Synthesis, nano-features, ex vivo anti-platelet aggregation and in vivo antithrombotic activities of poly-α,β-DL-aspartyl-L-arginine
Lin
Gui
a,
Ming
Zhao
*a,
Yuji
Wang
a,
Yinye
Wang
b,
Yang
Qin
a,
Li
Li
a and
Shiqi
Peng
*a
aCollege of Pharmaceutical Sciences, Capital Medical University, Beijing 100069, P.R. China. E-mail: mzhao@mail.bjum.edu.cn; sqpeng@mail.bjum.edu.cn; Tel: +86-10-8391-1528; Fax: +86-10-8391-1528; Tel: +86 10 83911535; Fax: +86 10 83911535
bCollege of Pharmaceutical Sciences, Peking University, Beijing 100083, P.R. China
First published on 26th October 2011
Abstract
In the nanoscale assembly-based design of antithrombotic agents, poly-α,β-DL-aspartyl-L-arginine (MW: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741) was prepared from the thermal polycondensation of DL-aspartic acid and the amidation of polysuccimide with L-arginine. In the pH environments corresponding to the stomach (pH 1.2), intestinal tract (pH 7.6), blood and tissue fluids (pH 7.4) 4.8 × 104,2,−4,−6,−8 nM of poly-α,β-DL-aspartyl-L-arginine assembled to form diverse nano-species. The sizes of the smallest nanoparticle, nanobell and nanomango were less than 100 nm. At the oral doses of 0.72, 1.44 and 2.89 μmol kg−1, poly-α,β-DL-aspartyl-L-arginine dose-dependently inhibited the ex vivo platelet aggregation and the in vivo thrombosis of the treated rats. By assembling to form diverse nano-species, the absorption of oral poly-α,β-DL-aspartyl-L-arginine in the stomach and intestinal tract could be assisted.
741) was prepared from the thermal polycondensation of DL-aspartic acid and the amidation of polysuccimide with L-arginine. In the pH environments corresponding to the stomach (pH 1.2), intestinal tract (pH 7.6), blood and tissue fluids (pH 7.4) 4.8 × 104,2,−4,−6,−8 nM of poly-α,β-DL-aspartyl-L-arginine assembled to form diverse nano-species. The sizes of the smallest nanoparticle, nanobell and nanomango were less than 100 nm. At the oral doses of 0.72, 1.44 and 2.89 μmol kg−1, poly-α,β-DL-aspartyl-L-arginine dose-dependently inhibited the ex vivo platelet aggregation and the in vivo thrombosis of the treated rats. By assembling to form diverse nano-species, the absorption of oral poly-α,β-DL-aspartyl-L-arginine in the stomach and intestinal tract could be assisted.
1. Introduction
α,β-Polyaspartic acid is used in a variety of fields for preparing a series of materials, such as superabsorbent hydrogels,1 a complex for 5-fluorouracil delivery,2 a vehicle of protein drug delivery,3 a promoter of octacalcium phosphate crystal growth,4 an inhibitor of the synthesis of octacalcium phosphate in aqueous medium,5,6 a chemical sand-fixing agent,7 a component of fusion proteins,8 a component for the surface of magnetite nanoparticles,9 a colloidal stabilizer of barium titanate aqueous suspensions,10 an analogue of naturally occurring soluble acidic proteins involved in biomineralization processes,11 a complexing agent in the removal of heavy metal ions,12 a control macromolecule in the growth of thin films of calcium carbonate polymorphs,13 a chelating agent for the dissolution of calcium salt deposits,14 an anionic component for layer-by-layer (LBL) assembly,15 and a polymer for generating demineralized eggshell membrane.16 These examples imply that the correlation of the nanoscale assembly and bioactivity represent a major advance in α,β-polyaspartic acid.On the other hand, the derivatives of α,β-polyaspartic acid have been reported to possess a number of important bioactivities. For instance poly-α,β-DL-aspartic acid 3-aminopropylamide showed antibacterial, antifungal and anticancer activities.17 Poly-β-L-aspartyl-L-arginine (mean MW, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) is also able to protect endothelial cells from injury,18 enhance prostacyclin synthesis in rat aortic endothelial cells19 and inhibit thrombosis of rats.20 However, the correlation of nanoscale assembly and bioactivity was not explored for these derivatives. To gain insight into the relationship of the possible nanoscale assembly and bioactivity, in the present paper we describe the preparation of a novel α,β-polyaspartic acid derivative, poly-α,β-DL-aspartyl-L-arginine, and measured its nano-features. After a series of pharmacological screenings, including antibacterial, antifungal, anticancer and antithrombotic actions, the pharmacological evaluation of poly-α,β-DL-aspartyl-L-arginine was focused on the ex vitro anti-platelet aggregation and the in vivo antithrombotic activities.
200) is also able to protect endothelial cells from injury,18 enhance prostacyclin synthesis in rat aortic endothelial cells19 and inhibit thrombosis of rats.20 However, the correlation of nanoscale assembly and bioactivity was not explored for these derivatives. To gain insight into the relationship of the possible nanoscale assembly and bioactivity, in the present paper we describe the preparation of a novel α,β-polyaspartic acid derivative, poly-α,β-DL-aspartyl-L-arginine, and measured its nano-features. After a series of pharmacological screenings, including antibacterial, antifungal, anticancer and antithrombotic actions, the pharmacological evaluation of poly-α,β-DL-aspartyl-L-arginine was focused on the ex vitro anti-platelet aggregation and the in vivo antithrombotic activities.
2. Materials
2.1. General
DL-Asp and L-Arg were purchased from Sigma Chemical Co. Chromatography was performed on Qingdao silica gel H. The purity of the intermediates and the products was confirmed by thin layer chromatography (TLC, Merck silica gel plates of type 60 F254, 0.25 mm layer thickness). The purity of poly-α,β-DL-aspartyl-L-arginine was determined by HPLC (Waters, C18 column 2.1 mm × 150 mm) using a UV detector. The retention time of poly-α,β-DL-aspartyl-L-arginine was 9.4 min, on analytical HPLC eluted with 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v MeOH/H2O at a 0.2 ml min−1 flow rate. The purity of poly-α,β-DL-aspartyl-L-arginine is 96%. Elemental analysis was carried out on a Perkin-Elmer 2400C instrument. 1H NMR spectra were recorded by Bruker Advance 500 spectrometers. IR spectra were recorded with a Perkin-Elmer 983 instrument. Amino acid analysis was carried out on a Sykam S433. For platelet counts and platelet aggregation, a Chrono-Log 490-D Optical Aggregometer (Havertown, PA, USA) was used. The statistical analysis of all the biological data was carried out by use of ANOVA test with p < 0.05 as significant cut-off.
20 v/v MeOH/H2O at a 0.2 ml min−1 flow rate. The purity of poly-α,β-DL-aspartyl-L-arginine is 96%. Elemental analysis was carried out on a Perkin-Elmer 2400C instrument. 1H NMR spectra were recorded by Bruker Advance 500 spectrometers. IR spectra were recorded with a Perkin-Elmer 983 instrument. Amino acid analysis was carried out on a Sykam S433. For platelet counts and platelet aggregation, a Chrono-Log 490-D Optical Aggregometer (Havertown, PA, USA) was used. The statistical analysis of all the biological data was carried out by use of ANOVA test with p < 0.05 as significant cut-off.
2.2. Preparing polysuccimide
2.2.1. 30 g (0.23 mol) of finely powdered DL-Asp was heated at 200 °C under vacuum (0.1–0.3 mm) for 120 h. The resulting tawny powders were triturated with saturated sodium bicarbonate solution (3 × 100 mL), the granular product was filtered and successively washed with water (5 × 100 mL), 1% hydrochloric acid (3 × 100 mL) and water (10 × 100 mL) to provide 16.5 g of polysuccimide as a yellowish powder. Its elemental analysis gave C: 48.70%, H: 3.64% and N: 14.22%.2.2.2. A suspension of 10 g (0.07 mol) of finely powdered DL-Asp in 50 mL of tetrahydronaphthalene was heated at 200 °C under vacuum (0.1–0.3 mm) for 100 h until no azeotropically distilling water was observed. The resulting tawny powders were triturated with ether (5 × 50 mL), and then successively washed with water (5 × 50 mL), 1% hydrochloric acid (3 × 50 mL) and water (10 × 50 mL) to provide 6.9 g of polysuccimide as a yellowish powder. Its elemental analysis gave C: 48.44%, H: 3.70% and N: 14.00%.
2.2.3. A syrup of 30 g (0.23 mol) of DL-Asp in 20 mL of phosphoric acid (85%) was heated at 180 °C under vacuum (0.1–0.3 mm) for 10 h. The resulting tawny powders were filtered and successively washed with water (5 × 100 mL), 1% hydrochloric acid (3 × 100 mL) and water (10 × 100 mL) to provide 24.2 g of polysuccimide as a yellowish powder. Its elemental analysis gave C: 48.60%, H: 3.62% and N: 14.20%.
2.3. Preparing poly-α,β-DL-aspartic acid
A solution of 0.97 g (10 mmole) of polysuccimide in 150 mL of sodium hydroxide (0.1 N) was stirred at room temperature for 1 h. This solution was neutralized with hydrochloric acid (0.1 N). With acetic acid (20%), the resulting solution was adjusted to pH 5.5 and heated to 50 °C. To this stirring warm solution a saturated solution of cupric acetate was added until precipitation occurred. The precipitates of the copper salt of poly-α,β-DL-aspartic acid were collected by filtration and suspended in 50 ml of water. To this suspension, hydrogen sulfide was added to provide a colorless solution. After filtration, the filtrate was evaporated under vacuum to 1 mL and dialyzed against ten 50 ml. portions of water over a period of 90 h. The dialyzing solution was evaporated under vacuum to 1 mL and lyophilized to provide 70 mg of poly-α,β-DL-aspartic acid.2.4. Preparing poly-α,β-DL-aspartyl-L-arginine
A suspension of 7.5 g (0.7 mmol) of polysuccimide and 14.4 g (82.6 mmol) of L-Arg in 40 ml of distilled water was stirred at room temperature for 8 h. After filtration, the filtrate was evaporated under vacuum. The residue was dissolved in 5 mL of distilled water, purified on a column of Sephadex G10 and the fraction was lyophilized to provide 7.1 g of powdery yellow poly-α,β-DL-aspartyl-L-arginine. Its purity was determined by HPLC (Waters, C18 column 2.1 mm × 150 mm) with a UV detector, eluted with 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v MeOH/H2O at a 0.2 ml min−1 flow rate. The HPLC analysis gave poly-α,β-DL-aspartyl-L-arginine a retention time of 9.4 min and 96% purity.
20 v/v MeOH/H2O at a 0.2 ml min−1 flow rate. The HPLC analysis gave poly-α,β-DL-aspartyl-L-arginine a retention time of 9.4 min and 96% purity.
2.5. Ex vivo anti-platelet aggregation assay of poly-α,β-DL-aspartyl-L-arginine
The assessments described here were performed based on a protocol reviewed and approved by the ethics committee of Capital Medical University. The committee assures the welfare of the animals was maintained in accordance to the requirements of the Animal Welfare Act and according to the guide for care and use of laboratory animals. Male Wister rats (purchased from Animal Center of Peking University) weighing 330–355 g were used after overnight fasting. The rats were administered orally with control (NS), poly-α,β-DL-aspartyl-L-arginine (0.72, 1.44 or 2.89 μmol kg−1) or aspirin (167 μmol kg−1). After 90 min, the rats were anesthetized with urethane (1.5 g kg−1 i.p.) and blood was drawn from the abdominal aorta into a syringe containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 volume of 3.8% sodium citrate. The platelet-rich plasma (PRP) was prepared by centrifugation of the blood at 120 × g for 15 min and at 850 × g for 15 min to prepare the platelet-poor plasma (PPP). The platelet concentration was adjusted to 2 × 109 ml−1 with PPP. The platelet aggregation was measured with a two-channel Chronolog aggregometer (Chrono-log model 490 optical aggregometer). ADP, collagen or thrombin were used as inducer, and the final concentrations of them were 10 μM, 100 μg protein/ml and 0.1 IU ml−1, respectively.
10 volume of 3.8% sodium citrate. The platelet-rich plasma (PRP) was prepared by centrifugation of the blood at 120 × g for 15 min and at 850 × g for 15 min to prepare the platelet-poor plasma (PPP). The platelet concentration was adjusted to 2 × 109 ml−1 with PPP. The platelet aggregation was measured with a two-channel Chronolog aggregometer (Chrono-log model 490 optical aggregometer). ADP, collagen or thrombin were used as inducer, and the final concentrations of them were 10 μM, 100 μg protein/ml and 0.1 IU ml−1, respectively.
2.6. In vivo antithrombotic activity assay of poly-α,β-DL-aspartyl-L-arginine
Aspirin (165 μmol kg−1) or poly-α,β-DL-aspartyl-L-arginine (0.72, 1.45 or 2.89 μmol kg−1) were dissolved in NS before administration and kept in an ice bath. Male Wister rats weighing 270–330 g (purchased from Animal Center of Peking University) were used. The rats were anesthetized with pentobarbital sodium (80.0 mg kg−1, i.p.) and the right carotid artery and left jugular vein were separated. A weighed 6 cm thread was inserted into the middle of a polyethylene tube. The polyethylene tube was filled with heparin sodium (50 IU ml−1 in NS) and one end was inserted into the left jugular vein. From the other end of the polyethylene tube heparin sodium was injected as anticoagulant, then NS or poly-α,β-DL-aspartyl-L-arginine were injected, and this end was inserted into the right carotid artery. Blood was allowed to flow from the right carotid artery to the left jugular vein through the polyethylene tube for 15 min. The thread was removed to obtain the weight of the wet thrombus.2.7. Animals
Male Wister rats and male ICR mice were purchased from Animal Center of Peking University. The described assessments were performed based on a protocol reviewed and approved by the ethics committee of Capital Medical University. The committee assures the welfare of the animals was maintained in accordance to the requirements of the Animal Welfare Act and according to the guide for care and use of laboratory animals.3. Results
3.1. Synthesis of poly-α,β-DL-aspartyl-L-arginine
As indicated in Fig. 1, poly-α,β-DL-aspartyl-L-arginine was prepared via the thermal polycondensation of DL-aspartic acid and the amidation of polysuccimide with L-arginine. The thermal polycondensation of DL-aspartic acid was carried out by following three procedures reported in the literature,1,21,22i.e. heating DL-aspartic acid at 200 °C under vacuum (0.1 mm) for 120 h, or heating a syrup of DL-aspartic acid in phosphoric acid (85%) at 180 °C under vacuum for 2.5 h, or azeotropically distilling a suspension of DL-aspartic acid in tetrahydronaphthalene for 100 h. The yields of polysuccimide from these polycondensations range from 63% to 93%. With stirring in aqueous sodium hydroxide (0.5 N) 1 h, polysuccimide was converted to poly-α,β-DL-aspartic acid in 90% yield. The amidation of polysuccimide and L-Arg provided 7.1 g (66%) of poly-α,β-DL-aspartyl-L-arginine. Its purity was determined by HPLC (Waters, C18 column 2.1 × 150 mm) with UV detection, eluted with 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 v/v MeOH/H2O at a 0.2 ml min−1 flow rate. The HPLC analysis gave poly-α,β-DL-aspartyl-L-arginine a retention time of 9.4 min and 97% purity. The 1H NMR analysis showed specific chemical shifts related to CO2H (δ = 11.3 ppm), CONH (δ = 8.1–8.5 ppm), α-CH of the aspartic acid residue (δ = 4.7–4.9 ppm), α-CH of the arginine residue (δ = 4.4–4.5 ppm), and CH2NHC(NH)NH2·2HCl (δ = 8.6–8.9 ppm, 8.2–8.4 ppm and 6.3–6.5 ppm). The IR showed specific peaks related to NH (3411 cm−1) and CO2H (2300–3000 cm−1). Amino acid analysis gave Asp/Arg = 1.1/0.9.
88 v/v MeOH/H2O at a 0.2 ml min−1 flow rate. The HPLC analysis gave poly-α,β-DL-aspartyl-L-arginine a retention time of 9.4 min and 97% purity. The 1H NMR analysis showed specific chemical shifts related to CO2H (δ = 11.3 ppm), CONH (δ = 8.1–8.5 ppm), α-CH of the aspartic acid residue (δ = 4.7–4.9 ppm), α-CH of the arginine residue (δ = 4.4–4.5 ppm), and CH2NHC(NH)NH2·2HCl (δ = 8.6–8.9 ppm, 8.2–8.4 ppm and 6.3–6.5 ppm). The IR showed specific peaks related to NH (3411 cm−1) and CO2H (2300–3000 cm−1). Amino acid analysis gave Asp/Arg = 1.1/0.9.
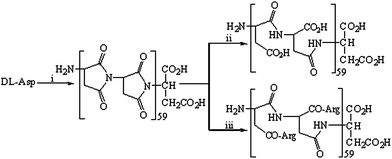 | ||
| Fig. 1 Synthetic route for poly-α,β-DL-aspartyl-L-arginine. (i) At 200 °C, or at 180 °C under vacuum in H3PO4 (85%), or azeotropic distillation in tetrahydronaphthalene; (ii) aqueous NaOH (2 N); (iii) L-arginine. | ||
3.2. Particle sizes of poly-α,β-DL-aspartyl-L-arginine in various conditions
The mean size and half-peak width of the assembled particles of poly-α,β-DL-aspartyl-L-arginine in water (pH 6.7) were recorded every 30 s for 5 min on a Malvern's Zeta Sizer (Nano-ZS90) with the DTS (Nano) Program, and the data are shown with Fig. 2. It was found that the particle diameter of poly-α,β-DL-aspartyl-L-arginine depended on temperature. For instance, at 4.8 × 10−2 nM the particle diameters at 25, 37 and 50 °C were 381, 192 and 122 nm, respectively. Additionally, the particle diameter of poly-α,β-DL-aspartyl-L-arginine depended on concentration at constant temperature. For instance, at 37 °C its particle diameters at 4.8 μM, 4.8 × 10−2 nM, 4.8 × 10−8 nM and 4.8 × 10−10 nM were 123, 192, 270 and 283 nm, respectively. | ||
| Fig. 2 Particle sizes of poly-α,β-DL-aspartyl-L-arginine in various conditions and temperatures. | ||
3.3. TEM images of poly-α,β-DL-aspartyl-L-arginine in various pH environment
To visualize the morphological features of poly-α,β-DL-aspartyl-L-arginine in the pH environment of the stomach, intestinal tract and blood or tissue fluid, transmission electron microscopy (TEM) was employed on copper micro grid substrates for pH 1.2, 7.6 and 7.4 aqueous poly-α,β-DL-aspartyl-L-arginine (4.8 × 10−8 nM). The TEM images are shown in Fig. 3–5. | ||
Fig. 3 TEM images of 4.8 × 10−8 nM poly-α,β-DL-aspartyl-L-arginine at pH 1.2; (A) nanoblocks, 64–100 nm in width and 100–250 nm in length; (B) nanorods, 64–96 nm in diameter and 184–360 nm in length; (C) nanoparticle of 5–16 nm in diameter and nanorod of 74 nm in diameter and 354 nm in length; A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 1.0 C = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0. 8.0. | ||
 | ||
Fig. 4 TEM images of 4.8 × 10−4 nM of poly-α,β-DL-aspartyl-L-arginine at pH 7.4; (A) nanobell of 79 nm in length; (B) nanoparticles, 91–205 nm in diameter; (C) nanorod, 200 nm in diameter and 833 nm in length. A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 7.5 C = 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8. 3.8. | ||
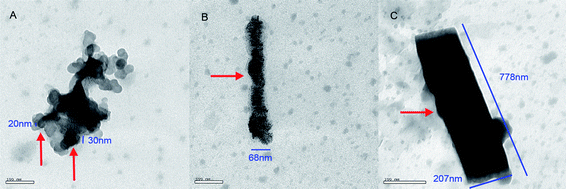 | ||
Fig. 5 TEM image of 4.8 × 10−4 nM of poly-α,β-DL-aspartyl-L-arginine at pH 7.6; (A) nanoparticle of 20–30 nm in diameter; (B) nanosausage, 68 nm in diameter and 500 nm in length; (C) nanorod, 207 nm in diameter and 778 nm in length. A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = 1.3 C = 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. 1.0. | ||
Fig. 3 shows that at the pH of the stomach (pH 1.2), the TEM test gives mixed images of nanoblocks of 64–100 nm in width and 100–250 nm in length, nanorods of 64–96 nm in diameter and 184–360 nm in length, as well as a mixture of nanoparticles of 5–16 nm in diameter and nanorod of 74 nm diameter and 354 nm in length. It should be noted that under these conditions, the nanoblocks were observed as the most abundant nano-species; a few nanorods were also observed, and only one nanorod accompanied by nanoparticles.
Fig. 4 shows that at the pH of blood (pH 7.4), the TEM test gives mixed images of the nanobells of 45–110 nm, of which a 79 nm in length nanobell is given as a representative here, nanoparticles of 91–205 nm in diameter as well as one nanorod of 200 nm in diameter and 833 nm in length.
Fig. 5 shows that at the pH of the intestinal tract (pH 7.6), the TEM test gives mixed images of nanoparticles of 20–30 nm in diameter, the nanosausage of 68 nm in diameter and 500 nm in length, as well as the nanorod of 207 nm in diameter and 778 nm in length.
3.4. TEM image of poly-α,β-DL-aspartyl-L-arginine at various concentrations
To visualize the effect of concentration on the morphological features, TEM was employed on copper micro grid substrates for 4.8 × 104,2,−4,−6,−8 nM aqueous poly-α,β-DL-aspartyl-L-arginine at pH 6.7. Fig. 6 and 7 show the corresponding images. | ||
Fig. 6 TEM image of 4.8 × 10−4 nM poly-α,β-DL-aspartyl-L-arginine at pH 6.7; (A) nanoparticles, 200 nm in diameter; (B) nanoparticles of 31–62 nm in diameter; (C) 750 nm nanonecklaces of pearls of 19–38 nm in diameter; (D) nanosausage, 300 nm in diameter and 2455 nm in length. A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = 2.5 D = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3 6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
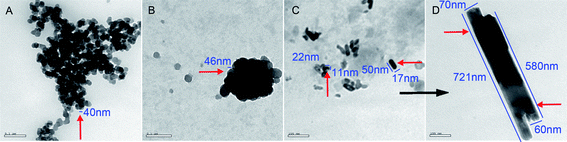 | ||
Fig. 7 TEM image of 4.8 × 10−6 nM of poly-α,β-DL-aspartyl-L-arginine at pH 6.7; (A) nanonecklaces of pearls of 40 nm in diameter; (B) nanoparticle of 46 nm in diameter; (C) nanorods, 11–17 nm in diameter and 22–50 nm in length; (D) nanopillars, 60–70 nm in diameter and 580–721 nm in length. A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = 1 D = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. 2. | ||
The TEM test of 4.8 × 104 nM poly-α,β-DL-aspartyl-L-arginine gives mixed images of a nanomango of 415 nm in diameter and 830 nm in length, as well as a nanobranch of 357 nm in diameter and 3.5 μm in length.
The TEM test of 4.8 × 102 nM poly-α,β-DL-aspartyl-L-arginine gives mixed images of nanoparticles of 30–37 nm in diameter, nanomangos of 11–178 in diameter and 44–367 nm in length as well as a nanowire of 4 nm in diameter and 571 nm in length.
Fig. 6 shows that the TEM test of 4.8 × 10−4 nM poly-α,β-DL-aspartyl-L-arginine gives mixed images of nanoparticles of 200 nm diameter, aggregates of nanoparticles of 31–62 nm diameter, nanonecklaces, 750 nm nanonecklaces of pearls of 19–27 nm in diameter, as well as the nanosausage of 386 nm in diameter and 2455 nm in length.
Fig. 7 shows that the TEM test of 4.8 × 10−6 nM of poly-α,β-DL-aspartyl-L-arginine gives mixed images of the nanonecklaces of pearls of 40 nm in diameter, a nanoparticle of 46 nm in diameter, nanorods of 11–17 nm in diameter and 22–50 nm in length, as well as nanopillars of 60–70 nm in diameter and 580–721 nm in length.
The TEM test of 4.8 × 10−8 nM poly-α,β-DL-aspartyl-L-arginine gives mixed images of nanoparticles of 6–18 nm in diameter, nanonecklaces of pearls of 54 nm in diameter, nanorods of 8–16 nm in diameter and 23–131 nm in length, as well as nanosausages of 21–36 nm in diameter and 86–136 nm in length.
3.5. Ex vivo anti-platelet aggregation of poly-α,β-DL-aspartyl-L-arginine
The ex vivo anti-platelet aggregation of poly-α,β-DL-aspartyl-L-arginine was evaluated by using a standard procedure of the antithrombotic assay23,24 with aspirin as the positive control and NS as the negative control. The data are shown in Fig. 8. As seen, 0.72, 1.45 or 2.89 μmol kg−1 of poly-α,β-DL-aspartyl-L-arginine significantly inhibited ADP-, collagen- and thrombin-induced platelet aggregation. Additionally, the efficacy of the anti-platelet aggregation progressively increases with increasing dose. Thus poly-α,β-DL-aspartyl-L-arginine shows dose-dependent ex vivo anti-platelet aggregation. | ||
| Fig. 8 Ex vivo anti-platelet aggregation activity of poly-α,β-DL-aspartyl-L-arginine (PDR).a (a) Asp = aspirin, n = 12; (b) compared to NS p < 0.01; (c) compared to NS and 1.45 μmol kg−1p < 0.01; (d) compared to NS p < 0.01, to 2.89 μmol kg−1p < 0.05. | ||
3.6. In vivo antithrombotic activity of oral poly-α,β-DL-aspartyl-L-arginine
The in vivo antithrombotic potency of oral poly-α,β-DL-aspartyl-L-arginine was evaluated on a rat antithrombotic model with aspirin as the positive control, NS as the negative control and the thrombus weight as the in vivo antithrombotic activity. The data are shown in Fig. 9. The thrombus weights of the rats orally receiving 0.72, 1.45 and 2.89 μmol kg−1 of poly-α,β-DL-aspartyl-L-arginine range from 23.00 to 16.83 mg and are significantly lower than that of the rats orally receiving NS (29.01 mg). Thus, poly-α,β-DL-aspartyl-L-arginine possesses antithrombotic activity. Additionally, with the increase of the dose, the thrombus weight is significantly decreased. Thus, poly-α,β-DL-aspartyl-L-arginine shows dose-dependent in vivo antithrombotic action. | ||
Fig. 9 Thrombus weight of poly-α,β-DL-aspartyl-L-arginine (PDR) treated rats.a (a) Asp = aspirin, NS = vehicle. Thrombus weight is represented by ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) ± SD mg, n = 12; (b) compared to NS p < 0.01; (c) compared to NS p < 0.01, to 0.72 μmol kg−1p < 0.05; (d) compared to NS p < 0.01, to 1.45 μmol kg−1p < 0.05. ± SD mg, n = 12; (b) compared to NS p < 0.01; (c) compared to NS p < 0.01, to 0.72 μmol kg−1p < 0.05; (d) compared to NS p < 0.01, to 1.45 μmol kg−1p < 0.05. | ||
4. Discussion
4.1. Molecular weight and purity of poly-α,β-DL-aspartyl-L-arginine
The HPLC analysis gave poly-α,β-DL-aspartyl-L-arginine a retention time of 9.4 min and 96% purity. The 1H NMR spectra showed specific chemical shifts related to CO2H (δ = 11.3 ppm), CONH (δ = 8.1–8.5 ppm), α-CH of the aspartic acid residue (δ = 4.7–4.9 ppm), α-CH of the arginine residue (δ = 4.4–4.5 ppm), and CH2NHC(NH)NH2·2HCl (δ = 8.6–8.9 ppm, 8.2–8.4 ppm and 6.3–6.5 ppm). The IR spectrum showed specific peaks related to NH (3411 cm−1) and CO2H (2300–3000 cm−1). Amino acid analysis carried out on Sykam S433 gave Asp/Arg = 1.1/0.9. Elemental analysis of polysuccimide gave values of C: 48.70%, H: 3.64% and N: 14.22%, which are close to the data in the literature. Top-MS gave a 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 672 for the mean molecular weight of polysuccimide, which was consistent with the reported value (11
672 for the mean molecular weight of polysuccimide, which was consistent with the reported value (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670) and n of polysuccimide, poly-α,β-DL-aspartic acid and poly-α,β-DL-aspartyl-L- arginine is 59.22 Top-MS gave 20
670) and n of polysuccimide, poly-α,β-DL-aspartic acid and poly-α,β-DL-aspartyl-L- arginine is 59.22 Top-MS gave 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 742 for the mean molecular weight of poly-α,β-DL-aspartyl-L-arginine, which was consistent with the calculated value (20
742 for the mean molecular weight of poly-α,β-DL-aspartyl-L-arginine, which was consistent with the calculated value (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741).
741).
4.2. Concentration- and temperature-dependent particle sizes of poly-α,β-DL-aspartyl-L-arginine
As indicated in Fig. 1, at a certain concentration the mean diameters of the particles of aqueous poly-α,β-DL-aspartyl-L-arginine significantly depended on temperature. For instance, at 4.8 × 10−2 nM the particle diameters at 25 °C, 37 °C and 50 °C were in the order 381 > 192 > 122 nm. Furthermore, the particle diameter of poly-α,β-DL-aspartyl-L-arginine significantly depended on its concentration. For instance, at 37 °C its particle diameters at 4.8 μM, 4.8 × 10−2 nM, 4.8 × 10−8 nM and 4.8 × 10−10 nM were in the order 123 < 192 < 270 < 283 nm. As mentioned above, poly-α,β-DL-aspartic acid derivatives are capable of self-assembly at the nanoscale, and this may be attributed to the hydrophobicity of the poly-α,β-DL-aspartyl residue. It is reasonable that the increases of both concentration and temperature could lead to stronger hydrophobic interaction and closer packing density.4.3. pH-dependent TEM images of poly-α,β-DL-aspartyl-L-arginine
At the pH of the stomach (pH 1.2), blood or tissue fluid (pH 7.4) and intestinal tract (pH 7.6), TEM tests of 4.8 × 10−4 nM aqueous poly-α,β-DL-aspartyl-L-arginine showed the coexistence of the nanoblock/nanorod/nanoparticle, nanobell/nanosausage/nanorod and nanoparticle/nanosausage/nanorod, respectively. The coexistence of various nano-species implies that in the stomach, intestinal tract, tissue fluid and blood, poly-α,β-DL-aspartyl-L-arginine-assembled nano-species are interconvertible. The smallest of the coexisting nano-species in the pH conditions of the stomach, blood and tissue fluid as well as intestinal tract are nanoparticles, nanobells and nanoparticles, respectively, while the diameters of the nanoparticles, the length of the nanobell and the diameters of the nanoparticles are 5–16 nm, 79 nm and 20–30 nm, respectively, less than 100 nm.4.4. Concentration-dependent TEM images of poly-α,β-DL-aspartyl-L-arginine
TEM images of aqueous poly-α,β-DL-aspartyl-L-arginine of 4.8 × 104,2,−4,−6,−8 nM show the coexistence of nanoparticle/nanomango/nanobranch, nanoparticle/aggregates of nanomango/nanowire, nanoparticles/nanoparticle/nanonecklaces/nanosausage, nanonecklaces/nanoparticle/nanorod/nanopillar, and nanoparticles/nanonecklaces/nanorod/nanosausage, respectively. The coexistence of various nano-species implies that in these concentrations, poly-α,β-DL-aspartyl-L-arginine-assembled nano-species are interconvertible.The smallest of the coexisting nano-species of 4.8 × 104,2,−4,−6,−8 nM aqueous poly-α,β-DL-aspartyl-L-arginine are nanoparticle, nanomango, nanoparticles, nanorod, and nanoparticles, respectively, while the diameters of the nanoparticles, the length of the nanomango, the diameter of the nanoparticles, the length of the nanorod and the length of the nanorod are 10, 44, 31, 22 and 8 nm, respectively, less than 100 nm.
4.5. Ex vivo anti-platelet aggregation of poly-α,β-DL-aspartyl-L-arginine
It was shown that poly-α,β-DL-aspartyl-L-arginine (0.72, 1.45, 2.89 μmol kg−1) dose-dependently inhibited platelet aggregation ex vivo. It was also shown that at 64 μM and 0.64 μM, which covered 4, 8 and 16 μM, of aqueous solution (pH 6.7) poly-α,β-DL-aspartyl-L-arginine assembled to form mixed nano-species including nanoblocks of 65 nm in breadth and 88 nm in length, as well as nanoglobes of 65 nm in diameter.4.6. In vivo antithrombotic action of poly-α,β-DL-aspartyl-L-arginine
It was shown that poly-α,β-DL-aspartyl-L-arginine (5, 0.5 or 0.05 μmol kg−1) dose-dependently reduced the thrombus weight in vivo. These doses correspond to 50, 5 and 0.5 μM blood concentrations of poly-α,β-DL-aspartyl-L-arginine of the treated rats, respectively. It was also shown that in 64 μM and 0.64 μM aqueous solution, which covered 50, 5 and 0.5 μM blood concentrations of treated rats, poly-α,β-DL-aspartyl-L-arginine assembled to mixed nano-species including nanoblocks of 65 nm in breadth and 88 nm in length, as well as nanoglobes of 65 nm in diameter. These nano-species, of which the sizes are less than 100 nm, should be responsible for the in vivo antithrombotic potency of poly-α,β-DL-aspartyl-L-arginine.In the onset and progression of acute coronary syndromes the rift, rupture and pathogenesis of atherosclerotic plaque with the formation of occlusive thrombus are critical steps, vascular damage, stimulus of platelets and initiation of the clotting cascade are the essential factors,25–27 and the discovery of oral antithrombotic drugs is of clinical interest. Poly-α,β-DL-aspartyl-L-arginine possesses oral ex vivo anti-platelet aggregation and in vivo antithrombotic activities. In general, oral poly-α,β-DL-aspartyl-L-arginine can be absorbed in the stomach and/or in the intestinal tract, and then consequently enters the blood and tissues. In the pH environments simulating that of the stomach (pH 1.2), blood and tissue fluid (pH 7.4), poly-α,β-DL-aspartyl-L-arginine assembled into the mixture of the nano-species of various features and sizes. In these pH environments either the diameter or the length of the smallest nano-species is less than 100 nm. The smallest nano-species of poly-α,β-DL-aspartyl-L-arginine are able to be absorbed in the stomach and intestinal tract. The nano-assembling property of poly-α,β-DL-aspartyl-L-arginine should be responsible for its desirable ex vivo anti-platelet aggregation and in vivo antithrombotic actions.
Acknowledgements
This work was finished in Beijing Area Major Laboratory of Peptide and Small Molecular Drugs, supported by PHR (IHLB, KM200910025-009), Special Project (2011ZX09302-007-01) and the Natural Scientific Foundation of China (81072522 & 21103115).References
- Y. Zhao, H. J. Su, L. Fang and T. W. Tan, Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH- responsiveness properties, Polymer, 2005, 46(14), 5368–5376 CrossRef CAS.
- Y. L. Zheng, W. L. Yang and C. C. Wang, et al., Nanoparticles based on the complex of chitosan and polyaspartic acid sodium salt: Preparation, characterization and the use for 5-fluorouracil delivery, Eur. J. Pharm. Biopharm., 2007, 67(3), 621–631 CrossRef CAS.
- T. W. Wang, Q. Xu, Y. Wua, A. J. Zeng, M. J. Li and H. X. Gao, Quarternized chitosan (QCS)/poly (aspartic acid) nanoparticles as a protein drug-delivery system, Carbohydr. Res., 2009, 344(7), 908–914 CrossRef CAS.
- E. M. Burke, Y. Guo, L. Colon, M. Rahima, A. Veis and G. H. Nancollas, Influence of polyaspartic acid and phosphophoryn on octacalcium phosphate growth kinetics, Colloids Surf., B, 2000, 17(1), 49–57 CrossRef CAS.
- A. Bigi, B. Bracci and S. Panzavolta, et al., Morphological and structural modifications of octacalcium phosphate induced by poly-L-aspartate, Cryst. Growth Des., 2004, 4(1), 141–146 CAS.
- L. E. Euliss, S. G. Grancharov and S. O'Brien, et al., Cooperative assembly of magnetic nanoparticles and block copolypeptides in aqueous media, Nano Lett., 2003, 3(11), 1489–1493 CrossRef CAS.
- J. Yang, F. Wang, L. Fang and T. W. Tan, The effects of aging tests on a novel chemical sand-fixing agent – polyaspartic acid, Compos. Sci. Technol., 2007, 67(10), 2160–2164 CrossRef CAS.
- Y. Fujita, M. Mie and E. Kobatake, Construction of nanoscale protein particle using temperature- sensitive elastin-like peptide and polyaspartic acid chain, Biomaterials, 2009, 30(20), 3450–3457 CrossRef CAS.
- N. Sadeghiani, L. S. Barbosa, L. P. Silva, R. B. Azevedo, P. C. Morais and Z. G. M. Lacava, Genotoxicity and inflammatory investigation in mice treated with magnetite nanoparticles surface coated with polyaspartic acid, J. Magn. Magn. Mater., 2005, 289, 466–468 CrossRef CAS.
- X. Y. Wang, B. I. Lee and L. Mann, Dispersion of barium titanate with polyaspartic acid in aqueous media, Colloids Surf., A, 2002, 202(1), 71–80 CrossRef CAS.
- B. Njegić-Džakula, L. Brecčević, G. Falini and D. Kralj, Calcite crystal growth kinetics in the presence of charged synthetic polypeptides, Cryst. Growth Des., 2009, 9(5), 2425–2434 Search PubMed.
- D. Kołodyńska, Z. Hubicki and M. Gęca, Polyaspartic acid as a new complexing agent in removal of heavy metal ions on polystyrene anion exchangers, Ind. Eng. Chem. Res., 2008, 47(16), 6221–6227 CrossRef.
- P. K. Ajikumar, R. Lakshminarayanan and S. Valiyaveettil, Controlled deposition of thin films of calcium carbonate on natural and synthetic templates, Cryst. Growth Des., 2004, 4(2), 331–335 CAS.
- Y. T. Wu and C. Grant, Effect of chelation chemistry of sodium poly-aspartate on the dissolution of calcite, Langmuir, 2002, 18(18), 6813–6820 CrossRef CAS.
- Y. N. Tan, J. Y. Lee and D. I. C. Wang, Morphosynthesis of gold nanoplates in polypeptide multilayer films, J. Phys. Chem. C, 2009, 113(25), 10887–10895 CAS.
- P. K. Ajikumar, B. J. M. Low and S. Valiyaveettil, Role of soluble polymers on the preparation of functional thin films of calcium carbonate, Surf. Coat. Technol., 2005, 198(1–3), 227–230 CrossRef CAS.
- H. K. Kovacs, J. Kovacs, M. A. Pisano and B. A. Shidolovsky, Synthesis and inhibitory activity of polyaspartic acid derivatives, J. Med. Chem., 1967, 10(5), 904–908 CrossRef CAS.
- J. Yang, Z. Y. Tang, Y. Y. Wang and S. Q. Peng, Polyaspartoyl-L-arginine protects endothelial cells against injury, Eur J Pharmacol., 2008, 599(1–3), 96–101 Search PubMed.
- Z. Y. Tang, J. Yang, X. Y. Liu, Y. Y. Wang and S. Q. Peng, Polyaspartoyl-L-arginine enhances prostacyclin synthesis in rat aortic endothelial cells, Eur. J. Pharmacol., 2008, 601(1–3), 124–128 CrossRef CAS.
- Z. Y. Tang, Y. Y. Wang, Y. L. Xiao, M. Zhao and S. Q. Peng, Anti-thrombotic activity of PDR, a newly synthesized L-Arg derivative, on three thrombosis models in rats, Thromb. Res., 2003, 110(1–3), 127–133 CrossRef CAS.
- A. Vegotsky, K. Harada and S. W. Fox, The characterization of polyaspartic acid and some related compounds, J. Am. Chem. Soc., 1958, 80(13), 3361–3366 CrossRef CAS.
- J. Kovacs, H. N. Kovacs, I. Könyves, J. Császár, T. Vajda and H. Mix, Chemical studies of polyaspartic acids, J. Org. Chem., 1961, 26(4), 1084–1091 CrossRef.
- S. Meair and C. E. Dempfle, In vitro models for assessing transcranial ultrasound-enhanced thrombolysis, Stroke, 2005, 36(5), 929–931 CrossRef.
- S. Pfaffenberger, B. Devcic-Kuhar and C. Kollmann, et al., Can a commercial diagnostic ultrasound device accelerate thrombolysis? An in vitro skull model, Stroke, 2005, 36(1), 124–128 CrossRef.
- B. Ashby, J. L. Daniel and J. B. Smith, Mechanisms of platelet activation and inhibition, Platelets Health Dis., 1990, 4(1), 1–26 CAS.
- M. J. Davies, A macro and micro view of coronary vascular insult in ischemic heart disease, Circulation, 1990, 82(3), 38–46 Search PubMed.
- M. J. Davies and A. C. Thomas, Plaque fissuring: the cause of acute myocardial infarction, sudden ischemic death, and crescendo angina, Br. Heart J., 1985, 53(4), 363–373 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
