of protein and DNA have been reported by using DNA aptamer–AgNCs and DNA-functionalised AgNCs, respectively.2 The similar model was that the oligonucletide-based recognition ligand was coupled with cytosine-rich sequence for target DNA or protein detection. These works had high selectivity for target species. However, to best of our knowledge, little has been done to develop a one-step analysis platform for tumor cell recognition with oligonucleotide-templated AgNCs, largely resulting from the difficulty in directly templating the fluorophores by using the traditional antibody based cell recognition ligands. With the advent of whole-cell-SELEX, more and more aptamers (DNA or RNA) have been selected and employed for cells analysis.3 If the strategy of one-step synthesis of fluorescent AgNCs–aptamer complexes for cell labeling could be achieved, there would be great effect on the field of cell labeling and imaging.
Herein, we reported a one-step engineering of intrinsically fluorescent AgNCs–aptamer assemblies that would allow developing facile and specific luminescent labels for target tumor cells recognition and analysis (Scheme 1).
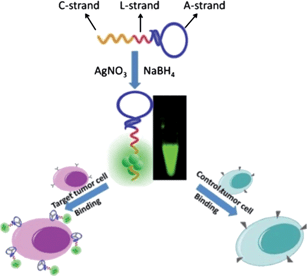 | ||
| Scheme 1 Schematic illustration of one-step AgNCs–aptamer assembly as a luminescent label for tumor cells. | ||
The in situ synthesis protocol reported here is a simple and inexpensive one-step labeling process without covalent conjugation of bio-recognition molecules to fluorophores. This engineering relies on the design of one single oligonucleotide structure, which is competent to work both on the AgNCs synthesis and the target bio-recognition. A cancer-targeted DNA aptamer sequence (A-strand) and cytosine-rich sequence for templated synthesis of fluorescent AgNCs (C-strand) were employed. Considering the conformational change in aptamers following cell binding and steric hindrance between aptamers and the synthesized AgNCs, we proposed to insert a linker sequence (L-strand), to associate the aptamer sequence with cytosine-rich sequence. These structures were used to form the fluorescent AgNCs in the later stage and then to recognize the target tumor cells. To demonstrate this principle, the binding of AgNCs to two different tumor cells, CCRF-CEM cells and Ramos cells, was studied in this work.
It was reported that the fluorescence of the DNA-templated AgNCs could be affected by the proximity of other nucleosides.1c,4 To obtain an AgNCs–aptamer assembly that could serve as both a fluorescent label and a specific binding probe, the effect of 16 different linkers, including adenine-rich sequences, guanine-rich sequences and thymine-rich sequences, on the fluorescence of AgNCs was firstly investigated in terms of base types and base numbers, as listed in Table S1.† The fluorescent AgNCs were synthesized under ambient atmosphere on ice (see SI for details†). And the suspension was directly measured by fluorescence spectroscopy. Although the excitation and emission maxima of the AgNCs generated by these linkers were all round 480 and 550 nm, respectively, the fluorescence intensities of the AgNCs prepared by using different linker–AgNCs templating DNA were obviously different (Fig. 1). Among the three types of bases, the thymine-rich sequence linker could remarkably suppress the fluorescence intensity of AgNCs. However, the fluorescence of AgNCs was enhanced when templating DNA was connected with an appropriate number of adenine-rich sequences or guanine-rich DNA sequences. By comparison, adenine-rich sequence linkers showed much stronger enhancement, and six-base adenine linker (A6) was the best, enchancing the photoluminescence (PL) quantum yields of AgNCs from 1.85% to 17.4%. As for guanine-rich linkers, the 8-base guanine linker (G8) enchanced the fluorescence intensity of AgNCs with the PL quantum yields from 1.85% to 16.2%, using rhodamine 6G as a reference.
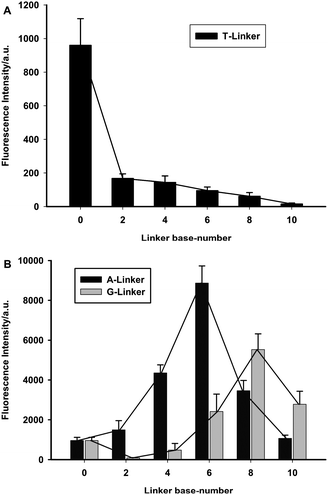 | ||
| Fig. 1 Effect of different linkers on the fluorescence of synthesized AgNCs. (A) linkers of different numbers of thymine; (B) linkers of different numbers of adenine (black) and guanine (grey). | ||
As for the engineered AgNCs–aptamer assemblies, the influence of aptamer on the fluorescence output was further investigated by using sgc8c aptamer, against human acute lymphoblastic leukemia CCRF-CEM cells, as a representative (Fig. 2). It was found that the fluorescence of AgNCs templated by both sgc8c-A6-C12 and sgc8c-G8-C12 was diminished, in comparison with those by A6-C12 and G8-C12, implying that the sgc8c aptamer could also affect the fluorescence of DNA-templated AgNCs. Nevertheless, the fluorescence of DNA-templated AgNCs by sgc8c-A6-C12 and sgc8c-G8-C12 was still much higher than that of sgc8c-C12. Furthermore, bright fluorescent AgNCs could not be synthsized by only sgc8c aptamer templated. By comparison, a higher fluorescence intensity of AgNCs was achieved for assemblies templated by sgc8c-A6-C12. These results declared that the spectral properties of these DNA-templated AgNCs are sensitive to the different base sequences. High Resolution Transmission Electron Microscopy (HRTEM) images confirmed that the AgNCs templated with sgc8c-A6-C12 are dispersed with a separation distance of ∼3 nm (Fig. 2(B)).
 | ||
| Fig. 2 (A) Fluorescence spectra of AgNCs templated by different oligonucleotides (sgc8c, sgc8c-C12, sgc8c-G8-C12, G8-C12, and sgc8c-A6-C12, A6-C12). (B) High response transmission electron microscopy (HRTEM) showing sgc8c-A6-C12-templated AgNCs. Scale bar is 50 nm. | ||
Up to now, the fluorescence variation mechanism is not clear. It has been reported that NaBH4 reduction could produce large Ag nanoparticles without fluorescence. So a tentative explanation is that the other bases could bind with comparable affinities to cytosine with Ag+. Before reduction, A or G bases could capture more Ag+ ions and form more fluorescent AgNCs. However, this possibility does not easily explain the trend shown in Fig. 1, where the fluorescence emission decreased with more adenine or guanine content. A second possibility is that the secondary structure of different sequences alter their optical properties. It was reported before that the secondary structure influences the fluorescence intensity of AgNCs.2b,4 To study whether the secondary structures influence the fluorescence intensities of AgNCs, every dimer of these sequences in our experiment was simulated by computer (not shown). The results showed that they had similar configurations of the hairpin loops with a tail. Thus, the secondary structure is not the cause of the fluorescence alteration. A third possibility is that complex strand deformation may induce the fluorescence alteration through inhibiting the specific interaction between Ag+ ions and C bases. Apparently, there would be more complicated strand deformations with longer oligonucleotide sequences. However, different fluorescence attenuations were found with the same aptamer (sgc8c) combined (Fig. 2). Therefore, the third possibility was ruled out as well. We are still investigating the real variation mechanism. And these results demonstrated that sgc8c-A6-C12 as an efficient template could obtain AgNCs with comparatively high fluorescence intensity for subsequent tumor cell recognition.
Before the application of the fluorescent AgNCs–aptamer assemblies, the stability assays of the aptamer–AgNcS assemblies were necessary. And they were investigated (Figure S1†). The results declared that the AgNCs–aptamer assemblies had good stability in binding buffer under light irradiation. To test the suitability of the one-step engineered intrinsically fluorescent AgNCs–aptamer assemblies for target tumor cell recognition and analysis, CCRF-CEM cells and Ramos cells (B cell line, human Burkitt's lymphoma) were used to bind with the sgc8c-AgNCs assembly, respectively. After incubating with cells at 4 °C for 40 min, the specificity of the sgc8c-AgNCs assembly to CCRF-CEM cells was measured using flow cytometry (FACScalibur, BD Bioscience, USA) and confocal laser scanning microscopy (FV500-IX70, Olympus, JP). The results from flow cytometry clearly confirmed that the fluorescent sgc8c-AgNCs assembly specifically recognized and labeled CCRF-CEM cells (Fig.3(A)). Furthermore, the confocal laser scanning microscopy data demonstrated the same trend of selective binding, as indicated by strong fluorescence in CEM cells incubated with sgc8c–AgNCs assembly (Fig.3(B)), rather than Ramos cells with the same treatment. These results demonstrated that the sgc8c–AgNCs assembly was able to recognize and analyse the CCRF-CEM cells with high specificity. In addition, we used another aptamer TD05 to engineer the assembly, and the binding of the TD05–AgNCs assembly to Ramos cells was also studied (Figure S2†). The results indicated that the TD05–AgNCs assembly also possessed high luminescence and specific binding to target Ramos cells.
 | ||
| Fig. 3 Specific recognition of CCRF-CEM cells with the sgc8c–AgNCs assembly. (A) The resulta of flow cytometry assays by counting 10,000 events. (B) The confocal laser scanning microscopy images of CCRF-CEM cells and Ramos cells incubated with sgc8c–AgNCs assembly. (a) and (b) represent the fluorescent image and merged image, respectively, of CCRF-CEM cells incubated with sgc8c–AgNCs assembly; (c) and (d) represented the fluorescent image and merged image, respectively, of Ramos cells incubated with sgc8c–AgNCs assembly. Scale bar is 20 μm. | ||
In summary, we have developed a strategy that allows generation of intrinsically fluorescent AgNCs–aptamer assemblies for cell recognition through a convenient one-step process. The fluorescent AgNCs–aptamer assemblies consist of fluorescent AgNCs on an aptamer-linker DNA. By considering the conformational change in the aptamer following cell binding and steric hindrance between the aptamer and the synthesized AgNCs, the effect of linkers with different base types and base numbers on the fluorescence of synthesized AgNCs has been investigated. A fluorescent sgc8c–AgNCs assembly with relatively high luminescence has been achieved and exhibited specific binding to target CCRF-CEM cells by using a six-base adenine linker. Additionally, a TD05–AgNCs assembly with specific binding to target Ramos cells was also engineered using this strategy, and showed specific recognition and labeling capabilities to target Ramos cells. The one-step engineering of fluorescent AgNCs–aptamer assemblies demonstrates a strategy to circumvent the conjugation of cell aptamers with fluorophores. When more and more aptamers are selected against tumor cells, we envision that this strategy holds great potential to be used in target cell recognition in either scientific research or clinical diagnosis.
This work was supported in part by the Key Project of Natural Science Foundation of China (90606003, 21175039, and 21190044), International Science & Technology Cooperation Program of China (2010DFB30300), and project supported by Hunan Provincial Natural Science Foundation and Hunan Provincial Science and Technology Plan of China (10JJ7002, 2011FJ2001), Hunan Provincial Graduate Research and Innovation projects (CX2011B132).
References
- (a) J. T. Petty, J. Zheng, N. V. Hud and R. M. Dickson, J. Am. Chem. Soc., 2004, 126, 5207 CrossRef CAS; (b) J. H. Yu, S. A. Patel and R. M. Dickson, Angew. Chem., Int. Ed., 2007, 46, 2028 CrossRef CAS; (c) C. M. Ritchie, K. R. Johnsen, J. R. Kiser, Y. Antoku, R. M. Dickson and J. T. Petty, J. Phys. Chem. C, 2007, 111, 175 CrossRef CAS; (d) J. H. Yu, S. M. Choi, C. I. Richards, Y. Antoku and R. M. Dickson, Photochem. Photobiol., 2008, 84, 1435 CrossRef CAS; (e) C. I. Richards, S. Choi, J.-C. Hsiang, Y. Antoku, T. Vosch, A. Bongiorno, Y.-L. Tzeng and R. M. Dickson, J. Am. Chem. Soc., 2008, 130, 5038 CrossRef CAS; (f) B. Sengupta, C. M. Ritchie, J. G. Buckman, K. R. Johnsen, P. M. Goodwin and J. T. Petty, J. Phys. Chem. C., 2008, 112, 18776 CAS; (g) J. H. Yu, S. Choi and R. M. Dickson, Angew. Chem., Int. Ed., 2009, 48, 318 CrossRef CAS; (h) W. W. Guo, J. P. Yuan and E. K. Wang, Chem. Commun., 2009, 3395 RSC; (i) J. Sharma, H. C. Yeh, H. Yoo, J. H. Werner and J. S. Martinez, Chem. Commun., 2010, 46, 3280 RSC; (j) Z. Huang, F. Pu, D. Hu, C. Wang, J. Ren and X. Qu, Chem.–Eur. J., 2011, 17, 3774 CrossRef CAS.
- (a) G. Y. Lan, W. Y. Chen and H. T. Chang, Biosens. Bioelectron., 2011, 26, 2431 CrossRef CAS; (b) W. W. Guo, J. P. Yuan, Q. Z. Dong and E. K. Wang, J. Am. Chem. Soc., 2010, 132, 932 CrossRef CAS; (c) H. C. Yeh, J. Sharma, J. J. Han, J. S. Martinez and J. H. Werner, Nano Lett., 2010, 10, 3106 CrossRef CAS; (d) K. Ma, Q. H. Cui, G. Y. Liu, F. Wu, S. J. Xu and Y. Shao, Nanotechnology, 2011, 22, 305502 CrossRef; (e) J. Sharma, H. C. Yeh, H. Yoo, J. H. Werner and J. S. Martinez, Chem. Commun., 2011, 47, 2294 RSC; (f) Z. X. Zhou, Y. Du and S. J. Dong, Biosens. Bioelectron., 2011, 28, 33, DOI:10.1016/j.bios.2011.06.028.
- (a) D. H. Shangguan, Y. Li, Z. W. Tang, Z. H. C. Cao, H. W. Chen, P. Mallikaratchy, K. Sefah, C. Y. J. Yang and W. H. Tan, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11838 CrossRef CAS; (b) X. H. Fang and W. H. Tan, Acc. Chem. Res., 2010, 43, 48 CrossRef CAS.
- E. G. Gwinn, P. O'Neill, A. J. Guerrero, D. Bouwmeester and D. K. Fygenson, Adv. Mater., 2008, 20, 279 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1nr11265a |
| ‡ Joint first author. |
| This journal is © The Royal Society of Chemistry 2012 |
