Development of novel ionic liquids based on ampicillin†
Ricardo
Ferraz
ab,
Luís C.
Branco
*a,
Isabel M.
Marrucho
*c,
João M. M.
Araújo
c,
Luis Paulo N.
Rebelo
c,
Manuel Nunes
da Ponte
a,
Cristina
Prudêncio
ad,
João Paulo
Noronha
a and
Željko
Petrovski
*a
aDepartamento de Química, REQUIMTE-CQFB, Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa, 2829-516 Caparica, Portugal. E-mail: z.petrovski@fct.unl.pt; l.branco@fct.unl.pt; imarrucho@itqb.unl.pt; Fax: +351 21 294 85 50
bCiências Químicas e das Biomoléculas, Escola Superior de Tecnologia da Saúde do Porto do Instituto Politécnico do Porto, Portugal
cInstituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa, Apartado 127, 2780-157 Oeiras, Portugal Web: www.itqb.unl.pt
dCentro de Farmacologia e Biopatologia Química (U38-FCT), Faculdade de Medicina da Universidade do Porto, Alameda Prof. Hernâni Monteiro, 4200-319 Porto, Portugal
First published on 15th February 2012
Abstract
Novel ionic liquids containing ampicillin as an active pharmaceutical ingredient anion were prepared with good yields by using a new, efficient synthetic procedure based on the neutralization of a moderately basic ammonia solution of ampicillin with different organic cation hydroxides. The relevant physical and thermal properties of these novel ionic liquids based on ampicillin were also evaluated.
Introduction
Ionic liquids (ILs) are generally defined as organic salts with melting points below 100 °C (some of them are liquid at room temperature) and composed entirely of ions.1,2 The large number of possible cation/anion combinations allows for a great variety of tuneable interactions and subsequent applications.1,3,4 Ionic liquids were initially used as greener alternatives to conventional, toxic and volatile organic solvents.1,5 Some properties generally attributed to ILs, such as high thermal and electrochemical stability, negligible vapour pressure,3,6 high ionic conductivity,3 non-flammability and a tuneable solvation capacity, encouraged their application across a wide range of areas, including organic chemistry,1,4 chemical engineering,1,3 materials science,3,7 physical chemistry,3,8,9 analytical chemistry,3,10 and biotechnology.1,3,11,12Recently, ILs have been combined with active pharmaceutical ingredients (APIs), and a so-called third generation of ILs has emerged.13 These IL–API compounds offer new and improved properties, such as stability, solubility, permeability and drug delivery, as compared to the corresponding solid pharmaceutical forms. The use of an active drug in the liquid form (at room temperature) can avoid some of the issues of polymorphism associated with crystalline solids and, thus, dramatically influence the drug's solubility and dosages.1,14,15 However, the entry of ILs into the biosciences has been delayed mainly because of the toxicity of the counterions.1 Most recent communications and reviews refer to the toxicity and activity of ILs against microorganism and cell cultures, especially their antimicrobial activity as well as drug delivery performance.1,15–18 ILs have recently been tested in the fight against multi-drug resistance18,19 and even against microbial biofilms, showing a broad and powerful spectrum of activities against several microbial pathogens, including Methicillin-resistant Staphylococcus aureus. The recent outbreak of E. coli O104 (ref. 20 and 21) in Germany as well as the appearance of multi-drug-resistant organisms such as Gram-negative Enterobacteriaceae given by the New Delhi metallo β-lactamase22–24 are becoming increasingly serious public health problems worldwide. Thus, the discovery of alternative and efficient pathways for the treatment of infections is one of the most urgent challenges of this century. Novel therapies using ILs as a drug delivery device1 offer interesting avenues for exploration.
From the pharmaceutical point of view, the possibility of eliminating the negative side effects of a given active compound by delivering it as an IL–API is extremely attractive. Taking advantage of the IL properties, the counter-ion can be meticulously selected in order to minimize those undesirable side effects or to open up novel treatment therapies in which two active ions are paired.
Results and discussion
This work intended to develop an efficient, synthetic methodology for preparing several ILs–APIs based on ampicillin [Amp]. Ampicillin has always been used as an anion combined with the following organic cations: 1-ethyl-3-methylimidazolium [EMIM], 1-hydroxy-ethyl-3-methylimidazolium [C2OHMIM], choline [cholin], tetraethylammonium [TEA], cetylpyridinium [C16pyr] and trihexyltetradecylphosphonium [P6,6,6,14]. These anions were chosen due to their low toxicity, except in the case of [P6,6,6,14] which was chosen to ensure that an IL–API in the liquid form could be obtained. The combination of the adequate anion or cation with a specific drug can lead to an alteration in the compound's biopharmaceutical drug classification25 as well as its drug formulation process.The most conventional synthetic preparation of ILs involves a metathesis reaction of an anion halide with an adequate alkaline salt and this was also used in the preparation of some bulky imidazolium and pyridinium ILs containing an ampicillin anion.26 The pure IL can be obtained by eliminating undesirable inorganic salts (mainly sodium, potassium or lithium chloride or bromide) using precipitation followed by filtration.27 The need to obtain pure ILs, especially halide-free ones, has been one of the central concerns within the IL community. The use of inorganic acids instead of salts is a potential approach to reducing these inorganic contaminations.28 In the case of a large number of inorganic or organic anions, alternatives need to be considered, due to the fact that an anion exchange by weaker acids than hydrohalic acids29 cannot be efficiently performed. In the case of imidazolium cations, Earle and Seddon proposed the use of imidazole carbenes30 as strong bases. Nevertheless, this process is restricted to imidazolium cations, due to the high reactivity and low stability of the carbene intermediate.28
Ion exchange resin methods recently developed by Ohno et al.31 are being successfully used as alternative anion exchange processes and they have been extended to other reactions. Amberlite resin (in the OH form) has been used in order to exchange halides (bromide or chloride) to the hydroxide form and then this basic solution is neutralized by the addition of an adequate acid solution. The acid–base reaction yields the desired salt or IL.28,29,31 Our first attempt to use this anion exchange method failed due to ampicillin's poor solubility in most common organic solvents (with the exception of DMSO), as well as the instability of the β-lactam ring in the presence of strong bases. The decomposition of ampicillin was always detected by NMR analysis after several attempts.
The initial synthetic procedure was modified by dissolving ampicillin in a moderately basic ammonia solution and then neutralizing it with different hydroxides prepared with the Ohno method. Using an ammonia solution buffer (pH = 11.6), the hydrolysis of the sensitive β-lactam ring by a possible hydroxide attack was avoided. Pure ILs–APIs based on ampicillin structure were obtained after eliminating the excess ammonia and/or ampicillin by evaporation and crystallization respectively. The organic cations were selected from substituted ammonium, phosphonium, pyridinium and methylimidazolium salts which were first transformed into hydroxides by the use of an ionic exchange column (Amberlite IRA-400 OH) in methanol.31 The prepared hydroxides were then neutralized with the β-lactam antibiotic (Scheme 1).
 | ||
| Scheme 1 Schematic synthetic procedure for the preparation of ampicillin-based ILs. | ||
The structures of the anions and the cations synthesised and studied in this work appear in Fig. 1.
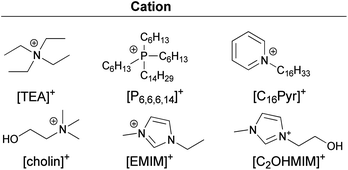 | ||
| Fig. 1 Structure of cations used. | ||
All isolated products were completely characterized by 1H and 13C NMR, FTIR and ESI mass spectra in order to check their expected structures and final purities. NMR studies also elucidate the expected cation/anion correlations by a quantitative integration of their characteristic 1H resonance peaks. Experimental and characterization details about prepared ILs–APIs are included as the ESI†.
Physical properties
Table 1 summarizes some properties of the synthesized ILs–APIs based on ampicillin. Our optimized synthetic procedure allowed us to obtain pure compounds in high yields (75 to 95%). All compounds are soluble in methanol, ethanol and water (except [P6,6,6,14][Amp]). It is important to emphasize that the use of appropriate ammonium and imidazolium cations can effectively change the initial trihydrate ampicillin water solubility (6 mg mL−1) and consequently its potential membrane permeability.| Compound | Yield (%)a | [α]27Db/° | Solubility | |
|---|---|---|---|---|
| Misciblec | Immisciblec | |||
| a Isolated yields. b Optical rotation values measured in methanol (2 mg mL−1) by polarimetry at 27 °C. c Observed complete solubilisation (miscible), partial solubilisation (pm) or non-solubilisation (immiscible) by adding solvent to a small amount of ionic liquid, MeOH (methanol), EtOH (ethanol), iPrOH (iso-propanol), Ac (acetone) and AcOEt (ethyl acetate). d The water content of [P6,6,6,14][Amp] was 14.7 ppm (determined by Karl Fisher titration). | ||||
| [TEA][Amp] | 76.0 | +48.7 ± 2.5 | MeOH, EtOH, iPrOH, H2O, | Ac, AcOEt(pm) |
| [P6,6,6,14][Amp]d | 80.0 | +23.3 ± 1.5 | MeOH, EtOH, iPrOH, Ac, AcOEt | H2O |
| [C16Pyr][Amp] | 76.4 | +51.7 ± 0.9 | MeOH, EtOH, iPrOH | Ac, AcOEt, H2O(pm) |
| [Cholin][Amp] | 70.7 | +52.3 ± 0.8 | MeOH, EtOH, H2O | iPrOH, Ac, AcOEt |
| [EMIM][Amp] | 94.6 | +89.3 ± 5.5 | MeOH, EtOH, iPrOH, H2O | Ac, AcOEt(pm) |
| [C2OHMIM][Amp] | 86.8 | +86.3 ± 4.5 | MeOH, EtOH, H2O | iPrOH(pm), Ac, AcOEt(pm) |
[Cholin][Amp] and [C2OHMIM][Amp] are immiscible in isopropanol contrary to other ILs–APIs. [P6,6,6,14][Amp] is the only IL–API completely miscible in acetone and ethyl acetate.
Optical rotation values of the prepared ILs–APIs based on ampicillin (+23.3° ± 1.5 to +89.3° ± 4.5 in methanol, Table 1) are significantly lower compared with the initial trihydrate ampicillin (+163.0° ± 2.0 in water) but are of the same order of magnitude as commercial sodium ampicillin (+40.0° ± 4.0). The highest optical rotations of the prepared compounds were observed when imidazolium cation structures were used.
Thermal properties
The thermal properties of synthesized ILs–APIs based on ampicillin are summarized in Table 2.| Compound | Physical state | T m /°C | T g /°C | T dec /°C |
|---|---|---|---|---|
| a Melting temperature (Tm) was determined by a melting point apparatus (Stuart Scientific). b Glass transition temperature (Tg) was determined by DSC measurements at a heating/cooling rate of 1 °C min−1 for all ILs. c Decomposition temperature (Tdec) was determined by TGA studies. | ||||
| [TEA][Amp] | Pale yellow solid | 79.0 | −18.64 | 214.75 |
| [P6,6,6,14][Amp] | Yellow viscous liquid | — | — | 297.65 |
| [C16Pyr][Amp] | Pale yellow solid | 86.0 | −19.64 | 269.39 |
| [Cholin][Amp] | Pale yellow solid | 58.0 | −20.12 | 221.29 |
| [EMIM][Amp] | Pale yellow solid | 72.0 | −17.86 | 239.64 |
| [C2OHMIM][Amp] | Pale yellow solid | 117.0 | −20.84 | 246.40 |
All the ILs–APIs were obtained as pale yellow solids (melting temperature (Tm) between 58 °C and 86 °C) except in the case of [P6,6,6,14][Amp] (viscous yellow liquid). [C2OHMIM][Amp] was obtained as a yellow molten salt (Tm = 117 °C). Particularly relevant is the successful reduction of the initial melting point of commercial ampicillin or sodium ampicillin (higher than 300 °C) by the appropriate selection of organic cations. The glass transition temperature (Tg) was determined at a heating/cooling rate of 1 °C min−1 for all compounds. Similar values of glass transition temperatures were detected for all ILs–APIs (−17.86 °C to −20.84 °C) except in the case of [P6,6,6,14][Amp].
In the case of [P6,6,6,14][Amp], DSC curves showed two transition peaks at low temperature which can be attributed to crystallization (−80.07 °C) and melting (−23.01 °C) processes, respectively.
Decomposition studies were performed by TGA analysis for all the synthesized compounds. As expected, these studies indicated that the selection of the organic cation influences the thermal stability of ILs–APIs based on ampicillin. [P6,6,6,14][Amp] and [C16Pyr][Amp] presented higher thermal stability than those ILs–APIs based on imidazolium and ammonium cations.
NMR studies
A preliminary 1H NMR study was performed in the case of IL [cholin][Amp] for different temperatures (25 °C to 85 °C). Fig. 2 illustrates a comparative 1H NMR study of [cholin][Amp] at four temperatures (25, 45, 65 and 85 °C) in d-DMSO. Two different regions of the 1H NMR spectra between 1 to 2 ppm and 6.5 to 8.8 ppm were particularly selected to check the aliphatic (methyl peaks) and aromatic (phenyl peaks) signals of the ampicillin anion, respectively.![Comparative 1H NMR study of [cholin][Amp] for four temperatures (25, 45, 65 and 85 °C) and two NMR regions (1 to 2 ppm and 6.5 to 8.8 ppm) in DMSO.](/image/article/2012/MD/c2md00269h/c2md00269h-f2.gif) | ||
| Fig. 2 Comparative 1H NMR study of [cholin][Amp] for four temperatures (25, 45, 65 and 85 °C) and two NMR regions (1 to 2 ppm and 6.5 to 8.8 ppm) in DMSO. | ||
In the case of two methyl peaks (1.10 and 1.41 ppm) of ampicillin, a chemical shift to the left was observed between 25 °C and 85 °C. This variation of the chemical shift (at least 0.04 ppm of difference between 25 °C and 85 °C) can be explained by the possible interaction of the carboxylate group of the ampicillin anion with the choline cation.
In the case of the aromatic peaks from ampicillin (7.20 to 7.50), no significant variation of chemical shift was observed, indicating that the phenyl ring of ampicillin does not interact with the choline cation.
Conclusions
The present work reports a new and efficient method for the synthesis of β-lactam antibiotics like ampicillin, which may prove useful for the development of new bioactive materials1 (antiseptics and anti-biofilm, for example) or to reduce drug resistance in microorganisms. With the careful selection of the organic cation, it is possible to provoke important physical and thermal properties of ILs–APIs based on ampicillin, such as water solubility, melting point and thermal stability. [Cholin][Amp] is the most interesting example of a prepared API–IL in what concerns its particular IL properties – low melting point (see Table 2), very high water solubility, as well as the biocompatibility and low toxicity of the choline cation and therefore probably will be most useful and interesting in further medicinal investigation.Acknowledgements
The authors acknowledge the Fundação para a Ciência e Tecnologia for its support through the Projects PEst-C/EQB/LA0006/2011, PTDC/EQU-EPR/104554/2008 and the grant SFRH/BPD/65981/2009. The NMR spectrometers are part of the National NMR Network (RNRMN) and are funded by Fundação para a Ciência e a Tecnologia (FCT).Notes and references
- R. Ferraz, L. C. Branco, C. Prudêncio, J. P. Noronha and Ž. Petrovski, ChemMedChem, 2011, 6, 975–985 CrossRef CAS
.
- T. Torimoto, T. Tsuda, K. Okazaki and S. Kuwabata, Adv. Mater., 2010, 22, 1196–1221 CrossRef CAS
.
- P. S. Kulkarni, L. C. Branco, J. G. Crespo, M. C. Nunes, A. Raymundo and C. A. M. Afonso, Chem.–Eur. J., 2007, 13, 8478–8488 CrossRef CAS
.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef
.
-
P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis (Green Chemistry (Wiley)(2 vol. set)), Wiley-VCH, 2007 Search PubMed
.
- M. J. Earle, J. M. S. S. Esperanca, M. A. Gilea, J. N. Canongia Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS
.
- Y. Ito and T. Nohira, Electrochim. Acta, 2000, 45, 2611–2622 CrossRef CAS
.
- J. Dupont and P. A. Z. Suarez, Phys. Chem. Chem. Phys., 2006, 8, 2441–2452 RSC
.
- F. Endres and S. Zein El Abedin, Phys. Chem. Chem. Phys., 2006, 8, 2101–2116 RSC
.
- J. L. Anderson, D. W. Armstrong and G. T. Wei, Anal. Chem., 2006, 78, 2892–2902 CrossRef
.
- D. R. MacFarlane and K. R. Seddon, Aust. J. Chem., 2007, 60, 3–5 CrossRef CAS
.
- F. van Rantwijk and R. A. Sheldon, Chem. Rev., 2007, 107, 2757–2785 CrossRef CAS
.
- W. L. Hough, M. Smiglak, H. Rodriguez, R. P. Swatloski, S. K. Spear, D. T. Daly, J. Pernak, J. E. Grisel, R. D. Carliss, M. D. Soutullo, J. H. Davis and R. D. Rogers, New J. Chem., 2007, 31, 1429–1436 RSC
.
- K. Bica, C. Rijksen, M. Nieuwenhuyzen and R. D. Rogers, Phys. Chem. Chem. Phys., 2010, 12, 2011–2017 RSC
.
- J. Stoimenovski, D. R. MacFarlane, K. Bica and R. D. Rogers, Pharm. Res., 2010, 27, 521–526 CrossRef CAS
.
- W. L. Hough-Troutman, M. Smiglak, S. Griffin, W. M. Reichert, I. Mirska, J. Jodynis-Liebert, T. Adamska, J. Nawrot, M. Stasiewicz, R. D. Rogers and J. Pernak, New J. Chem., 2009, 33, 26–33 RSC
.
- D. Demberelnyamba, K. S. Kim, S. J. Choi, S. Y. Park, H. Lee, C. J. Kim and I. D. Yoo, Bioorg. Med. Chem., 2004, 12, 853–857 CrossRef CAS
.
- L. Carson, P. K. W. Chau, M. J. Earle, M. A. Gilea, B. F. Gilmore, S. P. Gorman, M. T. McCann and K. R. Seddon, Green Chem., 2009, 11, 492–497 RSC
.
- N. Iwai, K. Nakayama and T. Kitazume, Bioorg. Med. Chem. Lett., 2011, 21, 1728–1730 CrossRef CAS
.
- M. Bielaszewska, A. Mellmann, W. Zhang, R. Köck, A. Fruth, A. Bauwens, G. Peters and H. Karch, Lancet Infect. Dis., 2011, 11, 671–676 CAS
.
- H. Pennington, Lancet Infect. Dis., 2011, 11, 652–653 CrossRef
.
- S. H. Tseng, C. M. Lee, T. Y. Lin, S. C. Chang and F. Y. Chang, J. Microbiol., Immunol. Infect., 2011, 44, 157–165 CrossRef
.
- K. K. Kumarasamy, M. A. Toleman, T. R. Walsh, J. Bagaria, F. Butt, R. Balakrishnan, U. Chaudhary, M. Doumith, C. G. Giske, S. Irfan, P. Krishnan, A. V. Kumar, S. Maharjan, S. Mushtaq, T. Noorie, D. L. Paterson, A. Pearson, C. Perry, R. Pike, B. Rao, U. Ray, J. B. Sarma, M. Sharma, E. Sheridan, M. A. Thirunarayan, J. Turton, S. Upadhyay, M. Warner, W. Welfare, D. M. Livermore and N. Woodford, Lancet Infect. Dis., 2010, 10, 597–602 CrossRef CAS
.
- A. B. Mochon, O. B. Garner, J. A. Hindler, P. Krogstad, K. W. Ward, M. A. Lewinski, J. K. Rasheed, K. F. Anderson, B. M. Limbago and R. M. Humphries, J. Clin. Microbiol., 2011, 49, 2386 CrossRef
.
- G. L. Amidon, H. Lennernäs, V. P. Shah and J. R. Crison, Pharm. Res., 1995, 12, 413–420 CrossRef CAS
.
- M. R. Cole, M. Li, B. El-Zahab, M. E. Janes, D. Hayes and I. M. Warner, Chem. Biol. Drug Des., 2011, 78, 33–41 CAS
.
- E. Alcalde, I. Dinares, A. Ibanez and N. Mesquida, Chem. Commun., 2011, 47, 3266–3268 RSC
.
- I. Dinares, C. G. de Miguel, A. Ibanez, N. Mesquida and E. Alcalde, Green Chem., 2009, 11, 1507–1510 RSC
.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9, 1155–1157 RSC
.
- J. M. Earle and K. R. Seddon, US Pat., 6939974, 2005.
- J. K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398–2399 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2md00269h |
| This journal is © The Royal Society of Chemistry 2012 |
