Squaric acid: a valuable scaffold for developing antimalarials?†
S. Praveen
Kumar
a,
Paulo M. C.
Glória
a,
Lídia M.
Gonçalves
a,
Jiri
Gut
b,
Philip J.
Rosenthal
b,
Rui
Moreira
a and
Maria M. M.
Santos
*a
aResearch Institute for Medicines and Pharmaceutical Sciences (iMed.UL), Faculty of Pharmacy, University of Lisbon, Av. Prof. Gama Pinto, 1649-003 Lisbon, Portugal. E-mail: mariasantos@ff.ul.pt; Fax: 351 21 794 6470; Tel: 351 21 794 6400
bDepartment of Medicine, San Francisco General Hospital, University of California, San Francisco, CA 94143, USA
First published on 8th February 2012
Abstract
We describe here the synthesis of a library of thirty-eight squaric derivatives and the evaluation of activity against papain-, falcipain-2- and a chloroquine-resistant strain of P. falciparum. The most active compounds combine significant antiplasmodial activity with minimal cytotoxicity.
Introduction
Malaria is one of the most widespread infectious diseases of our time, causing nearly 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths each year.1 The most lethal of the Plasmodium species that infects humans—Plasmodium falciparum—has acquired resistance to most clinically used antimalarials.2 Furthermore, the first cases of putative resistance to artemisinin-based combination therapy (ACT) have been reported. For that reason, there is an urgent need for novel antimalarial drugs. In fact, a major challenge is to make sure that each new generation of molecules provides antiparasitic activity against the resistant strains.3
000 deaths each year.1 The most lethal of the Plasmodium species that infects humans—Plasmodium falciparum—has acquired resistance to most clinically used antimalarials.2 Furthermore, the first cases of putative resistance to artemisinin-based combination therapy (ACT) have been reported. For that reason, there is an urgent need for novel antimalarial drugs. In fact, a major challenge is to make sure that each new generation of molecules provides antiparasitic activity against the resistant strains.3
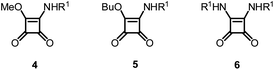
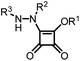
Following our studies on Michael acceptors as cysteine protease inhibitors we become interested in the squaric acid scaffold.4–8 Our interest was triggered by reports showing that dimethyl squarate reacts efficiently with thiols to give the corresponding addition–elimination products,9 suggesting that a similar process might also occur within the active site of a cysteine protease. Moreover, since the first synthesis of squaric acid a number of derivatives have been reported in the fields of bioorganic and medicinal chemistry. This scaffold has already been used with success to develop metalloenzyme inhibitors.10–13 However, the potential of this scaffold in antimalarial agents remained to be explored. With this in mind, we developed a library of squaramide derivatives by incorporating substituents with different stereoelectronic properties and lipophilicities at C-3 and C-4 (Fig. 1) in order to perform a structure–activity study for this scaffold.
 | ||
| Fig. 1 General structure of squaric acid derivatives synthesized. | ||
Meanwhile, in 2010, GlaxoSmithKline's chemical library for inhibitors of P. falciparum was made public to encourage additional drug lead identification efforts and further research into malaria. In that library were included four compounds containing a squaric moiety, with activities ranging from 62 to 878 nM against P. falciparum strain 3D7 (Fig. 2).14 Two of these squaric acid derivatives were annotated as antagonists of serotonin receptor, a drug target without obvious orthologue in the malarial genome. More extended structure–activity data are required to evaluate the usefulness of the squaric acid scaffold as a starting point to develop novel antimalarials and to gain a better understanding of their putative targets.
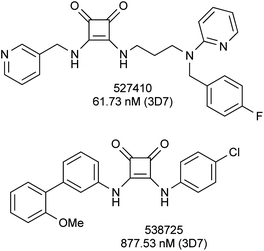 | ||
| Fig. 2 Examples of squaric derivatives from the GSK library. | ||
Results and discussion
To explore the potential of the squaric acid scaffold as an antiplasmodial agent we synthesized a series of compounds containing different aromatic and aliphatic amines (Scheme 1). Commercially available squarates 1 and 2 and amines 3a–q were used as starting materials. Squaramates 4a–f and squaramides 6g–q were obtained from dimethyl squarate 1 using one or two equivalents of the appropriate amines. In contrast, when we used the butyl squarate 2 and one equivalent of amines 3e–f as the starting materials, we obtained squaramides 6e–f instead of the corresponding squaramates 5. Squaramide 6a was obtained from reaction of two equivalents of butyl squarate 2 and amine 3a. Interestingly, compound 6b was obtained by reflux of a mixture of one equivalent of butyl squarate 2 and amine 3b. The same reaction at room temperature led to compound 5b.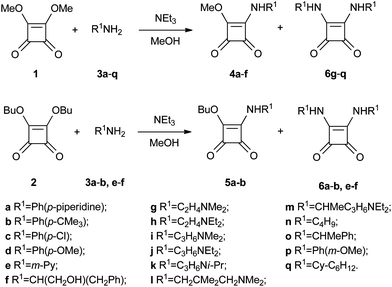 | ||
| Scheme 1 Synthesis of squaramides 4a–f, 5a–b, 6a–b and 6e–q. | ||
Compounds 4a–f, 5a–b, 6a–b and 6e–q were then screened against papain-, falcipain-2- and the chloroquine-resistant W2 strain of P. falciparum (Table 1). Most of the compounds were devoid of activity against P. falciparum. However, compounds 5a–b and 6a–b had low micromolar antiplasmodial activity, with IC50 values ranging from 0.99 to 4.16 μM. Except for compounds 6a–b and 6p, all compounds were inactive against papain, the prototype for clan CA cysteine proteases. In fact, we already observed a similar result for squaric vinyl sulfones.6
|
|
||||
|---|---|---|---|---|
| Compounds | R1 | Papain IC50/μM | Falcipain-2 IC50a/μM | P. falciparum W2 IC50/μM |
| a ND = not done. | ||||
| 4a | Ph(p-piperidin-1-yl) | >10 | ND | >10 |
| 4b | Ph(p-CMe3) | >10 | ND | >10 |
| 4c | Ph(p-Cl) | >10 | NDs | >10 |
| 4d | Ph(p-OMe) | >10 | ND | >10 |
| 4e | m-Py | >10 | ND | >10 |
| 4f | CH(CH2OH)(CH2Ph) | >10 | >50 | >10 |
| 5a | Ph(p-piperidin-1-yl) | >10 | ND | 3.13 ± 0.35 |
| 5b | Ph(p-CMe3) | >10 | >50 | 2.65 ± 0.19 |
| 6a | Ph(p-piperidin-1-yl) | 2.8 ± 1.3 | >50 | 4.16 ± 0.54 |
| 6b | Ph(p-CMe3) | 8.1 ± 0.4 | 24.3 ± 3.98 | 0.99 ± 0.23 |
| 6e | m-Py | >10 | ND | >10 |
| 6f | CH(CH2OH)(CH2Ph) | >10 | ND | >10 |
| 6g | C2H4NMe2 | >10 | ND | >10 |
| 6h | C2H4NEt2 | >10 | >50 | >10 |
| 6i | C3H6NMe2 | >10 | ND | >10 |
| 6j | C3H6NEt2 | >10 | >50 | >10 |
| 6k | C3H6Ni-Pr | >10 | >50 | >10 |
| 6l | CH2CMe2CH2NMe2 | >10 | ND | >10 |
| 6m | CHMeC3H6NEt2 | >10 | ND | >10 |
| 6n | C4H9 | >10 | ND | >10 |
| 6o | CHMePh | >10 | ND | >10 |
| 6p | Ph(m-OMe) | 5 ± 1 | >50 | >10 |
| 6q | Cy-C6H12 | >10 | ND | >10 |
| E−64 | 0.12 ± 0.02 | 0.06 ± 0.01 | 1.94 ± 0.004 | |
Based on our previous results showing IC50 values of around 1 μM for squaric-Leu-azaPhe-VSPh derivatives,6 we tried to improve the activity of these compounds, by synthesizing two unsymmetrical squaramides. Ph(p-CMe3) and CH(CH2OH)(CH2Ph) were chosen as substituents due to the good activities of compounds 5b and 6b and to test the presence of a hydroxyl group, respectively. However, compounds 8b and 8f were devoid of antiplasmodial activity (IC50, >10 μM) (Scheme 2).
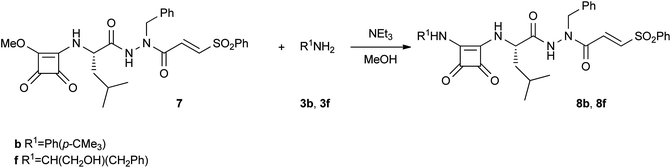 | ||
| Scheme 2 Synthesis of squaramides 8b and 8f. | ||
The next step was to evaluate the potential of the squaric acid scaffold in the design of selective falcipain inhibitors, by incorporating the appropriate recognition moiety at the double bond. A series of compounds containing an aza-dipeptide recognition moiety were synthesized in which the squaric moeity acts as a replacement of the P1 carbonyl group. Gly, Phe and homoPhe residues were selected for P1. The recognition moiety was obtained starting with N-BocLeu and the corresponding hydrazine.
The aza-Gly compound was obtained from reaction of Boc protected hydrazine 9a with dimetyl squarate 1. Deprotection of the Boc group, followed by coupling with BocLeu, led to aza squaric derivative 11a containing Gly in P1 (Scheme 3).
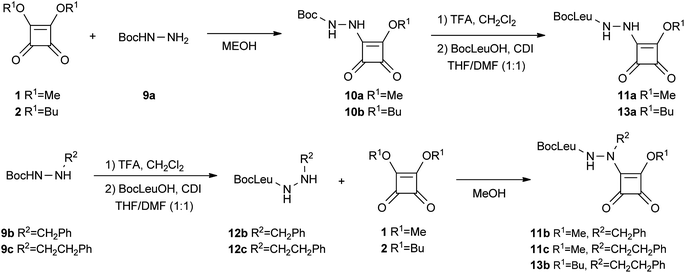 | ||
| Scheme 3 Synthesis of aza squaric derivatives 11a–c and 13a–b. | ||
The aza squaric acid derivatives containing Phe and homoPhe in P1 were synthesized starting from the corresponding Boc protected hydrazines 9b–c. Deprotection of the Boc group with TFA, followed by coupling with BocLeuOH, led to amino acid hydrazides 12b–c. Finally, coupling with the dimetyl squarate 1 led to aza squaric derivatives 11b–c in 42–47% yields (Scheme 3). All of the proposed structures were established by NMR (1H, 13C, COSY and HMQC), IR, and MS.
Compounds 11a–c were then screened against the W2 strain of P. falciparum (Table 2). The results show that the recognition moiety seems to be essential for antiplasmodial activity, as compounds 4a–f and 6e–q, lacking the aza-dipeptidyl moiety, were devoid of activity. In contrast, compounds 11a and 11c had good antiplasmodial activity, with IC50 values of 9 and 6 μM, respectively. Probably FP-2 inhibition is not the primary mode by which derivatives 11 exert their antiplasmodial activities, since compound 11c was not active against falcipain-2 for the tested concentrations. In fact, all compounds were inactive against papain.
|
|
||||||
|---|---|---|---|---|---|---|
| Compounds | R1 | R2 | R3 | Aza-dipeptide | Papain C50/μM | P. falciparum W2 IC50/μM |
| a Insoluble, saturated solution of 1 μM was used. | ||||||
| 11a | Me | H | BocLeu | BocLeu-azaGly | >10 | 8.53 ± 0.71 |
| 11b | Me | CH2Ph | BocLeu | BocLeu-azaPhe | >10 | >1a |
| 11c | Me | CH2CH2Ph | BocLeu | BocLeu-azahomoPhe | >10 | 6.36 ± 0.78 |
| 11d | Me | H | MuLeu | MuLeu-azaGly | >10 | >10 |
| 11e | Me | CH2CH2Ph | MuLeu | MuLeu-azahomoPhe | >10 | >10 |
| 11f | Me | CH2CH2Ph | CbzLeu | CbzLeu-azahomoPhe | >10 | 2.99 ± 0.23 |
| 11g | Me | CH2CH2Ph | CbzGly | CbzGly-azahomoPhe | >10 | 9.36 ± 0.1 |
| 11h | Me | CH2CH2Ph | CbzPhe | CbzPhe-azahomoPhe | >10 | 3.14 ± 0.07 |
| 13a | Bu | H | BocLeu | BocLeu-azaGly | >10 | >10 |
| 13b | Bu | CH2CH2Ph | BocLeu | BocLeu-azahomoPhe | >10 | >10 |
| 13c | Bu | H | MuLeu | MuLeu-azaGly | >10 | 9.45 ± 0.02 |
| 13d | Bu | CH2CH2Ph | MuLeu | MuLeu-azahomoPhe | >10 | >10 |
| 13e | Bu | CH2CH2Ph | CbzLeu | CbzLeu-azahomoPhe | >10 | 4.51 ± 0.25 |
| 13f | Bu | CH2CH2Ph | CbzGly | CbzGly-azahomoPhe | >10 | 5.17 ± 0.04 |
| 13g | Bu | CH2CH2Ph | CbzPhe | CbzPhe-azahomoPhe | >10 | 4.69 ± 0.13 |
| E−64 | 0.3 ± 0.2 (nM) | 1.94 ± 0.004 | ||||
To further explore the potential of the aza-dipeptidyl squaric acid derivatives as antimalarials we completed our structure–activity study by synthesizing other derivatives of compounds 11a (aza-Gly) and 11c (aza-homoPhe).
We synthesized a new series of compounds containing Cbz-protected amino acids in P2. Gly, Leu and Phe residues in P2 were selected in order to modulate the interaction with P2 (Scheme 4). Two compounds were also synthesized with a 4-morpholinecarbonyl (Mu) protected Leu in P2 and an aza-Gly or aza-homoPhe in P1 (Scheme 4). Commercially available methyl 1 and butyl 2 squarates were used as starting materials.
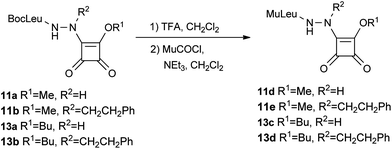 | ||
| Scheme 4 Synthesis of N-Mu aza squaric derivatives 11d–e and 13c–d. | ||
The BocLeu butoxy squaric derivatives 13a and 13b were synthesized using the same methodology applied for the synthesis of compounds 11a and 11c, respectively (Scheme 3). Compounds 11d–e and 13c–d were obtained from the corresponding BocLeu aza squaric acid derivatives with 69–76% yields, by deprotection of the Boc group with TFA, followed by reaction with 4-morpholinecarbonyl chloride (Scheme 4). The N-Cbz protected aza squaric acid derivatives were synthesized from the corresponding amino acid hydrazides in 71–83% yields (Scheme 5).
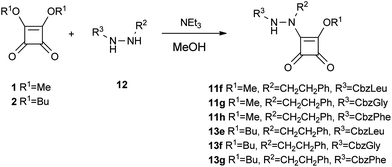 | ||
| Scheme 5 Synthesis of N-Cbz aza squaric derivatives 11f–h and 13e–g. | ||
Compounds 11d–h and 13a–g were then tested against papain and against the W2 strain of P. falciparum (Table 2). Inspection of the data in Table 2 allows the following observations:
(1) As observed before for compound 11c, azadipeptidyl squaric derivatives did not inhibit papain at the tested concentrations.
(2) For OMe compounds containing an aza-homoPhe or an aza-Gly in P1 and Leu in P2, the presence of a Mu protective group resulted in the loss of activity against P. falciparum (i.e. compounds 11d and 11eversus their Boc-protected counterparts 11a and 11c). However, the opposite result was observed for the inhibitor containing an aza-homoPhe in P1 and a Cbz-Leu in P2 (i.e. compound 11f). In this case, activity was increased ca. 2-fold higher than that of its Boc-protected counterpart 11c.
(3) The order of inhibitory activity against P. falciparum for the methoxy series depended on the nature of the P2 residue and varied in the order Leu > Phe > Gly. However, this effect had no major impact on activity for the butoxy series (13evs.13fvs.13g).
(4) Related to the Cbz series, substitution of the OMe group for an OBu group in the squaric moiety improved the activity ca. 2-fold only for the inhibitors containing a CbzGly amino acid (i.e. compound 13fversus the OMe counterpart 11g).
(5) For compounds containing an aza-homoPhe or an aza-Gly in P1 and BocLeu, the substitution of the OMe group for an OBu group in the squaric moiety resulted in the loss of activity against P. falciparum (i.e. compounds 13a and 13bversus their OMe counterparts 11a and 11c).
(6) For compounds containing the Mu-Leu-azaGly sequence (i.e. compounds 11d and 13c) substitution of the OMe for an OBu group was beneficial. In fact, compound 11d was inactive but compound 13c presented an IC50 of 9.5 μM.
The in vitro cytotoxicity of the most active compounds for P. falciparum (compounds 5a–b, 6a–b, 11a, 11c, 11f–h, 13c and 13e–g) was evaluated using NIH 3T3 and Hek 293T cells. Remarkably, most of the squaric derivatives were non-cytotoxic up to 100 μM (Table 3). Only, squaramate 5b led to some cytotoxicity when compared to the other squaric derivatives; but even in this case, the selectivity index (SI) is ca. 5, which indicates only modest toxicity.
| Compounds | W2 IC50/μM | NIH 3T3 IC50/μM | Hek 293T IC50/μM | SIa | log Pb |
|---|---|---|---|---|---|
| a Selectivity index toward W2 strain, which is expressed by the ratio IC50Hek/IC50W2. b Estimated by the ALOGPS 2.1 algorithm.15 | |||||
| 5a | 3.13 ± 0.35 | >100 | >100 | >32 | 3.48 |
| 5b | 2.65 ± 0.19 | 17.51 ± 1.27 | 12.35 ± 1.20 | 4.7 | 4.07 |
| 6a | 4.16 ± 0.54 | >50 | >50 | >12 | 4.53 |
| 6b | 0.99 ± 0.23 | >100 | >100 | >101 | 5.70 |
| 11a | 8.53 ± 0.71 | >100 | >100 | >12 | 1.30 |
| 11c | 6.36 ± 0.78 | >100 | >100 | >16 | 2.85 |
| 11f | 2.99 ± 0.23 | >100 | >100 | >33 | 3.28 |
| 11g | 9.36 ± 0.1 | >100 | >100 | >11 | 1.82 |
| 11h | 3.14 ± 0.07 | >100 | >100 | >32 | 3.56 |
| 13c | 9.45 ± 0.02 | >100 | >100 | >11 | 1.03 |
| 13e | 4.51 ± 0.25 | >100 | >100 | >22 | 4.48 |
| 13f | 5.17 ± 0.04 | >100 | >100 | >19 | 2.97 |
| 13g | 4.69 ± 0.13 | >100 | >100 | >21 | 4.72 |
Importantly, the calclogP values15 for the most active compounds range from 1.0 to 5.7 (Table 3) suggesting that they present appropriate cell permeability properties.
Conclusions
We have synthesized a library of 38 squaric derivatives and evaluated activity against papain-, falcipain-2- and a chloroquine-resistant strain of P. falciparum.The first series of compounds was obtained by reaction of dimethyl or dibutyl squaramates with the appropriate amines in moderate to good yields.
Aza-dipeptidyl squaric acid derivatives displayed good antiplasmodial activity against P. falciparum. The most active compounds contained an azahomoPhe moiety at the peptidic sequence and presented IC50 values of around 3 μM. No cysteine protease inhibition was observed for compounds containing a recognition moiety, suggesting that FP-2 inhibition is not the primary mode by which derivatives 11 and 13 exert antiplasmodial activities.
However, the recognition moiety seems to be important for the antiplasmodial activities of the squaric derivatives, as most of the non-peptidic compounds were inactive against P. falciparum. For this reason, one might speculate that the compounds are inhibiting other proteases involved in the parasite life cycle. In fact, in silico approaches have identified 92 putative proteases in the P. falciparum genome.16 Further investigations are necessary to clarify the target of these compounds.
In conclusion, several squaric acid derivatives showed significant antiplasmodial activity against a CQ-resistant P. falciparum strain and minimal cytotoxicity. Overall, aza dipeptide squaric derivatives are promising lead compounds for the development of new agents against P. falciparum malaria.
Acknowledgements
This work was supported by Fundação para a Ciência e a Tecnologia (Portugal) through postdoctoral fellowships to P.M.C.G. and S.P.K. and grant PEst-OE/SAU/UI4013/2011. We also thank Fundação para a Ciência e a Tecnologia (Portugal) for the project REDE/1518/REM/2005 for the mass experiments at LCLEM, Faculdade de Farmácia da Universidade de Lisboa. PJR is a Distinguished Clinical Scientist of the Doris Duke Charitable Foundation.Notes and references
- World Malaria Report 2010, http://www.who.int/malaria/world_malaria_report_2010/worldmalariareport2010.pdf, accessed December 15, 2011 Search PubMed.
- T. Rodrigues, R. Moreira and F. Lopes, Future Med. Chem., 2011, 3, 1 CrossRef CAS.
- J. N. Burrows, K. Chibale and T. N. C. Wells, Curr. Top. Med. Chem., 2011, 11, 1226 CrossRef CAS.
- A. S. Newton, P. M. C. Glória, L. M. Gonçalves, D. J. V. A. dos Santos, R. Moreira, R. C. Guedes and M. M. M. Santos, Eur. J. Med. Chem., 2010, 45, 3858 CrossRef CAS.
- P. M. C. Glória, I. Coutinho, L. M. Gonçalves, C. Baptista, J. Soares, A. S. Newton, R. Moreira, L. Saraiva and M. M. M. Santos, Eur. J. Med. Chem., 2011, 46, 2141 CrossRef.
- P. M. C. Glória, J. Gut, L. M. Gonçalves, P. J. Rosenthal, R. Moreira and M. M. M. Santos, Bioorg. Med. Chem., 2011, 19, 7635 CrossRef.
- S. P. Kumar, J. Gut, R. C. Guedes, P. J. Rosenthal, M. M. M. Santos and R. Moreira, Eur. J. Med. Chem., 2011, 46, 927 CrossRef.
- M. M. M. Santos and R. Moreira, Mini-Rev. Med. Chem., 2007, 7, 1040 CrossRef CAS.
- T. Shinada, A. Yamasaki, Y.-I. Kiniwa, K. Shimamoto and Y. Ohfune, Tetrahedron Lett., 2009, 50, 4354 CrossRef CAS.
- J. A. Butera, D. J. Jenkins, J. R. Lennox, J. H. Sheldon, N. W. Norton, D. Warga and T. M. Argentieri, Bioorg. Med. Chem. Lett., 2005, 15, 2495 CrossRef CAS.
- S. Hanessian, V. Vinci, L. Auzzas, M. Marzi and G. Giannini, Bioorg. Med. Chem. Lett., 2006, 16, 4784 CrossRef CAS.
- J. Charton, B. P. Deprez and R. F. Deprez-Poulain, Bioorg. Med. Chem. Lett., 2008, 18, 4968 CrossRef CAS.
- R. I. Storer, A. Caroline and L. H. Jones, Chem. Soc. Rev., 2011, 40, 2330 RSC.
- W. A. Guiguemde, A. A. Shelat, D. Bouck, S. Duffy, G. J. Crowther, P. H. Davis, D. C. Smithson, M. Connelly, J. Clark, F. Zhu, M. B. Jimenez-Diaz, M. S. Martinez, E. B. Wilson, A. K. Tripathi, J. Gut, E. R. Sharlow, I. Bathurst, F. El Mazouni, J. W. Fowble, I. Forquer, P. L. McGinley, S. Castro, I. Angulo-Barturen, S. Ferrer, P. J. Rosenthal, J. L. DeRisi, D. J. Sullivan, Jr, J. S. Lazo, D. S. Roos, M. K. Riscoe, M. A. Phillips, P. K. Rathod, W. C. V. Voorhis, V. M. Avery and R. K. Guy, Nature, 2010, 465, 311 CrossRef CAS.
- ALOGPS 2.1; Virtual Computational Chemistry Laboratory, http://www.vcclab.org/lab/alogps/, accessed December 15, 2011 Search PubMed.
- Y. Wu, X. Wang, X. Liu and Y. Wang, Genome Res., 2003, 4, 601 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2md20011b |
| This journal is © The Royal Society of Chemistry 2012 |